The Dipeptide Monoester Prodrugs of Floxuridine and Gemcitabine—Feasibility of Orally Administrable Nucleoside Analogs
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. The Synthesis of Gemcitabine Prodrug and Floxuridine Prodrug
2.3. Cell Culture
2.4. Hydrolysis Studies
2.4.1. Enzymatic Stability
2.4.2. Chemical Stability
2.5. Uptake Studies
2.6. Solution for Single-Pass Intestinal Perfusion
2.7. Single-Pass Intestinal Perfusion Studies in Mice
2.8. Cell Proliferation Assays
2.9. Data Analysis
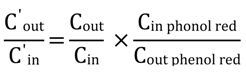
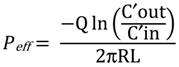
2.10. HPLC Analysis
2.11. LC-MS Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Floxuridine and Gemcitabine Prodrugs
| Prodrug | Purity (%) (HPLC) | ESI-MS (M+H)+ | LogP a | |
|---|---|---|---|---|
| Required | Observed | |||
| Gemcitabine | 95.3 | 263.2 | 263.9 | −1.14 |
| 5′-L-Phenylalanyl-L-tyrosylgemcitabine | 95.5 b | 574.5 | 574.4 | 1.04 |
| Floxuridine | 100.0 | 246.2 | 245.0 | −0.51 |
| 5′-L-Phenylalanyl-L-tyrosyl-floxuridine | 99.0 | 557.2 | 557.2 | 0.12 |
3.2. The Stability of Floxuridine, 5′-l-Phenylalanyl-l-Tyrosyl-floxuridine, Gemcitabine, and 5′-l-Phenylalanyl-l-Tyrosyl-gemcitabine in Three Buffers (Acidic pH, SIF (pH 6.0), and pH 7.4), and Caco-2, Panc-1, and AsPC-1 Cell Homogenates
| Prodrug | 0.01 N HCl t1/2 (min) | SIF pH 6.0 t1/2 (min) | Buffer pH 7.4 t1/2 (min) | Caco-2 cell homogenates t1/2 (min) | Panc-1 cell homogenates t1/2 (min) | AsPC-1 cell homogenates t1/2 (min) |
|---|---|---|---|---|---|---|
| Gemcitabine | >120 | >120 | >120 | 105.0 ± 6.1 | >120 | 33.7 ± 14.5 |
| 5′-l-Phenylalanyl-l-tyrosyl-gemcitabine | >120 | >120 | 33.6 ± 1.4 | 14.7 ± 4.4 | 30.2 ± 1.1 | 8.1 ± 0.6 |
| Floxuridine | >120 | >120 | >120 | 14.3 ± 7.0 a | 41.7 ± 6.8 | 6.4 ± 3.2 a |
| 5′-l-Phenylalanyl-l-tyrosyl-floxuridine | >120 | >120 | >120 | 103.8 ± 55.5 b | 40.4 ± 0.2 | 59.7 ± 1.4 b |
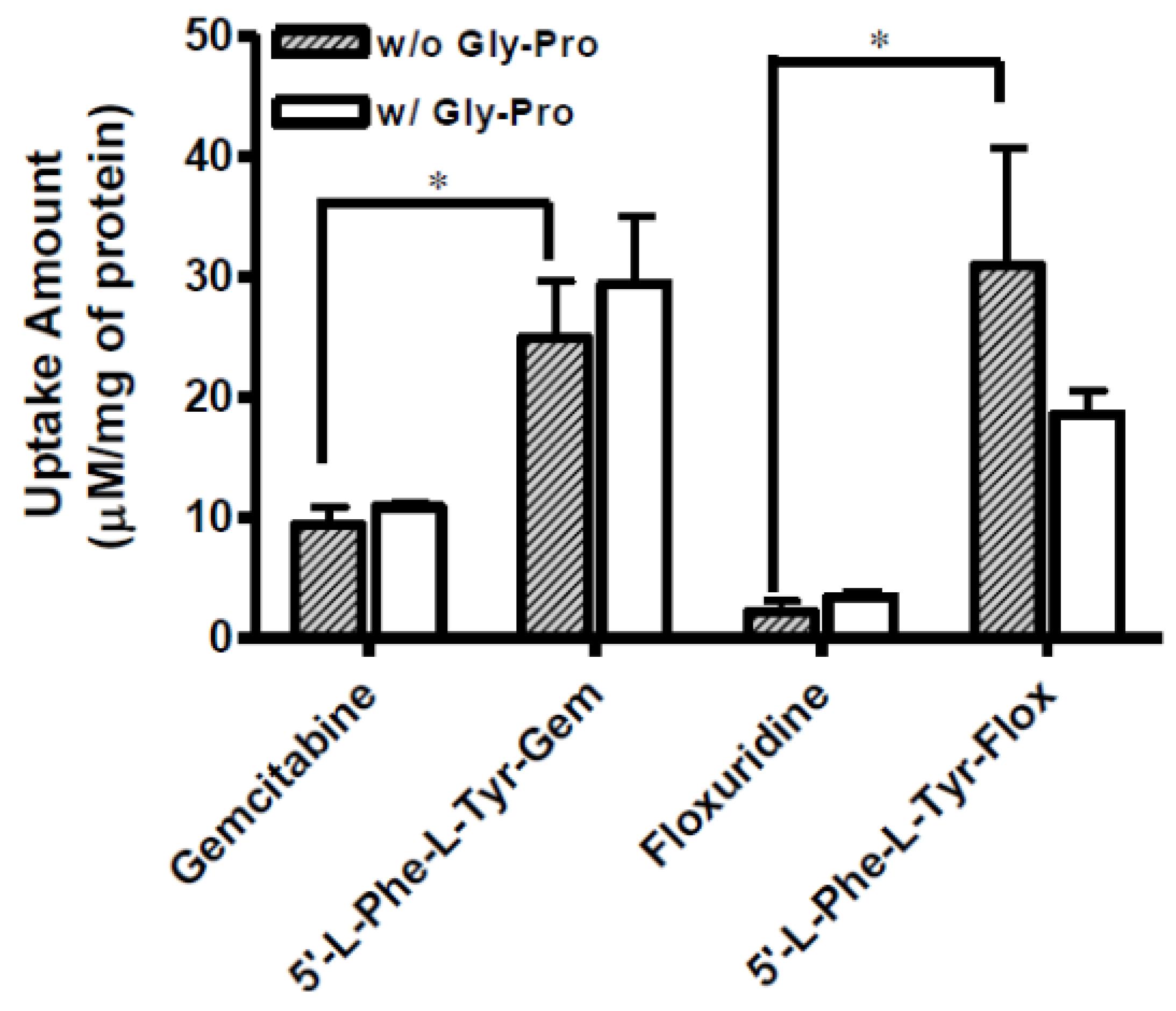
3.3. Uptake Study of Floxuridine, 5′-l-Phenylalanyl-l-Tyrosyl-floxuridine, Gemcitabine, and 5′-l-Phenylalanyl-l-Tyrosyl-gemcitabine in Caco-2, Panc-1, and AsPC-1 Cells with Both the Presence and the Absence of 10 mM Gly-Pro
| Compound | Cells | Metabolite * (%) | Parent drug * (%) | Prodrug (%) |
|---|---|---|---|---|
| Gemcitabine | Caco-2 | 5 | 95 | - |
| 5′-l-Phenylalanyl-l-tyrosyl-gemcitabine | 3 | 91 | 6 | |
| Floxuridine | 100 | 0 | - | |
| 5′-l-Phenylalanyl-l-tyrosyl-floxuridine | 8 | 47 | 45 | |
| Gemcitabine | Panc-1 | 80 | 20 | - |
| 5′-l-Phenylalanyl-l-tyrosyl-gemcitabine | 76 | 15 | 9 | |
| Floxuridine | 0 | 0 | - | |
| 5′-l-Phenylalanyl-l-tyrosyl-floxuridine | 0 | 2 | 98 | |
| Gemcitabine | AsPC-1 | 10 | 90 | - |
| 5′-l-Phenylalanyl-l-tyrosyl-gemcitabine | 28 | 33 | 39 | |
| Floxuridine | 67 | 33 | - | |
| 5′-l-Phenylalanyl-l-tyrosyl-floxuridine | 40 | 0 | 60 |


3.4. In Situ Permeability of Floxuridine, 5′-l-Phenylalanyl-l-Tyrosylfloxuridine, Gemcitabine, and 5′-l-Phenylalanyl-l-Tyrosylgemcitabine in the Single-Pass Intestinal Perfusion Study and the Drug Concentration in Plasma in Mice
| Prodrug/drug | Peff, mouse perfusion (×10−5 cm/s) |
|---|---|
| Gemcitabine | 0.2 ± 0.2 |
| 5′-l-Phenylalanyl-l-tyrosyl-gemcitabine | 2.2 ± 0.4 * |
| Floxuridine | 0.1 ± 0.8 |
| 5′-l-Phenylalanyl-l-tyrosyl-floxuridine | 1.9 ± 0.1 * |
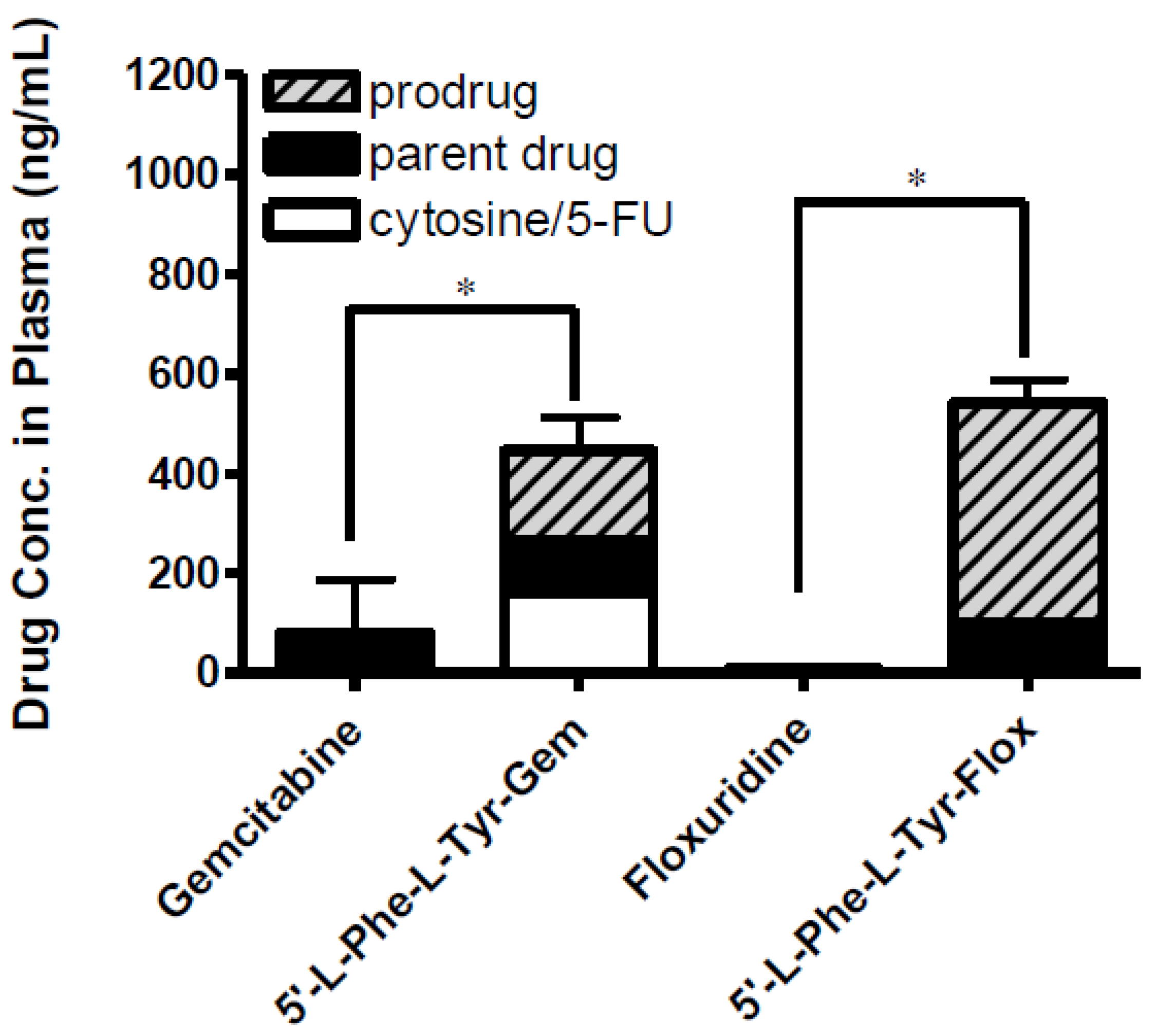

3.5. Cell Proliferation Assay
| Prodrug/drug | GI50 AsPC-1 (mM) | GI50 Panc-1 (mM) |
|---|---|---|
| Gemcitabine | 10.2 ± 1.6 | ND |
| 5′-l-Phenylalanyl-l-tyrosyl-gemcitabine | 5.0 ± 0.3 | 3.2 ± 0.7 |
| Floxuridine | 22.9 ± 5.7 # | ND |
| 5′-l-Phenylalanyl-l-tyrosyl-floxuridine | 4.2 ± 0.1 | 3.0 ± 0.3 |
4. Conclusions
Supplementary Files
Acknowledgments
Conflicts of Interest
References
- Melichar, B. Hepatic arterial infusion in colorectal carcinoma: Is anatomical targeting still relevant in an era of molecularly targeted therapy? Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2012, 156, 81–92. [Google Scholar] [CrossRef]
- Scheithauer, W.; Rosen, H.; Kornek, G.V.; Sebesta, C.; Depisch, D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993, 306, 752–755. [Google Scholar] [CrossRef]
- Van Moorsel, C.J.; Peters, G.J.; Pinedo, H.M. Gemcitabine: Future prospects of single-agent and combination studies. Oncologist 1997, 2, 127–134. [Google Scholar]
- Zhang, Y.; Kim, W.Y.; Huang, L. Systemic delivery of gemcitabine triphosphate via LCP nanoparticles for NSCLC and pancreatic cancer therapy. Biomaterials 2013, 34, 3447–3458. [Google Scholar] [CrossRef]
- Han, H.; de Vrueh, R.L.; Rhie, J.K.; Covitz, K.M.; Smith, P.L.; Lee, C.P.; Oh, D.M.; Sadee, W.; Amidon, G.L. 5′-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm. Res. 1998, 15, 1154–1159. [Google Scholar] [CrossRef]
- Han, H.K.; Oh, D.M.; Amidon, G.L. Cellular uptake mechanism of amino acid ester prodrugs in Caco-2/hPEPT1 cells overexpressing a human peptide transporter. Pharm. Res. 1998, 15, 1382–1386. [Google Scholar] [CrossRef]
- Song, X.; Lorenzi, P.L.; Landowski, C.P.; Vig, B.S.; Hilfinger, J.M.; Amidon, G.L. Amino acid ester prodrugs of the anticancer agent gemcitabine: Synthesis, bioconversion, metabolic bioevasion, and hPEPT1-mediated transport. Mol. Pharm. 2005, 2, 157–167. [Google Scholar] [CrossRef]
- Song, X.; Vig, B.S.; Lorenzi, P.L.; Drach, J.C.; Townsend, L.B.; Amidon, G.L. Amino acid ester prodrugs of the antiviral agent 2-bromo-5,6-dichloro-1-(beta-d-ribofuranosyl)benzimidazole as potential substrates of hPEPT1 transporter. J. Med. Chem. 2005, 48, 1274–1277. [Google Scholar] [CrossRef]
- Talluri, R.S.; Samanta, S.K.; Gaudana, R.; Mitra, A.K. Synthesis, metabolism and cellular permeability of enzymatically stable dipeptide prodrugs of acyclovir. Int. J. Pharm. 2008, 361, 118–124. [Google Scholar] [CrossRef]
- Tolle-Sander, S.; Lentz, K.A.; Maeda, D.Y.; Coop, A.; Polli, J.E. Increased acyclovir oral bioavailability via a bile acid conjugate. Mol. Pharm. 2004, 1, 40–48. [Google Scholar] [CrossRef]
- Tsume, Y.; Hilfinger, J.M.; Amidon, G.L. Enhanced cancer cell growth inhibition by dipeptide prodrugs of floxuridine: Increased transporter affinity and metabolic stability. Mol. Pharm. 2008, 5, 717–727. [Google Scholar] [CrossRef]
- Tsume, Y.; Vig, B.S.; Sun, J.; Landowski, C.P.; Hilfinger, J.M.; Ramachandran, C.; Amidon, G.L. Enhanced absorption and growth inhibition with amino acid monoester prodrugs of floxuridine by targeting hPEPT1 transporters. Molecules 2008, 13, 1441–1454. [Google Scholar] [CrossRef]
- Vig, B.S.; Lorenzi, P.J.; Mittal, S.; Landowski, C.P.; Shin, H.C.; Mosberg, H.I.; Hilfinger, J.M.; Amidon, G.L. Amino acid ester prodrugs of floxuridine: Synthesis and effects of structure, stereochemistry, and site of esterification on the rate of hydrolysis. Pharm. Res. 2003, 20, 1381–1388. [Google Scholar] [CrossRef]
- Cao, F.; Gao, Y.; Wang, M.; Fang, L.; Ping, Q. Propylene glycol-linked amino acid/dipeptide diester prodrugs of oleanolic acid for PepT1-mediated transport: Synthesis, intestinal permeability, and pharmacokinetics. Mol. Pharm. 2013, 10, 1378–1387. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Saito, M.; Suzuki, Y.; Nambu, N.; Nagai, T. Specificity of esterases and structure of prodrug esters. II. Hydrolytic regeneration behavior of 5-fluoro-2′-deoxyuridine (FUdR) from 3′,5′-diesters of FUdR with rat tissue homogenates and plasma in relation to their antitumor activity. Chem. Pharm. Bull. 1985, 33, 1652–1659. [Google Scholar] [CrossRef]
- Landowski, C.P.; Vig, B.S.; Song, X.; Amidon, G.L. Targeted delivery to PEPT1-overexpressing cells: Acidic, basic, and secondary floxuridine amino acid ester prodrugs. Mol. Cancer Ther. 2005, 4, 659–667. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Casida, J.E. 3′,5′-diesters of 5-fluoro-2′-deoxyuridine: Synthesis and biological activity. Biochem. Pharmacol. 1965, 14, 1605–1619. [Google Scholar] [CrossRef]
- Wang, Z.; Pal, D.; Mitra, A.K. Stereoselective evasion of P-glycoprotein, cytochrome P450 3A, and hydrolases by peptide prodrug modification of saquinavir. J. Pharm. Sci. 2012, 101, 3199–3213. [Google Scholar] [CrossRef]
- Anand, B.S.; Patel, J.; Mitra, A.K. Interactions of the dipeptide ester prodrugs of acyclovir with the intestinal oligopeptide transporter: Competitive inhibition of glycylsarcosine transport in human intestinal cell line-Caco-2. J. Pharmacol. Exp. Ther. 2003, 304, 781–791. [Google Scholar] [CrossRef]
- Friedrichsen, G.M.; Chen, W.; Begtrup, M.; Lee, C.P.; Smith, P.L.; Borchardt, R.T. Synthesis of analogs of l-valacyclovir and determination of their substrate activity for the oligopeptide transporter in Caco-2 cells. Eur. J. Pharm. Sci. 2002, 16, 1–13. [Google Scholar] [CrossRef]
- Guo, A.; Hu, P.; Balimane, P.V.; Leibach, F.H.; Sinko, P.J. Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPEPT1) expressed in a mammalian cell line. J. Pharmacol. Exp. Ther. 1999, 289, 448–454. [Google Scholar]
- Hatanaka, T.; Haramura, M.; Fei, Y.J.; Miyauchi, S.; Bridges, C.C.; Ganapathy, P.S.; Smith, S.B.; Ganapathy, V.; Ganapathy, M.E. Transport of amino acid-based prodrugs by the Na+- and Cl(−) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J. Pharmacol. Exp. Ther. 2004, 308, 1138–1147. [Google Scholar]
- Phan, D.D.; Chin-Hong, P.; Lin, E.T.; Anderle, P.; Sadee, W.; Guglielmo, B.J. Intra- and interindividual variabilities of valacyclovir oral bioavailability and effect of coadministration of an hPEPT1 inhibitor. Antimicrob. Agents Chemother. 2003, 47, 2351–2353. [Google Scholar] [CrossRef]
- Umapathy, N.S.; Ganapathy, V.; Ganapathy, M.E. Transport of amino acid esters and the amino-acid-based prodrug valganciclovir by the amino acid transporter ATB(0,+). Pharm. Res. 2004, 21, 1303–1310. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Daniel, H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol. Sci. 2002, 23, 434–440. [Google Scholar] [CrossRef]
- Tsume, Y.; Hilfinger, J.M.; Amidon, G.L. Potential of amino acid/dipeptide monoester prodrugs of floxuridine in facilitating enhanced delivery of active drug to interior sites of tumors: A two-tier monolayer in vitro study. Pharm. Res. 2011, 28, 2575–2588. [Google Scholar] [CrossRef]
- Anand, B.S.; Katragadda, S.; Mitra, A.K. Pharmacokinetics of novel dipeptide ester prodrugs of acyclovir after oral administration: Intestinal absorption and liver metabolism. J. Pharmacol. Exp. Ther. 2004, 311, 659–667. [Google Scholar] [CrossRef]
- Meredith, D.; Temple, C.S.; Guha, N.; Sword, C.J.; Boyd, C.A.; Collier, I.D.; Morgan, K.M.; Bailey, P.D. Modified amino acids and peptides as substrates for the intestinal peptide transporter PepT1. Eur. J. Biochem. 2000, 267, 3723–3728. [Google Scholar] [CrossRef]
- Nielsen, C.U.; Andersen, R.; Brodin, B.; Frokjaer, S.; Taub, M.E.; Steffansen, B. Dipeptide model prodrugs for the intestinal oligopeptide transporter. Affinity for and transport via hPepT1 in the human intestinal Caco-2 cell line. J. Control Release 2001, 76, 129–138. [Google Scholar] [CrossRef]
- Satake, M.; Enjoh, M.; Nakamura, Y.; Takano, T.; Kawamura, Y.; Arai, S.; Shimizu, M. Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2002, 66, 378–384. [Google Scholar] [CrossRef]
- Surendran, N.; Covitz, K.M.; Han, H.; Sadee, W.; Oh, D.M.; Amidon, G.L.; Williamson, R.M.; Bigge, C.F.; Stewart, B.H. Evidence for overlapping substrate specificity between large neutral amino acid (LNAA) and dipeptide (hPEPT1) transporters for PD 158473, an NMDA antagonist. Pharm. Res. 1999, 16, 391–395. [Google Scholar] [CrossRef]
- Wenzel, U.; Gebert, I.; Weintraut, H.; Weber, W.M.; Clauss, W.; Daniel, H. Transport characteristics of differently charged cephalosporin antibiotics in oocytes expressing the cloned intestinal peptide transporter PepT1 and in human intestinal Caco-2 cells. J. Pharmacol. Exp. Ther. 1996, 277, 831–839. [Google Scholar]
- Wenzel, U.; Thwaites, D.T.; Daniel, H. Stereoselective uptake of beta-lactam antibiotics by the intestinal peptide transporter. Br. J. Pharmacol. 1995, 116, 3021–3027. [Google Scholar] [CrossRef]
- Yang, B.; Smith, D.E. Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice. Drug Metab. Dispos. 2013, 41, 608–614. [Google Scholar] [CrossRef]
- Gonzalez, D.E.; Covitz, K.M.; Sadee, W.; Mrsny, R.J. An oligopeptide transporter is expressed at high levels in the pancreatic carcinoma cell lines AsPc-1 and Capan-2. Cancer Res. 1998, 58, 519–525. [Google Scholar]
- Garcia-Manteiga, J.; Molina-Arcas, M.; Casado, F.J.; Mazo, A.; Pastor-Anglada, M. Nucleoside transporter profiles in human pancreatic cancer cells: Role of hCNT1 in 2′,2′-difluorodeoxycytidine- induced cytotoxicity. Clin. Cancer Res. 2003, 9, 5000–5008. [Google Scholar]
- Grem, J.L. 5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Investig. N. Drugs 2000, 18, 299–313. [Google Scholar] [CrossRef]
- Kahramanogullari, O.; Fantaccini, G.; Lecca, P.; Morpurgo, D.; Priami, C. Algorithmic modeling quantifies the complementary contribution of metabolic inhibitions to gemcitabine efficacy. PloS One 2012, 7, e50176. [Google Scholar]
- Veltkamp, S.A.; Pluim, D.; van Eijndhoven, M.A.; Bolijn, M.J.; Ong, F.H.; Govindarajan, R.; Unadkat, J.D.; Beijnen, J.H.; Schellens, J.H. New insights into the pharmacology and cytotoxicity of gemcitabine and 2′,2′-difluorodeoxyuridine. Mol. Cancer Ther. 2008, 7, 2415–2425. [Google Scholar] [CrossRef]
- O’Neill, V.J.; Twelves, C.J. Oral cancer treatment: Developments in chemotherapy and beyond. Br. J. Cancer 2002, 87, 933–937. [Google Scholar] [CrossRef]
- Stuurman, F.E.; Nuijen, B.; Beijnen, J.H.; Schellens, J.H. Oral anticancer drugs: Mechanisms of low bioavailability and strategies for improvement. Clin. Pharmacokinet. 2013, 52, 399–414. [Google Scholar] [CrossRef]
- Birnie, G.D.; Kroeger, H.; Heidelberger, C. Studies of fluorinated pyrimidines. Xviii. The degradation of 5-fluoro-2′-deoxyuridine and related compounds by nucleoside phosphorylase. Biochemistry 1963, 2, 566–572. [Google Scholar] [CrossRef]
- Guise, C.P.; Abbattista, M.R.; Tipparaju, S.R.; Lambie, N.K.; Su, J.; Li, D.; Wilson, W.R.; Dachs, G.U.; Patterson, A.V. Diflavin oxidoreductases activate the bioreductive prodrug PR-104A under hypoxia. Mol. Pharmacol. 2012, 81, 31–40. [Google Scholar] [CrossRef]
- Rossolillo, P.; Winter, F.; Simon-Loriere, E.; Gallois-Montbrun, S.; Negroni, M. Retrovolution: HIV-driven evolution of cellular genes and improvement of anticancer drug activation. PLoS Genet. 2012, 8, e1002904. [Google Scholar] [CrossRef]
- Stiborova, M.; Rupertova, M.; Frei, E. Cytochrome P450- and peroxidase-mediated oxidation of anticancer alkaloid ellipticine dictates its anti-tumor efficiency. Biochim. Biophys. Acta 2011, 1814, 175–185. [Google Scholar]
- Travica, S.; Pors, K.; Loadman, P.M.; Shnyder, S.D.; Johansson, I.; Alandas, M.N.; Sheldrake, H.M.; Mkrtchian, S.; Patterson, L.H.; Ingelman-Sundberg, M. Colon cancer-specific cytochrome P450 2W1 converts duocarmycin analogues into potent tumor cytotoxins. Clin. Cancer Res. 2013, 19, 2952–2961. [Google Scholar] [CrossRef]
- Tsume, Y.; Amidon, G.L. The feasibility of enzyme targeted activation for amino acid/dipeptide monoester prodrugs of floxuridine; cathepsin D as a potential targeted enzyme. Molecules 2012, 17, 3672–3689. [Google Scholar] [CrossRef]
- Bonastre, J.; Jan, P.; Barthe, Y.; Koscielny, S. Metastatic breast cancer: We do need primary cost data. Breast 2012, 21, 384–388. [Google Scholar] [CrossRef]
- Incecayir, T.; Tsume, Y.; Amidon, G.L. Comparison of the permeability of metoprolol and labetalol in rat, mouse, and Caco-2 cells: Use as a reference standard for BCS classification. Mol. Pharm. 2013, 10, 958–966. [Google Scholar] [CrossRef]
- Tsume, Y.; Provoda, C.J.; Amidon, G.L. The achievement of mass balance by simultaneous quantification of floxuridine prodrug, floxuridine, 5-fluorouracil, 5-dihydrouracil, alpha-fluoro-beta-ureidopropionate, alpha-fluoro-beta-alanine using LC-MS. J. Chromatogr. B 2011, 879, 915–920. [Google Scholar] [CrossRef]
- Tsume, Y.; Amidon, G.L. Selection of suitable prodrug candidates for in vivo studies via in vitro studies; the correlation of prodrug stability in between cell culture homogenates and human tissue homogenates. J. Pharm. Pharm. Sci. 2012, 15, 433–446. [Google Scholar]
- Landowski, C.P.; Lorenzi, P.L.; Song, X.; Amidon, G.L. Nucleoside ester prodrug substrate specificity of liver carboxylesterase. J. Pharmacol. Exp. Ther. 2006, 316, 572–580. [Google Scholar]
- Gasparini, G. Metronomic scheduling: The future of chemotherapy? Lancet Oncol. 2001, 2, 733–740. [Google Scholar] [CrossRef]
- Hanahan, D.; Bergers, G.; Bergsland, E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J. Clin. Investig. 2000, 105, 1045–1047. [Google Scholar] [CrossRef]
- Kamen, B.A.; Rubin, E.; Aisner, J.; Glatstein, E. High-time chemotherapy or high time for low dose. J. Clin. Oncol. 2000, 18, 2935–2937. [Google Scholar]
- Kerbel, R.S.; Kamen, B.A. The anti-angiogenic basis of metronomic chemotherapy. Nat. Rev. Cancer 2004, 4, 423–436. [Google Scholar] [CrossRef]
- Kerbel, R.S.; Klement, G.; Pritchard, K.I.; Kamen, B. Continuous low-dose anti-angiogenic/metronomic chemotherapy: From the research laboratory into the oncology clinic. Ann. Oncol. 2002, 13, 12–15. [Google Scholar] [CrossRef]
- Emmenegger, U.; Man, S.; Shaked, Y.; Francia, G.; Wong, J.W.; Hicklin, D.J.; Kerbel, R.S. A comparative analysis of low-dose metronomic cyclophosphamide reveals absent or low-grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res. 2004, 64, 3994–4000. [Google Scholar] [CrossRef]
- Bender, D.M.; Bao, J.; Dantzig, A.H.; Diseroad, W.D.; Law, K.L.; Magnus, N.A.; Peterson, J.A.; Perkins, E.J.; Pu, Y.J.; Reutzel-Edens, S.M.; et al. Synthesis, crystallization, and biological evaluation of an orally active prodrug of gemcitabine. J. Med. Chem. 2009, 52, 6958–6961. [Google Scholar] [CrossRef]
- Koolen, S.L.; Witteveen, P.O.; Jansen, R.S.; Langenberg, M.H.; Kronemeijer, R.H.; Nol, A.; Garcia-Ribas, I.; Callies, S.; Benhadji, K.A.; Slapak, C.A.; et al. Phase I study of Oral gemcitabine prodrug (LY2334737) alone and in combination with erlotinib in patients with advanced solid tumors. Clin. Cancer Res. 2011, 17, 6071–6082. [Google Scholar] [CrossRef]
- Moysan, E.; Bastiat, G.; Benoit, J.P. Gemcitabine versus Modified Gemcitabine: A review of several promising chemical modifications. Mol. Pharm. 2013, 10, 430–444. [Google Scholar] [CrossRef] [Green Version]
- Pratt, S.E.; Durland-Busbice, S.; Shepard, R.L.; Donoho, G.P.; Starling, J.J.; Wickremsinhe, E.R.; Perkins, E.J.; Dantzig, A.H. Efficacy of low-dose oral metronomic dosing of the prodrug of gemcitabine, LY2334737, in human tumor xenografts. Mol. Cancer Ther. 2013, 12, 481–490. [Google Scholar] [CrossRef]
- Stuurman, F.E.; Voest, E.E.; Awada, A.; Witteveen, P.O.; Bergeland, T.; Hals, P.A.; Rasch, W.; Schellens, J.H.; Hendlisz, A. Phase I study of oral CP-4126, a gemcitabine derivative, in patients with advanced solid tumors. Investig. N. Drugs 2013, 31, 959–966. [Google Scholar] [CrossRef]
- Wickremsinhe, E.; Bao, J.; Smith, R.; Burton, R.; Dow, S.; Perkins, E. Preclinical absorption, distribution, metabolism, and excretion of an oral amide prodrug of gemcitabine designed to deliver prolonged systemic exposure. Pharmaceutics 2013, 5, 261–276. [Google Scholar] [CrossRef]
- Yamamoto, N.; Nokihara, H.; Yamada, Y.; Uenaka, K.; Sekiguchi, R.; Makiuchi, T.; Slapak, C.A.; Benhadji, K.A.; Tamura, T. Phase I study of oral gemcitabine prodrug (LY2334737) in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013, 71, 1645–1655. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Qin, Y.; Wang, R.; Li, G.; Sun, C.; Qu, X.; Li, W. Pharmacokinetics and metabolism of SL-01, a prodrug of gemcitabine, in rats. Cancer Chemother. Pharmacol. 2013, 71, 1541–1550. [Google Scholar] [CrossRef]
- Huang, P.; Chubb, S.; Hertel, L.W.; Grindey, G.B.; Plunkett, W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991, 51, 6110–6117. [Google Scholar]
- Plunkett, W.; Huang, P.; Gandhi, V. Preclinical characteristics of gemcitabine. Anti-Cancer Drugs 1995, 6, 7–13. [Google Scholar] [CrossRef]
- Plunkett, W.; Huang, P.; Xu, Y.Z.; Heinemann, V.; Grunewald, R.; Gandhi, V. Gemcitabine: Metabolism, mechanisms of action, and self-potentiation. Semin. Oncol. 1995, 22, 3–10. [Google Scholar]
- Ruiz van Haperen, V.W.; Veerman, G.; Vermorken, J.B.; Peters, G.J. 2′,2′-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochem. Pharmacol. 1993, 46, 762–766. [Google Scholar] [CrossRef]
- DeFeo-Jones, D.; Garsky, V.M.; Wong, B.K.; Feng, D.M.; Bolyar, T.; Haskell, K.; Kiefer, D.M.; Leander, K.; McAvoy, E.; Lumma, P.; et al. A peptide-doxorubicin “prodrug” activated by prostate-specific antigen selectively kills prostate tumor cells positive for prostate-specific antigen in vivo. Nat. Med. 2000, 6, 1248–1252. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Isaacs, J.T. Enzymatic activation of prodrugs by prostate-specific antigen: Targeted therapy for metastatic prostate cancer. Cancer J. Sci. Am. 1998, 4, S15–S21. [Google Scholar]
- Denmeade, S.R.; Nagy, A.; Gao, J.; Lilja, H.; Schally, A.V.; Isaacs, J.T. Enzymatic activation of a doxorubicin-peptide prodrug by prostate-specific antigen. Cancer Res. 1998, 58, 2537–2540. [Google Scholar]
- Kumar, S.K.; Williams, S.A.; Isaacs, J.T.; Denmeade, S.R.; Khan, S.R. Modulating paclitaxel bioavailability for targeting prostate cancer. Bioorg. Med. Chem. 2007, 15, 4973–4984. [Google Scholar] [CrossRef]
- Luan, Y.; Jing, F.; Zhang, J.; Zou, M.; Wang, X.; Jia, Y.; Liu, N.; Mou, J.; Xu, W. Design, synthesis, and activity evaluation of a new 5-fluorouracil prodrug containing an Asn-Gly-Arg(NO2)COOCH3 tripeptide. Protein Pept. Lett. 2012, 19, 1122–1131. [Google Scholar] [CrossRef]
- Mhaka, A.; Denmeade, S.R.; Yao, W.; Isaacs, J.T.; Khan, S.R. A 5-fluorodeoxyuridine prodrug as targeted therapy for prostate cancer. Bioorg. Med. Chem. Lett. 2002, 12, 2459–2461. [Google Scholar] [CrossRef]
- Sotiropoulou, G.; Pampalakis, G. Targeting the kallikrein-related peptidases for drug development. Trends Pharmacol. Sci. 2012, 33, 623–634. [Google Scholar] [CrossRef]
- Huang, Y. Pharmacogenetics/genomics of membrane transporters in cancer chemotherapy. Cancer Metastasis Rev. 2007, 26, 183–201. [Google Scholar] [CrossRef]
- Mackey, J.R.; Mani, R.S.; Selner, M.; Mowles, D.; Young, J.D.; Belt, J.A.; Crawford, C.R.; Cass, C.E. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998, 58, 4349–4357. [Google Scholar]
- Hagmann, W.; Jesnowski, R.; Faissner, R.; Guo, C.; Lohr, J.M. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology 2009, 9, 136–144. [Google Scholar] [CrossRef]
- Hagmann, W.; Jesnowski, R.; Lohr, J.M. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia 2010, 12, 740–747. [Google Scholar]
- Konig, J.; Nies, A.T.; Cui, Y.; Leier, I.; Keppler, D. Conjugate export pumps of the multidrug resistance protein (MRP) family: Localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophys. Acta 1999, 1461, 377–394. [Google Scholar]
- Oguri, T.; Achiwa, H.; Sato, S.; Bessho, Y.; Takano, Y.; Miyazaki, M.; Muramatsu, H.; Maeda, H.; Niimi, T.; Ueda, R. The determinants of sensitivity and acquired resistance to gemcitabine differ in non-small cell lung cancer: A role of ABCC5 in gemcitabine sensitivity. Mol. Cancer Ther. 2006, 5, 1800–1806. [Google Scholar] [CrossRef]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40. [Google Scholar] [CrossRef]
- Sweet, R.; Paul, A.; Zastre, J. Hypoxia induced upregulation and function of the thiamine transporter, SLC19A3 in a breast cancer cell line. Cancer Biol. Ther. 2010, 10, 1101–1111. [Google Scholar] [CrossRef]
- Wang, C.; Uray, I.P.; Mazumdar, A.; Mayer, J.A.; Brown, P.H. SLC22A5/OCTN2 expression in breast cancer is induced by estrogen via a novel intronic estrogen-response element (ERE). Breast Cancer Res. Treat. 2012, 134, 101–115. [Google Scholar] [CrossRef]
- Kwak, E.Y.; Shim, W.S.; Chang, J.E.; Chong, S.; Kim, D.D.; Chung, S.J.; Shim, C.K. Enhanced intracellular accumulation of a non-nucleoside anti-cancer agent via increased uptake of its valine ester prodrug through amino acid transporters. Xenobiotica 2012, 42, 603–613. [Google Scholar] [CrossRef]
- Hagmann, W.; Faissner, R.; Schnolzer, M.; Lohr, M.; Jesnowski, R. Membrane drug transporters and chemoresistance in human pancreatic carcinoma. Cancers 2010, 3, 106–125. [Google Scholar] [CrossRef]
- Nambaru, P.K.; Hubner, T.; Kock, K.; Mews, S.; Grube, M.; Payen, L.; Guitton, J.; Sendler, M.; Jedlitschky, G.; Rimmbach, C.; et al. Drug efflux transporter multidrug resistance-associated protein 5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metab. Dispos. 2011, 39, 132–139. [Google Scholar] [CrossRef]
- Reid, G.; Wielinga, P.; Zelcer, N.; de Haas, M.; van Deemter, L.; Wijnholds, J.; Balzarini, J.; Borst, P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol. Pharmacol. 2003, 63, 1094–1103. [Google Scholar] [CrossRef]
- Engel, J.B.; Schally, A.V.; Halmos, G.; Baker, B.; Nagy, A.; Keller, G. Targeted therapy with a cytotoxic somatostatin analog, AN-238, inhibits growth of human experimental endometrial carcinomas expressing multidrug resistance protein MDR-1. Cancer 2005, 104, 1312–1321. [Google Scholar] [CrossRef]
- Keller, G.; Schally, A.V.; Nagy, A.; Halmos, G.; Baker, B.; Engel, J.B. Targeted chemotherapy with cytotoxic bombesin analogue AN-215 can overcome chemoresistance in experimental renal cell carcinomas. Cancer 2005, 104, 2266–2274. [Google Scholar] [CrossRef]
- Michaelsen, S.R.; Christensen, C.L.; Sehested, M.; Cramer, F.; Poulsen, T.T.; Patterson, A.V.; Poulsen, H.S. Single agent- and combination treatment with two targeted suicide gene therapy systems is effective in chemoresistant small cell lung cancer cells. J. Gene Med. 2012, 14, 445–458. [Google Scholar] [CrossRef]
- Tanino, T.; Nawa, A.; Kondo, E.; Kikkawa, F.; Daikoku, T.; Tsurumi, T.; Luo, C.; Nishiyama, Y.; Takayanagi, Y.; Nishimori, K.; et al. Paclitaxel-2′-Ethylcarbonate prodrug can circumvent P-glycoprotein-mediated cellular efflux to increase drug cytotoxicity. Pharm. Res. 2007, 24, 555–565. [Google Scholar] [CrossRef]
- Zollner, G.; Wagner, M.; Fickert, P.; Silbert, D.; Fuchsbichler, A.; Zatloukal, K.; Denk, H.; Trauner, M. Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver Int. 2005, 25, 367–379. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsume, Y.; Borras Bermejo, B.; Amidon, G.L. The Dipeptide Monoester Prodrugs of Floxuridine and Gemcitabine—Feasibility of Orally Administrable Nucleoside Analogs. Pharmaceuticals 2014, 7, 169-191. https://doi.org/10.3390/ph7020169
Tsume Y, Borras Bermejo B, Amidon GL. The Dipeptide Monoester Prodrugs of Floxuridine and Gemcitabine—Feasibility of Orally Administrable Nucleoside Analogs. Pharmaceuticals. 2014; 7(2):169-191. https://doi.org/10.3390/ph7020169
Chicago/Turabian StyleTsume, Yasuhiro, Blanca Borras Bermejo, and Gordon L. Amidon. 2014. "The Dipeptide Monoester Prodrugs of Floxuridine and Gemcitabine—Feasibility of Orally Administrable Nucleoside Analogs" Pharmaceuticals 7, no. 2: 169-191. https://doi.org/10.3390/ph7020169
APA StyleTsume, Y., Borras Bermejo, B., & Amidon, G. L. (2014). The Dipeptide Monoester Prodrugs of Floxuridine and Gemcitabine—Feasibility of Orally Administrable Nucleoside Analogs. Pharmaceuticals, 7(2), 169-191. https://doi.org/10.3390/ph7020169




