Toxicological Studies of 212Pb Intravenously or Intraperitoneally Injected into Mice for a Phase 1 Trial
Abstract
:1. Introduction
2. Results
2.1. Mortality of Normal Balb/c Mice Injected i.p. or i.v. with 212Pb
| Activity (MBq) | Necropsy Groups | Hematology Groups | ||||
|---|---|---|---|---|---|---|
| i.v. | i.p. | i.v. | i.p. | |||
| Day 7 | Day 90 | Day 7 | Day 90 | Day 90 | ||
| 0.0925 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| 0.185 | 10/10 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| 0.278 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| 0.370 | 5/5 | 10/10 | 5/5 | 10/10 | 5/5 | 5/5 |
| 0.555 | 5/5 | 5/5 | 5/5 | 7/10 | 5/5 | 5/5 |
| 0.740 | 5/5 | 3/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| 1.110 | 5/5 | 5/5 | 5/5 | 4/5 | 5/5 | 4/5 |
| 1.488 | ND a | 1/4 | 3/5 | |||
| 1.850 | ND | 2/5 | 0/5 | |||
2.2. Effect of 212Pb Given i.p. or i.v. on the Body Weight of Normal Balb/c Mice
| Body Weight (g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | 7 Days | 90 Days | % Change | ||||||
| Untreated | 21.1 ± 2.1 | 25.4 ± 3.6 | 20.4 | ||||||
| MBq | Time Weighed | Body Weight (g) | |||||||
| I.P. Injected | I.V. Injected | ||||||||
| 7 Days | % Change | 90 Days | % Change | 7 Days | % Change | 90 Days | % Change | ||
| 0.0925 | Initial a | 21.8 ± 1.1 b | 0.5 | 20.6 ± 0.8 | 13.1 | 26.1 ± 2.4 | 3.1 | 20.8 ± 1.5 | 12.0 |
| Final | 21.9 ± 1.6 | 23.3 ± 0.9 | 26.9 ± 1.5 | 23.3 ± 0.9 | |||||
| 0.185 | Initial | 20.8 ± 1.6 | 2.4 | 21.2 ± 1.1 | 14.2 | 28.1 ± 3.0 | −0.4 | 19.9 ± 1.1 | 17.6 |
| Final | 21.3 ± 1.6 | 24.3 ± 1.5 | 28.0 ± 3.3 | 23.4 ± 2.1 | |||||
| 0.278 | Initial | 22.8 ± 1.6 | 0.9 | 21.3 ± 1.2 | 2.8 | 27.1 ± 2.8 | 0 | 20.8 ± 0.8 | 7.2 |
| Final | 23.0 ± 1.5 | 21.9 ± 1.3 | 27.1 ± 2.2 | 22.3 ± 1.0 | |||||
| 0.370 | Initial | 26.7 ± 2.5 | −9.7 | 21.8 ± 1.7 | 8.7 | 25.8 ± 1.0 | −2.3 | 22.1 ± 0.9 | 3.6 |
| Final | 24.1 ± 1.7 | 23.7 ± 2.1 | 25.2 ± 0.6 | 22.9 ± 0.8 | |||||
| 0.555 | Initial | 26.4 ± 2.9 | −3.4 | 22.0 ± 1.2 | 4.5 | 25.6 ± 1.8 | −3.1 | 20.2 ± 0.5 | 1.0 |
| Final | 25.5 ± 3.3 | 23.0 ± 1.4 | 24.8 ± 2.1 | 20.4 ± 1.5 | |||||
| 0.740 | Initial | 21.9 ± 1.0 | −6.4 | 24.8 ± 2.1 | 33.6 | 26.0 ± 1.8 | −9.6 | 25.6 ± 2.0 | −6.6 |
| Final | 20.5 ± 0.8 | 32.2 ± 1.9 | 23.5 ± 0.5 | 23.9 ± 1.5 | |||||
| 1.110 | Initial | 25.4 ± 1.8 | −9.8 * | 19.7 ± 2.4 | 10.7 | 28.3 ± 2.0 | −17.6 * | 26.5 ± 1.9 | −6.4 * |
| Final | 22.9 ± 2.5 | 21.8 ± 2.5 | 23.3 ± 3.2 | 24.8 ± 1.6 | |||||
| 1.488 | Initial | ND c | 23.5 ± 1.5 | 4.3 | |||||
| Final | 24.5 | ||||||||
| 1.850 | Initial | ND | 24.2 ± 1.9 | ||||||
| Final | |||||||||
2.3. Hematological Analysis of Normal Balb/c Mice Injected i.p. or i.v. with 212Pb
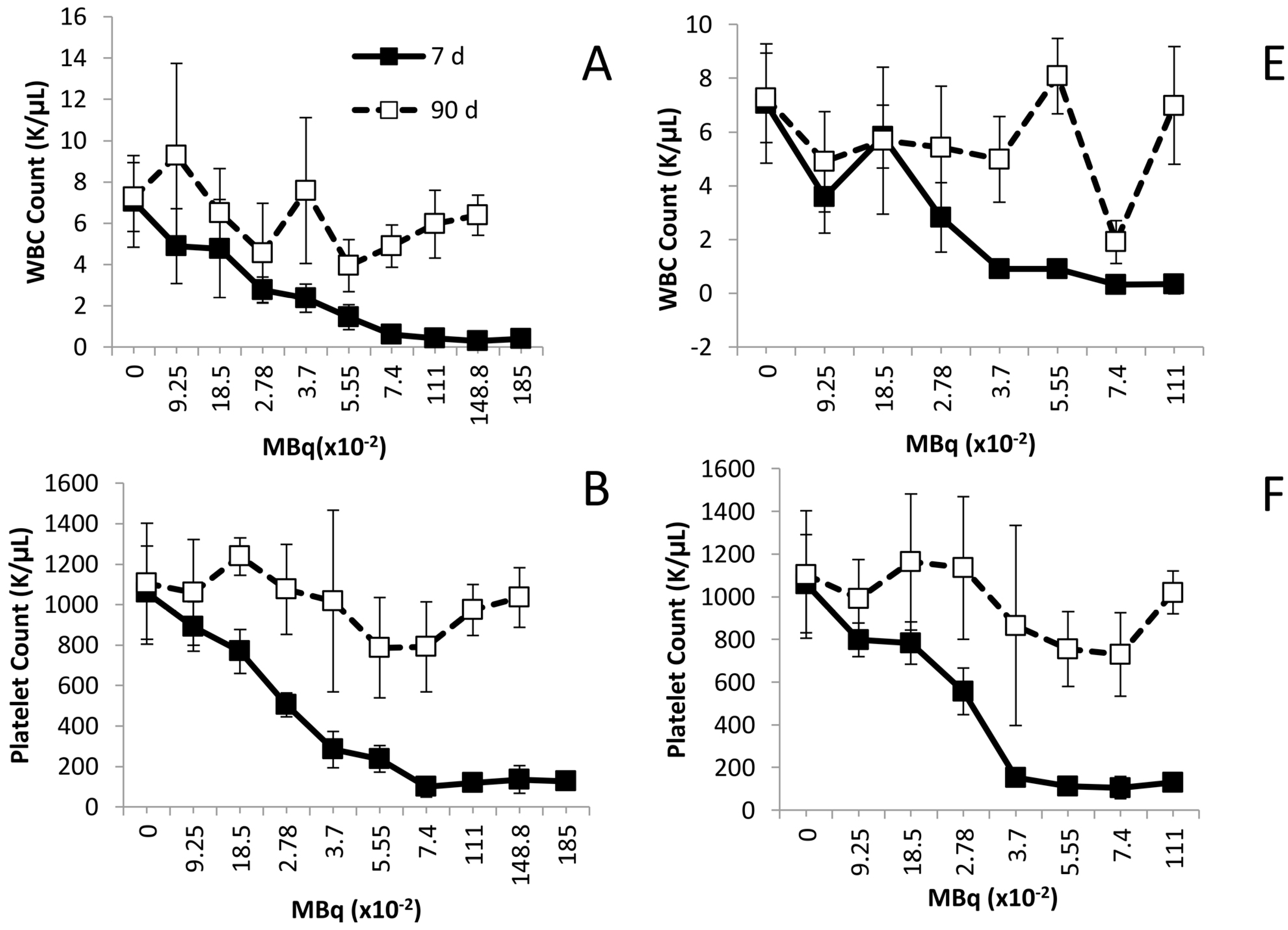
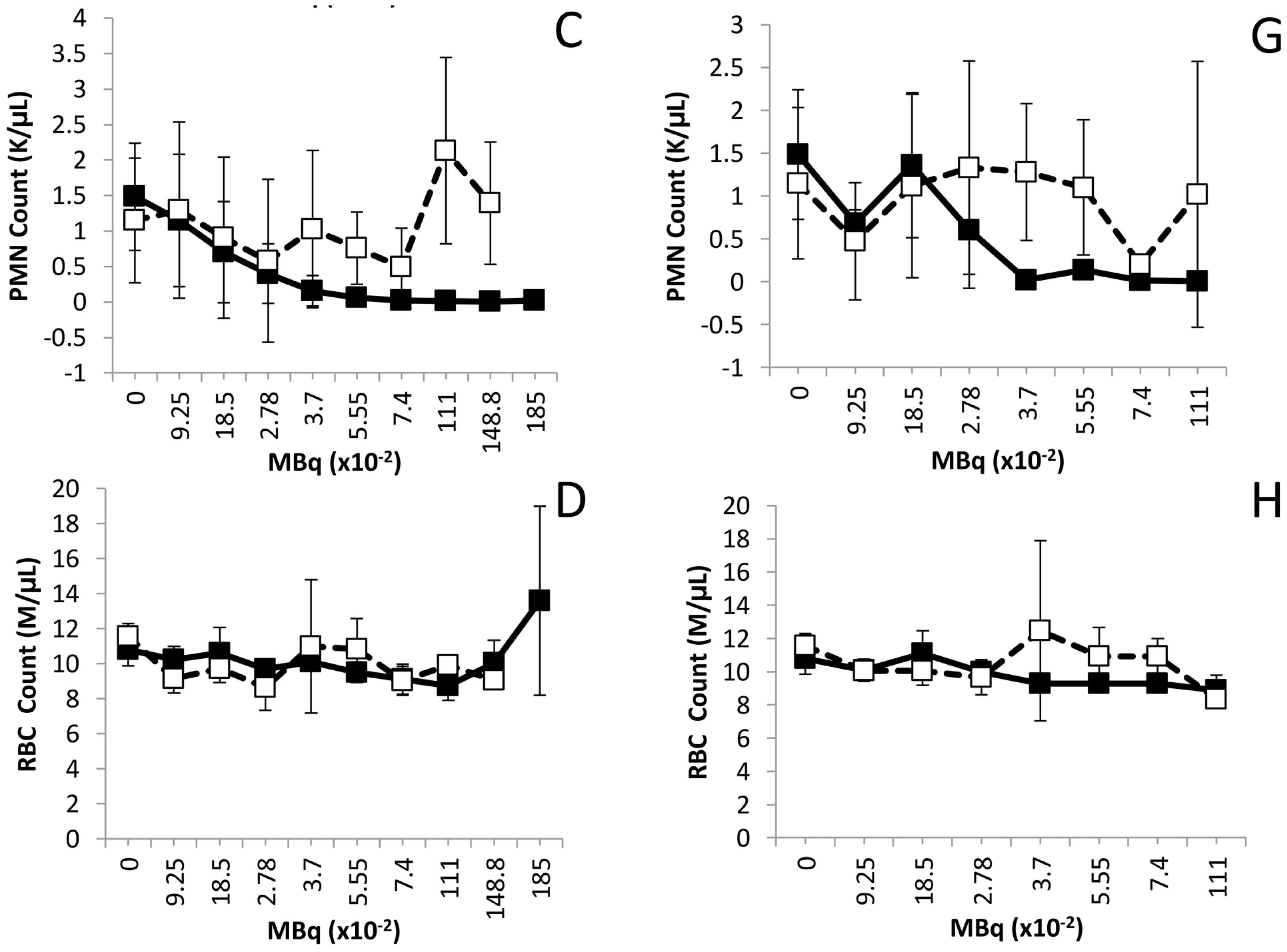

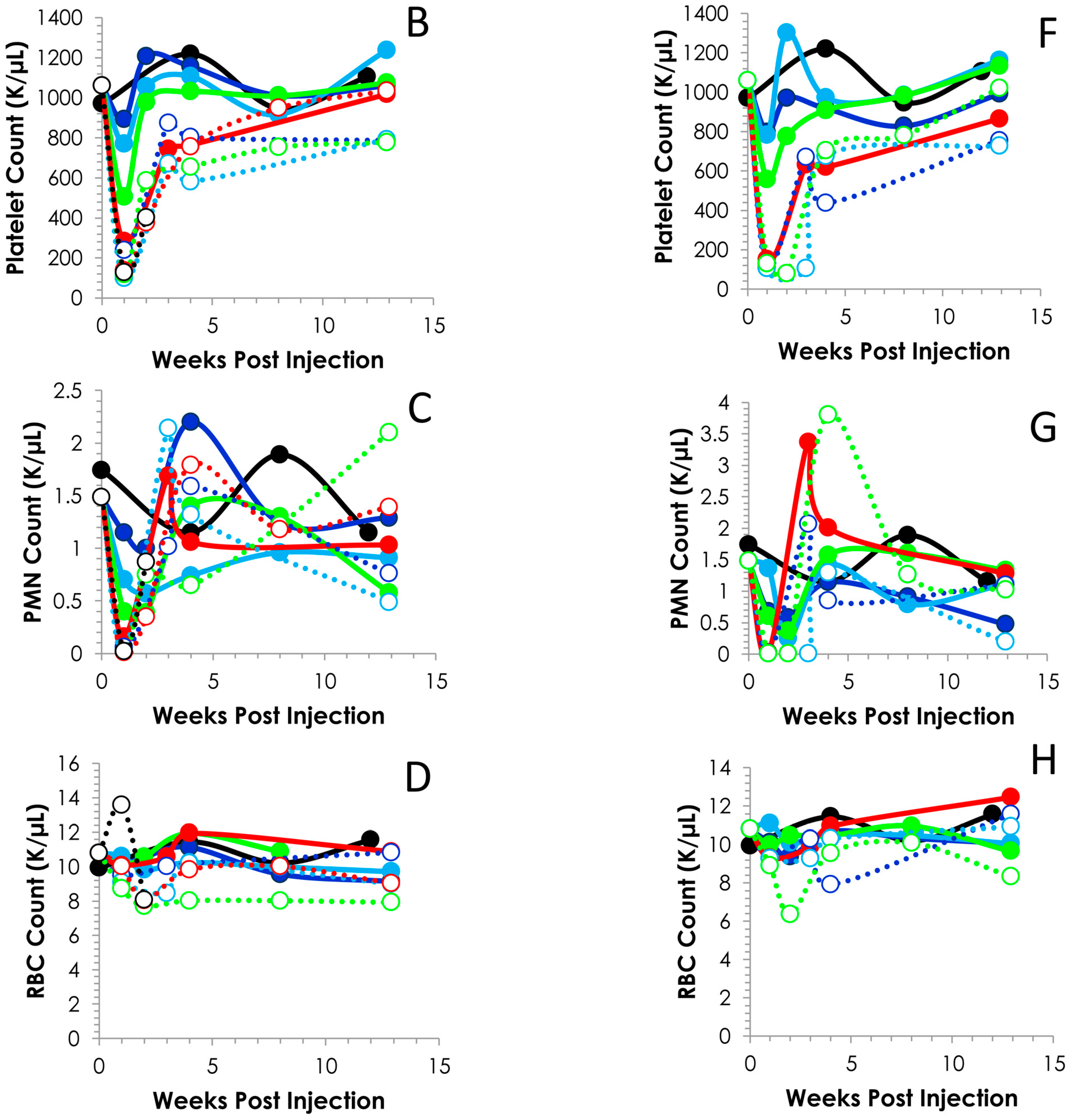
2.4. Effects of Increasing 212Pb Activity on Blood Chemistry
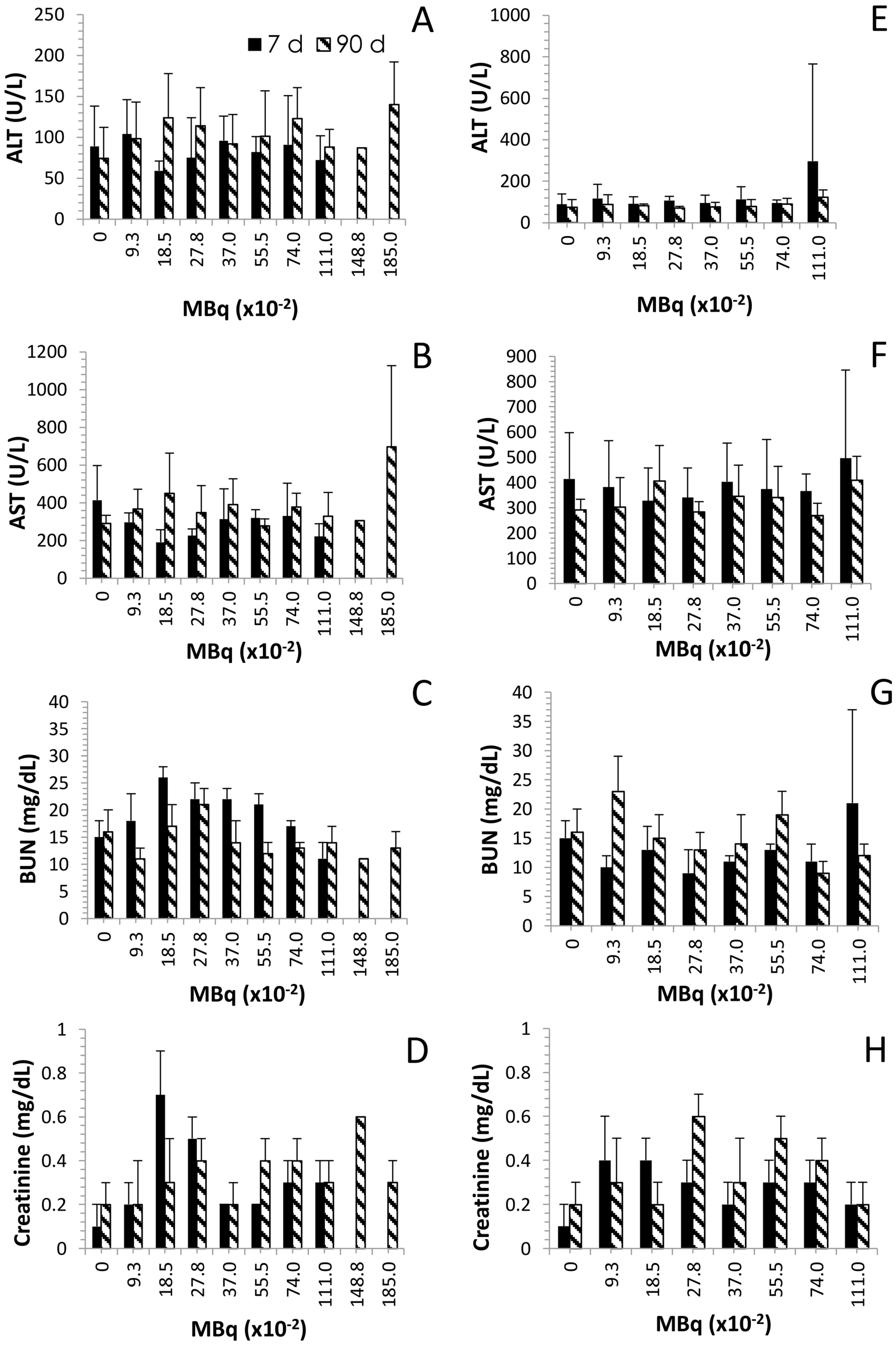
2.5. Histological Findings Following Injection of Normal Balb/c Mice with 212Pb
| Injection Route | Activity (MBq) | Timepoint (day) | Kidneys (Chronic Nephritis) | Decreases in WBC and Platelet Counts | Bone Marrow Hypoplasia a | Spleen (EMH, Hyperplasia of Red Pulp) b | Increases in Liver Parameters | Hepatic Vacuolization |
|---|---|---|---|---|---|---|---|---|
| None | 0 | 7 | 0/5 | 0 | 0/5 | 0/5 | 0 | 0 |
| I.V. | 0.185 | 7 | 3/5 | 0 | 0/5 | 0/5 | 0 | 1/5 |
| 0.278 | 7 | 4/5 | Yes | 3/5 | 4/5 | 0 | 5/5 | |
| 0.555 | 7 | 1/5 | Yes+ | 4/5 | 0/5 | 0 | 2/5 | |
| 0.555 | 90 | 5/5 | 0 | 0/5 | 4/5 | 0 | 2/5 | |
| I.P. | 0.185 | 7 | 0/5 | 0 | 0/5 | 3/5 | 0 | 0 |
| 0.278 | 7 | 1/5 | Yes | 5/5 | 4/5 | 0 | 0 | |
| 0.740 | 7 | 3/5 | Yes | 5/5 | 0/5 | 0 | 2/5 | |
| 0.740 | 90 | 5/5 | Yes | 5/5 | 2/5 | 0 | 4/5 | |
| 1.850c | 90 | 2/2 | ND d | 0/2 | 0/2 | ++ | 2/2 |
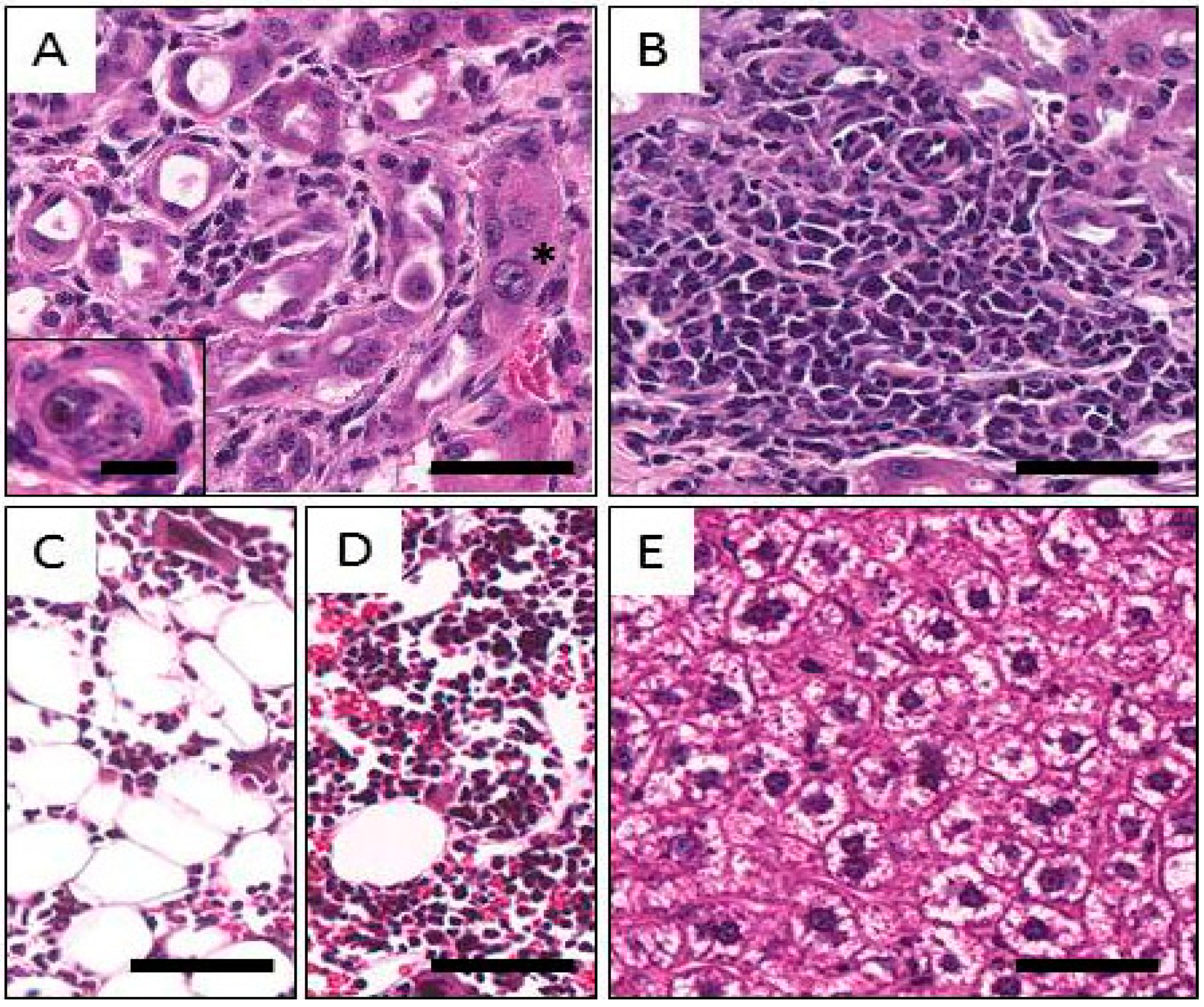
3. Discussion
4. Experimental Section
4.1. Preparation of 212Pb and Sampling
4.2. Animal Model
4.3. Measurements
4.3.1. Body Weight
4.3.2. Hematology
4.3.3. Clinical Chemistry
4.3.4. Histopathology
4.4. Statistical Analysis
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Horak, E.; Hartmann, F.; Garmestani, K.; Wu, C.; Brechbiel, M.; Gansow, O.A.; Landolfi, N.F.; Waldmann, T.A. Radioimmunotherapy targeting of HER2/neu oncoprotein on ovarian tumor using lead-212-DOTA-AE1. J. Nucl. Med. 1997, 38, 1944–1950. [Google Scholar]
- Milenic, D.E.; Baidoo, K.E.; Kim, Y.S.; Brechbiel, M.W. Evaluation of cetuximab as a candidate for targeted alpha-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. MAbs 2015, 7, 255–264. [Google Scholar]
- Milenic, D.E.; Baidoo, K.E.; Shih, J.H.; Wong, K.J.; Brechbiel, M.W. Evaluation of platinum chemotherapy in combination with HER2-targeted alpha-particle radiation. Cancer Biother. Radiopharm. 2013, 28, 441–449. [Google Scholar]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Abdulla, A.; Flynn, J.; Brechbiel, M.W. Potentiation of high-LET radiation by gemcitabine: Targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin. Cancer Res. 2007, 13, 1926–1935. [Google Scholar]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Ma, D.; Abdulla, A.; Brechbiel, M.W. Alpha-particle radioimmunotherapy of disseminated peritoneal disease using a 212Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother. Radiopharm. 2005, 20, 557–568. [Google Scholar]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Baidoo, K.E.; Albert, P.S.; Wong, K.J.; Flynn, J.; Brechbiel, M.W. Multimodality therapy: Potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2008, 14, 5108–5115. [Google Scholar] [CrossRef]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. 212Pb-radioimmunotherapy induces G(2) cell-cycle arrest and delays DNA damage repair in tumor xenografts in a model for disseminated intraperitoneal disease. Mol. Cancer Ther. 2012, 11, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. 212Pb-radioimmunotherapy potentiates paclitaxel-induced cell killing efficacy by perturbing the mitotic spindle checkpoint. Br. J. Cancer 2013, 108, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Sensitization of tumor to 212Pb radioimmunotherapy by gemcitabine involves initial abrogation of G2 arrest and blocked DNA damage repair by interference with Rad51. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Impact of alpha-targeted radiation therapy on gene expression in a pre-clinical model for disseminated peritoneal disease when combined with paclitaxel. PLoS ONE 2014, 9, e108511. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Kim, Y.S.; Brechbiel, M.W. Gene expression profiling upon (212)Pb-TCMC-trastuzumab treatment in the LS-174T i.p. xenograft model. Cancer Med. 2013, 2, 646–653. [Google Scholar] [PubMed]
- Boudousq, V.; Bobyk, L.; Busson, M.; Garambois, V.; Jarlier, M.; Charalambatou, P.; Pelegrin, A.; Paillas, S.; Chouin, N.; Quenet, F.; et al. Comparison between internalizing anti-HER2 mAbs and non-internalizing anti-CEA mAbs in alpha-radioimmunotherapy of small volume peritoneal carcinomatosis using 212Pb. PLoS ONE 2013, 8, e69613. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, P.; Schneider, N.; Glover, S.; Cui, L.; Torgue, J.; Rixe, O.; Spitz, H.B.; Dong, Z. Significant systemic therapeutic effects of high-LET immunoradiation by 212Pb-trastuzumab against prostatic tumors of androgen-independent human prostate cancer in mice. Int. J. Oncol. 2012, 40, 1881–1888. [Google Scholar]
- Chappell, L.L.; Dadachova, E.; Milenic, D.E.; Garmestani, K.; Wu, C.; Brechbiel, M.W. Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl. Med. Biol. 2000, 27, 93–100. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Bae, O.N.; Noh, J.Y.; Kim, K.; Kang, S.; Shin, Y.J.; Lim, K.M.; Chung, J.H. Erythrophagocytosis of lead-exposed erythrocytes by renal tubular cells: Possible role in lead-induced nephrotoxicity. Environ. Health Perspect. 2015, 123, 120–127. [Google Scholar] [CrossRef]
- Matovic, V.; Buha, A.; Ethukic-Cosic, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Roselli, M.; Brechbiel, M.W.; Pippin, C.G.; McMurray, T.J.; Carrasquillo, J.A.; Colcher, D.; Lambrecht, R.; Gansow, O.A.; Schlom, J. In vivo evaluation of a lead-labeled monoclonal antibody using the DOTA ligand. Eur. J. Nucl. Med. 1998, 25, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Soderland, P.; Lovekar, S.; Weiner, D.E.; Brooks, D.R.; Kaufman, J.S. Chronic kidney disease associated with environmental toxins and exposures. Adv. Chronic. Kidney Dis. 2010, 17, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Cuenot, F.; Meyer, M.; Espinosa, E.; Bucaille, A.; Burgat, R.; Guilard, R.; Marichal-Westrich, C. New Insights into the complexation of lead(II) by 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane (DOTAM): Structural, thermodynamic, and kinetic studies. Eur. J. Inorg. Chem. 2008, 2008, 267–283. [Google Scholar] [CrossRef]
- Naruki, Y.; Carrasquillo, J.A.; Reynolds, J.C.; Maloney, P.J.; Frincke, J.M.; Neumann, R.D.; Larson, S.M. Differential cellular catabolism of 111In, 90Y and 125I radiolabeled T101 anti-CD5 monoclonal antibody. Int. J. Rad. Appl. Instrum. B 1990, 17, 201–207. [Google Scholar] [CrossRef]
- Yao, Z.; Garmestani, K.; Wong, K.J.; Park, L.S.; Dadachova, E.; Yordanov, A.; Waldmann, T.A.; Eckelman, W.C.; Paik, C.H.; Carrasquillo, J.A. Comparative cellular catabolism and retention of astatine-, bismuth-, and lead-radiolabeled internalizing monoclonal antibody. J. Nucl. Med. 2001, 42, 1538–1544. [Google Scholar] [PubMed]
- Rogers, B.E.; Franano, F.N.; Duncan, J.R.; Edwards, W.B.; Anderson, C.J.; Connett, J.M.; Welch, M.J. Identification of metabolites of 111In-diethylenetriaminepentaacetic acid-monoclonal antibodies and antibody fragments in vivo. Cancer Res. 1995, 55, 5714s–5720s. [Google Scholar] [PubMed]
- Wu, C.; Jagoda, E.; Brechbiel, M.; Webber, K.O.; Pastan, I.; Gansow, O.A.; Eckelman, W.C. Biodistribution and catabolism of Ga-67-labeled anti-Tac dsFv fragment. Bioconjugate Chem. 1997, 8, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Schlom, J.; Hand, P.H.; Greiner, J.W.; Colcher, D.; Shrivastav, S.; Carrasquillo, J.A.; Reynolds, J.C.; Larson, S.M.; Raubitschek, A. Innovations that influence the pharmacology of monoclonal antibody guided tumor targeting. Cancer Res. 1990, 50, 820s–827s. [Google Scholar] [PubMed]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M., Jr. Dose escalation and dosimetry of first-in-human alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Revised Guides for Organ Sampling and Trimming in Rats and Mice. Available online: http://www.niehs.nih.gov/research/atniehs/labs/lep/path-support/necropsy-support/guides (accessed on 21 July 2015).
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenic, D.E.; Molinolo, A.A.; Solivella, M.S.; Banaga, E.; Torgue, J.; Besnainou, S.; Brechbiel, M.W.; Baidoo, K.E. Toxicological Studies of 212Pb Intravenously or Intraperitoneally Injected into Mice for a Phase 1 Trial. Pharmaceuticals 2015, 8, 416-434. https://doi.org/10.3390/ph8030416
Milenic DE, Molinolo AA, Solivella MS, Banaga E, Torgue J, Besnainou S, Brechbiel MW, Baidoo KE. Toxicological Studies of 212Pb Intravenously or Intraperitoneally Injected into Mice for a Phase 1 Trial. Pharmaceuticals. 2015; 8(3):416-434. https://doi.org/10.3390/ph8030416
Chicago/Turabian StyleMilenic, Diane E., Alfredo A. Molinolo, María S. Solivella, Eileen Banaga, Julien Torgue, Sarah Besnainou, Martin W. Brechbiel, and Kwamena E. Baidoo. 2015. "Toxicological Studies of 212Pb Intravenously or Intraperitoneally Injected into Mice for a Phase 1 Trial" Pharmaceuticals 8, no. 3: 416-434. https://doi.org/10.3390/ph8030416





