Inactivation of Caliciviruses
Abstract
:1. Introduction
2. Literature Survey
3. Results and Discussion
3.1. Inactivation at Various Temperatures
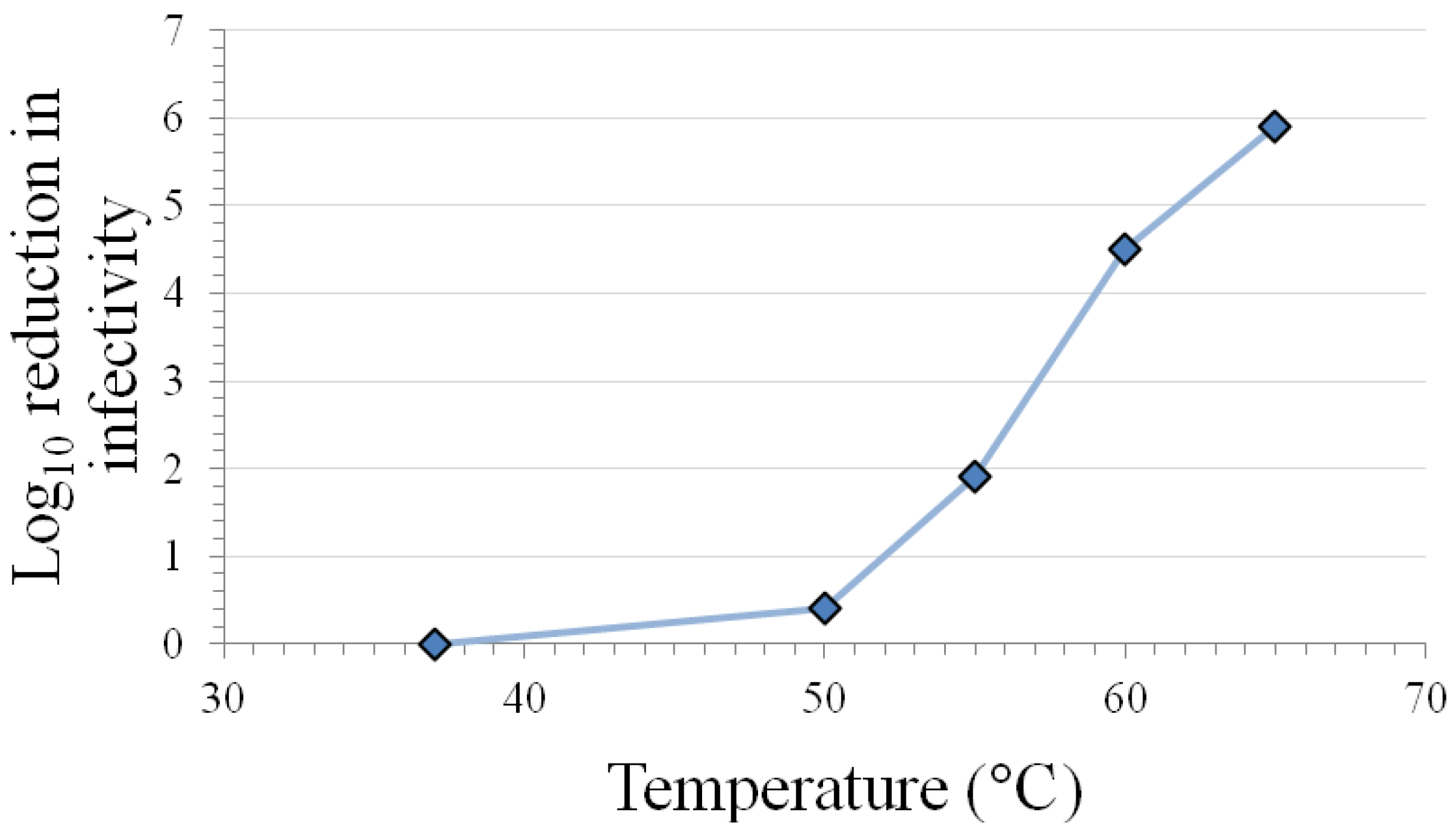
| Inactivation Approach | Coupon material / Test matrix | Log10 Reduction in Infectivity Titer | Ref. | |||
|---|---|---|---|---|---|---|
| FeCV | CaCV | MNV | VESV | |||
| Coupon Studies | ||||||
| 25 °C for 7 days | Stainless steel | >5 * | − | ~5 | − | [12] |
| 25 °C for 9 days | Stainless steel | − | − | ~2.2 | − | [19] |
| 25 °C for 30 days | Stainless steel | − | − | 6.2 | − | [19] |
| Solution Studies | ||||||
| 25 °C for 7 days | Virus suspension | ~3.5 * | − | >1 | − | [12] |
| 25 °C for 21 days | Tap water | ≥6 * | − | − | − | [10] |
| 37 °C for 7 days | Tap water | ≥6 * | − | − | − | [10] |
| 50 °C for 30 min | water | ~3.5 † | − | − | − | [6] |
| 50 °C for 60 min | water | − | − | − | 2–3 | [5] |
| 55 °C for 3 min | Virus stock | 0.5 * | − | 0.8 | − | [24] |
| 56 °C for 3 min | Virus stock | 0 * | − | − | − | [8] |
| 56 °C for 8 min | 3-4 µg/mL protein virus stock | 3 * | 3 | − | − | [9] |
| 56 °C for 30 min | Virus stock | ≥7 * | − | − | − | [13] |
| 56 °C for 60 min | Virus stock | ≥7.5 * | − | − | − | [8] |
| 59 °C for 7 min | Virus + 10% FBS | 4 ‡ | − | − | − | [11] |
| 60 °C for 5 min | Culture medium or water | ~5 § | − | − | − | [15] |
| 60 °C for 10 min | Virus in PBS | − | − | ~3.6 | − | [22] |
| 60 °C for 30 min | Virus in PBS | ~2.1 * | − | 2.2 | − | [20] |
| 62 °C for 30 min | Virus stock | ≥7 * | − | − | − | [13] |
| 63 °C for 10 min | Virus in water | - | − | 3.3 | − | [16] |
| 65 °C for 2 min | Virus stock | >6.7 * | − | >6.7 | − | [24] |
| Inactivation Approach | Coupon material / Test matrix | Log10 Reduction in Infectivity Titer | Ref. | |||
| FeCV | MNV | VESV | ||||
| Solution Studies | ||||||
| 70 °C for 1.5 min | Culture medium or water | 6 § | − | − | − | [15] |
| 70 °C for 2.5 min | Virus in PBS | − | − | ~4.2 | − | [22] |
| 70 °C for 3 min | Virus stock | 6.5 * | − | − | − | [8] |
| 71 °C for 1 min | 3-4 µg/mL protein virus stock | 3 * | 3 | − | − | [9] |
| 72 °C for 1 min | Virus stock | >6.7 * | − | >6.7 | − | [24] |
| 72 °C for 1 min | Virus in water | − | − | ≥5 | − | [16] |
| 72 °C for 3 min | Virus in PBS | − | − | ≥3.5 | − | [18] |
| 80 °C for 2.5 min | Culture medium or water | − | − | 6.5 | − | [14] |
3.2. Inactivation by UV Irradiation
| Irradiation Approach and Test Matrix | Inactivation Constant (K) and R2 | Ref. | |||
|---|---|---|---|---|---|
| FeCV a | CaCV | MNV | BoCV | ||
| UV-C (254 nm) irradiation (K = log10 reduction in titer per unit fluence [mJ/cm2]) | |||||
| Water | – | – | – | K = 0.19 b R2 = 0.96 b | [41] |
| Low protein virus stock, ambient temperature | K = 0.20 a | K = 0.17 a | – | – | [40] |
| R2 = 0.96 a | R2 = 0.93 a | ||||
| Low protein virus stock, ambient temperature | K = 0.16 b | – | K = 0.14 b | – | [44] |
| R2 = 0.99 b | R2 = 1.00 b | ||||
| Phosphate buffered saline, ambient temperature | – | – | K = 0.13 a | – | [43] |
| R2 = 0.96 a | |||||
| Phosphate buffered saline, ambient temperature | K = 0.027 a | – | – | – | [38] |
| R2 = 0.75a | |||||
| Treated drinking water, ambient temperature | K = 0.12 b | – | – | – | [39] |
| R2 = 0.96 b | |||||
| Buffered demand-free water, ambient temperature | K = 0.10 b | – | – | – | [39] |
| R2 = 0.92 b | |||||
| Secondary effluent wastewater, ambient temperature | K = 0.21 a | – | – | – | [42] |
| R2 = 0.99 b | |||||
| 3–4 µg/mL Protein virus stock, ambient temperature | K = 0.13 a | K = 0.15 a | – | – | [40] |
| R2 = 0.87 a | R2 = 0.91 a | ||||
| UV-B irradiation (K = log10 reduction in titer per unit fluence [mJ/cm2]) | |||||
| 3–4 µg/mL Protein virus stock, 4° C | K = 0.072 a | K = 0.072 a | – | – | [9] |
| R2 = 0.98 a | R2 = 0.96 a | ||||
3.3. Photodynamic and Photocatalytic Inactivation
| Irradiation approach and test matrix | Log10 reduction in titer | Ref. | ||
|---|---|---|---|---|
| FeCV | NoV | MNV | ||
| Photodynamic Inactivation | ||||
| 1 µM methylene blue; human plasma, 5 min illumination | >3.9 | – | – | [49] |
| Photocatalytic Inactivation | ||||
| TiO2 film plus visible light; virus stock, 24 hr at 30 °C | 2.0 * | – | – | [51] |
| TiO2 film plus visible light, virus stock + 1 mg/mL BSA, 24 hr at 30 °C | 0.9 * | – | – | [51] |
| TiO2 10 mg/L plus UV 254 nm; virus stock, 3.8 min at ambient temperature | – | – | 3.6 | [43] |
| TiO2 film plus UV 254 nm; secondary effluent at ambient temperature | – | ~1.0 a | – | [50] |
3.4. Inactivation by Ionizing Radiation
| Irradiation approach and test matrix | Inactivation constant (K) and R2 | Ref. | ||
|---|---|---|---|---|
| FeCV | CaCV | MNV | ||
| Gamma irradiation (K = log10 reduction in titer per unit fluence [kGy]) | ||||
| DMEM at ambient temperature | – | – | K = 0.33 a | [53] |
| R2 = 0.97 a | ||||
| Low protein virus stock at ambient temperature | K = 5.9 a | K = 7.4 a | – | [40] |
| R2 = 0.96 a | R2 = 0.86 a | |||
| 3–4 µg/mL Protein virus stock at ambient temperature | NDc | K = 7.0 a | – | [40] |
| R2 = 0.85 a | ||||
| Electron beam irradiation (K = log10 reduction in titer per unit fluence [kGy]) | ||||
| PBS at ambient temperature | – | – | K = 0.53 a | [54] |
| R2 = 0.99 a | ||||
| DMEM at ambient temperature | – | – | K = 0.31 a | [54] |
| R2 = 0.98 a | ||||
| Oyster homogenate | – | – | K = 0.20 a | [56] |
| Whole oysters | – | – | K = 0.22 a | [56] |
| Coupon (cabbage) at ambient temperature | – | – | K = 0.22 a | [54] |
| R2 = 0.98 a | ||||
| Coupon (strawberry) at ambient temperature | – | – | K = 0.18 a | [54] |
| R2 = 0.93 a | ||||
| Coupon (lettuce surface) at ambient temperature | K = 0.34 a | – | – | [55] |
| R2 = 0.98 b | ||||
3.5. High Pressure Inactivation
| Pressure | Temperature and Time | Test matrix | Log10 reduction in infectivity titer | ||
|---|---|---|---|---|---|
| FeCV | MNV | Ref. | |||
| 200 MPa | −10 °C, 4 min | Virus stock in DMEM + 10% FBS | 5.0 ‡ | − | [11] |
| 0 °C, 4 min | Virus stock in DMEM + 10% FBS | 4.4 ‡ | − | [11] | |
| 4 °C, 2 min | Virus + DMEM | − | ~2.5 | [62] | |
| 4 °C, 5 min | Virus stock in DMEM + 10% FBS | 4.7 ‡ | − | [11] | |
| 5 °C, 6 min | Virus + DMEM | 3 § | − | [15] | |
| 250 MPa | −10 °C, 4 min | Virus stock in DMEM + 10% FBS | 4.6 ‡ | - | [11] |
| 0 °C, 4 min | Virus stock in DMEM + 10% FBS | 4.8 ‡ | - | [11] | |
| 4 °C, 2 min | Virus + DMEM | − | ~5 | [62] | |
| 5 °C, 6 min | Virus + DMEM | ~6 § | - | [15] | |
| 300 MPa | 4 °C, 2 min | Virus + DMEM | − | ~5.4 | [62] |
| 10 °C, 3 min | Virus + DMEM | ~5 § | − | [15] | |
| Ambient temp.; 3 min | Virus + cell culture medium | 3.6 Φ | − | [63] | |
| 350 MPa | 5 °C, 5 min | Virus stock in DMEM + 10% FBS | − | 5.6 | [60] |
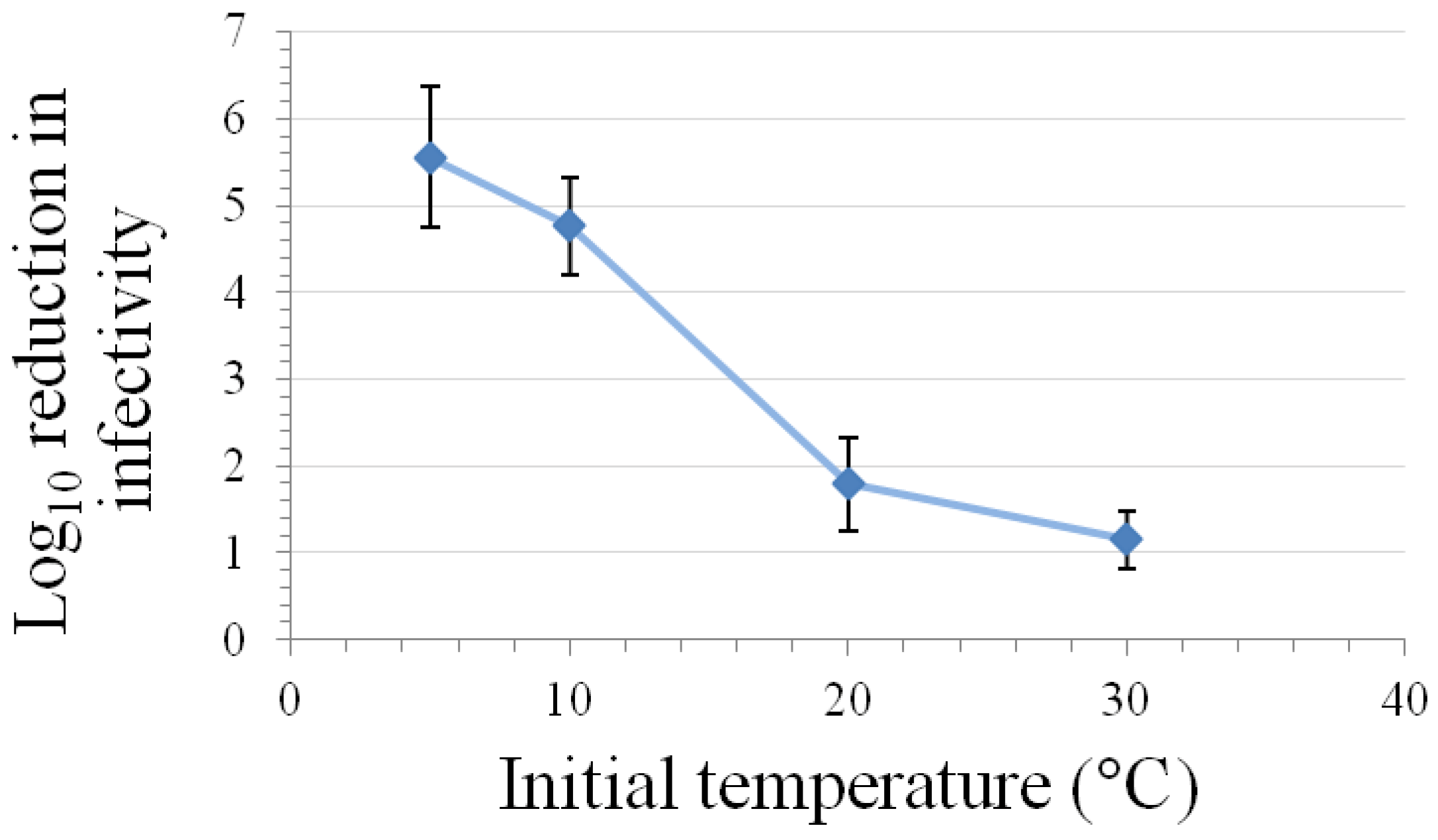
3.6. Inactivation by Alcohols
| Inactivation Approach | Conditions | Coupon Material / Test Matrix | Log10 Reduction in Infectivity Titer | Ref. | ||
|---|---|---|---|---|---|---|
| FeCV | CaCV | MNV | ||||
| Coupon studies | ||||||
| Ethanol | 62%; 30 s at ambient temp. | Skin | ~2.1 | − | ~3.5 | [78] |
| 70%; 30 s at ambient temp. | Skin | 3.8 | − | - | [67] | |
| 75%; 30 s at ambient temp. | Skin | ~2.3 | − | ~2.7 | [78] | |
| 75%; 30 s at ambient temp. | Skin | − | − | 0.9 | [71] | |
| 80%; 30 s at ambient temp. | Skin | − | ~1.7 | [78] | ||
| 90%; 30 s at ambient temp. | Skin | 2.8 | − | − | [67] | |
| 99.5%; 2 min at ambient temp. | Skin | 1.3 | − | − | [70] | |
| 60%; 1 min at ambient temp. | Plastic | 1.3 | − | − | [75] | |
| 60%; 5 min at ambient temp. | Stainless steel | − | − | ~6.2 | [72] | |
| 70%; 10 min at ambient temp. | Stainless steel | 1.3 | − | − | [68] | |
| 90%; 10 min at ambient temp. | Stainless steel | 2.3 | − | − | [68] | |
| 2-Propanol | 60%; 5 min at ambient temp. | Stainless steel | − | − | ~3.0 | [72] |
| 70%; 10 min at ambient temp. | Stainless steel | 2.3 | − | − | [68] | |
| 90%; 10 min at ambient temp. | Stainless steel | 2.4 | − | − | [68] | |
| 60%; 1 min at ambient temp. | Plastic | <0.5 | − | − | [75] | |
| 70%; 30 s at ambient temp. | Skin | 2.2 | − | − | [67] | |
| 90%; 30 s at ambient temp. | Skin | 0.8 | − | − | [67] | |
| 91%; 2 min at ambient temp. | Skin | 0.43 | − | − | [70] | |
| 1-Propanol | 60%; 5 min at ambient temp. | Stainless steel | − | − | ~6.2 | [72] |
| 70%; 30 s at ambient temp. | Skin | 3.6 | − | − | [67] | |
| 90%; 30 s at ambient temp. | Skin | 1.4 | − | − | [67] | |
| Solution studies | ||||||
| Ethanol | 30%; 3 min at ambient temp. | Virus stock + disinfectant | − | − | 0.29 | [69] |
| 50%; 30 s at ambient temp. | Virus stock + disinfectant | 2.2 | − | − | [67] | |
| 50%; 5 min at ambient temp. | Virus stock + disinfectant | 2.2 | − | 0.4 | [74] | |
| 50%; 20 min at 20 °C | Virus stock + disinfectant | <2 | − | <2 | [76] | |
| 60%; 30 s at ambient temp. | Virus stock + disinfectant | − | − | >4 | [69] | |
| Solution studies | ||||||
| Ethanol | 70%; 30 s at ambient temp. | Virus stock + disinfectant | <0.5 | − | ~3.5 | [77] |
| 70%; 30 s at ambient temp. | Virus stock + disinfectant | 3.6 | − | − | [67] | |
| 70%; 2 min at ambient temp. | Virus stock + disinfectant | ~1.2 | ~1.8 | − | [9] | |
| 70%; 5 min at ambient temp. | Virus stock + disinfectant | 2.6 | − | >3.6 | [74] | |
| 75%; 1 min at ambient temp. | Virus stock + disinfectant | 1.3 | − | − | [8] | |
| 90%; 30 s at ambient temp. | Virus stock + disinfectant | <0.5 | − | ~3.6 | [77] | |
| 90%; 5 min at ambient temp. | Virus stock + disinfectant | 0.3 | − | >3.6 | [74] | |
| Sterillium® gel | 85% ethanol; 30 s at ambient temp. | Virus stock + disinfectant | − | − | >4 | [69] |
| 1-Propanol | 30%; 20 min at 20 °C | Virus stock + disinfectant | >4 | − | >4 | [76] |
| 70%; 30 s at ambient temp. | Virus stock + disinfectant | ≥4.1 | − | - | [67] | |
| 2-Propanol | 30%; 3 min at ambient temp. | Virus stock + disinfectant | − | − | 1.6 | [69] |
| 50%; 5 min at ambient temp. | Virus stock + disinfectant | 0.8 | − | 1.0 | [74] | |
| 60%; 30 s at ambient temp. | Virus stock + disinfectant | − | − | 3.9 | [69] | |
| 70%; 30 s at ambient temp. | Virus stock + disinfectant | 2.4 | − | − | [67] | |
| 70%; 5 min at ambient temp. | Virus stock + disinfectant | 0.2 | − | >2.6 | [74] | |
| 90%; 5 min at ambient temp. | Virus stock + disinfectant | 0.1 | - | >2.6 | [74] | |
| Purell® VF447 | 70%; 30 s at ambient temp. | Virus stock + disinfectant | ≥4.8 | - | ≥3.7 | [71] |
3.7. Inactivation by Oxidizing Agents
| Inactivation Approach | Conditions | Coupon Material / Test Matrix | Log10 Reduction in Infectivity Titer | Ref. | |||
| FeCV | VESV | MNV | SMSV | ||||
| Coupon Studies | |||||||
| Sodium hypochlorite | 100 ppm; 10 min at 20 °C | Plastic | 2.8 * | − | − | − | [85] |
| 1000 ppm chlorine; 1 min at ambient temp. | Plastic | >4.2 * | − | − | − | [75] | |
| 1000 ppm; 10 min at 20 °C | Plastic | 6.4 * | − | − | − | [85] | |
| 3%; 10 min at ambient temp. | Stainless steel | − | − | ≥4.8 | − | [93] | |
| 500 ppm free chlorine; 5 min at ambient temp. | Stainless steel | − | − | ~2.3 | − | [19] | |
| 500 ppm free chlorine; 10 min at ambient temp. | Stainless steel | 1.9 * | − | 1.0 | − | [100] | |
| 800 ppm free chlorine; 10 min at ambient temp. | Stainless steel | 1.1 * | − | − | − | [81] | |
| 1000 ppm free chlorine; 5 min at ambient temp. | Stainless steel | − | − | ≥6.2 | − | [19] | |
| 5000 ppm free chlorine; 2 min at ambient temp. | Stainless steel | 3.2 * | − | 1.5 | − | [100] | |
| 5000 ppm free chlorine; 10 min at ambient temp. | Stainless steel | 3.4 * | − | − | − | [81] | |
| Swan topical antiseptic | 1% iodine; 30 s at ambient temp. | Skin | 2.7 * | − | − | − | [70] |
| Hypochlorous acid | Fog; 5 min at ambient temp. | Ceramic tile | − | − | >3.5 | − | [87] |
| Coupon Studies | |||||||
| Hydrogen peroxide vapor | 127 ppm; 1 hr at ambient temp. | Stainless steel | − | − | ~5.2 | [108] | |
| 127 ppm; 1 hr at ambient temp. | Framing panels | − | − | ~4.8 | [108] | ||
| Fog; 15 min at ambient temp. | Stainless steel | 3.9 * | − | − | − | [101] | |
| Fog; 15 min at ambient temp. | Glass | ≥5.2 * | − | − | − | [101] | |
| Fog; 15 min at ambient temp. | Vinyl flooring | ≥5.2 * | − | − | − | [101] | |
| Fog; 15 min at ambient temp. | Plastic | ≥5.2 * | − | − | − | [101] | |
| Gaseous chlorine dioxide | 8 ppm; 6 hr, ≥75% humidity, at 20 °C | Glass | >6 * | − | − | − | [91] |
| 8 ppm; 24 hr, 45%–55% humidity, at 20 °C | Glass | 2.1 * | − | − | − | [91] | |
| Ceramic tile | ≥5.2 * | − | − | − | [91] | ||
| Peracetic acid | 1500 ppm; 5 min at ambient temp. | Stainless steel | − | − | ~4.3 | − | [72] |
| Ozone | 20-25 ppm; 20 min at ambient temp. | Plastic | ≥5.8 | − | − | − | [86] |
| Axen30® | 10 min at ambient temp. | Plastic | ≥4.7 * | − | − | − | [104] |
| Solution Studies | |||||||
| Betadine® | 1% iodine; 30 s at ambient temp. | Virus stock + disinfectant | − | − | >4 | − | [69] |
| Wescodyne® | 0.02% iodine; 2 min at 25 °C | Virus stock + disinfectant | − | ~4.5 | − | ≥6.3 | [79] |
| Sani-Chick | 0.8% iodine; 1 min at ambient temp. | Virus stock + disinfectant | ≥5 * | − | − | − | [8] |
| Chlorine dioxide | 0.2 ppm; 16 min at 20° C | Virus stock + disinfectant | ~4 * | − | − | − | [90] |
| 0.29 ppm; 1 min at 5 °C | Demand free water, pH 7.2 | − | − | ≥3.5 | − | [97] | |
| 0.26 ppm; 30 s at 20 °C | Demand free water, pH 7.2 | − | − | ≥3.5 | − | [97] | |
| 0.4 ppm; 10 min at 20° C | Virus stock + disinfectant | ~4 * | − | − | − | [90] | |
| 0.8 ppm; 2.1 min at 20° C | Virus stock + disinfectant | ~4 * | − | − | − | [90] | |
| Dent-a-Gene | 200 ppm; 10 min at ambient temp. | Virus stock + disinfectant | 6.7 | − | − | − | [82] |
| Solution Studies | |||||||
| Sodium hypochlorite | 0.1%; 2 min at 25 °C | Virus stock + disinfectant | − | ≥6.3 | − | ≥6.3 | [79] |
| 3%; 10 min at ambient temp. | Virus stock + disinfectant | ≥6.7 | − | − | − | [82] | |
| 3%; 10 min at ambient temp. | Virus stock + disinfectant | ≥6 | − | − | − | [107] | |
| 0.19 ppm; 2 min at 5 °C | Demand-free water atpH 7.2 | − | − | ≥2.5 | − | [97] | |
| 0.18 ppm; 1 min at 20 °C | Demand-free water at pH 7.2 | − | − | ≥2.5 | − | [97] | |
| 0.1 ppm free chlorine; 5 min at ambient temp. | Virus stock + disinfectant + 10 ng/mL protein | ≥4.6 * | − | − | − | [89] | |
| 0.1 ppm free chlorine; 5 min at ambient temp. | Virus stock + disinfectant + 120 ng/mL protein | 3.5 * | − | − | − | [89] | |
| 0.2 ppm free chlorine; 0.5 min at 5 °C | Buffered Water at pH 7 or 8 | − | − | ≥4 | − | [94] | |
| 0.2 ppm free chlorine; 0.25 min at 5 °C | Demand free tap water, pH 7 | − | − | ≥4 | − | [94] | |
| 0.5 ppm free chlorine; 15 s at 5 °C | Water at pH 6 | ≥4.3 * | − | − | − | [83] | |
| 0.5 ppm free chlorine; 30 s at ambient | Treated water at pH 7.2 | − | − | 4.1 | − | [95] | |
| 2.5 ppm free chlorine; 10 min at ambient temp. | Seawater | − | − | ~2.5 | − | [102] | |
| 8 ppm free chlorine; 5 min at ambient temp. | Primary effluent waste water | 3.5 * | − | − | − | [42] | |
| 75 ppm total chlorine; 30 s at ambient temp. | Virus stock + disinfectant | ~1 * | − | ~2.6 | − | [77] | |
| 250 ppm total chlorine; 30 s at ambient temp. | Virus stock + disinfectant | ~2.5 * | − | ~3.9 | − | [77] | |
| 300 ppm free chlorine; 10 min at ambient temp. | Virus stock + disinfectant | ~1.5 * | − | - | − | [9] | |
| 2600 ppm chlorine; 30 s at ambient temp. | Virus stock + disinfectant | − | − | >4 | − | [69] | |
| Solution Studies | |||||||
| Sodium hypochlorite | 3000 ppm free chlorine; 10 min at ambient temp. | Virus stock + disinfectant | >5 * | − | − | − | [9] |
| 5000 ppm chlorine; 1 min at ambient temp. | Virus stock + disinfectant | ≥5 * | − | − | − | [8] | |
| 5000 ppm free chlorine; 15 min at ambient temp. | Virus stock + disinfectant | >5 * | − | − | − | [88] | |
| 5500 ppm free chlorine; 15 min at ambient temp. | Virus stock + disinfectant + 25% feces | 4 * | − | − | − | [88] | |
| Monochloramine | 1ppm; 170 min at 5 °C | Buffered Water at pH 7 or 8 | − | − | 4 | − | [92] |
| Trifectant® | 1%; 10 min at ambient temp. | Virus stock + disinfectant | 6.7 | − | − | − | [82] |
| Potassium peroxy-monosulfate | 1%; 2 hr at ambient temp. | PBS + disinfectant | ≥7.1 * | − | ≥6.6 | − | [103] |
| Oxystrong FG | 0.1%; 15 min at 20 °C | Virus stock + disinfectant + 40% FBS | ≥3 * | − | − | − | [88] |
| Ozone | 0.06 mg/L; 5 min at 5 °C | Water at pH 7 | 2.8 * | − | − | − | [84] |
| 1 ppm; 15 s at 5 °C | Water at pH 7 | 4.3 * | − | − | − | [84] | |
| 1 ppm; 2 min at 20 °C | Demand free water at pH 7 | − | − | ~2 | − | [96] | |
| 1 ppm; 2 min at 20 °C | Demand free water at pH 5.6 | − | − | ~2.9 | − | [96] | |
| 6.3 ppm; 5 min at ambient temp. | Water | ≥6.8 ‡ | − | 4.7 | − | [99] | |
3.8. Inactivation by Other Classes of Disinfectants
3.9. Low pH Inactivation
| Inactivant and Conditions | Class of Disinfectant | Coupon Material/ Test Matrix | Log10 Reduction in Infectivity Titer | Ref. | |||
|---|---|---|---|---|---|---|---|
| FeCV | MNV | VESV | SMSV | ||||
| Coupon studies | |||||||
| Glutaraldehyde; 2500 ppm, 5 min at ambient temp. | Aldehyde | Stainless steel | − | ~4.5 | − | − | [72] |
| MicroQuat®; 1800 ppm, 10 min at ambient temp. | Quaternary ammonium | Stainless steel | 2.3 * | − | − | − | [81] |
| Oasis® 144; 1600 ppm, 10 min at ambient temp. | Quaternary ammonium | Stainless steel | 2.0 * | − | − | − | [81] |
| UMQ; 3120 ppm, 10 min at ambient temp. | Quaternary ammonium | Stainless steel | 3.4* | − | − | − | [81] |
| 0.08%/0.02%, 10 min at ambient temp. | Quaternary ammonium | Stainless steel | − | ~1.5 | − | − | [93] |
| Formulation R-82; 0.39%, 10 min at ambient temp. | Quaternary ammonium | Plastic | 6.5 * | − | − | − | [85] |
| 1000 ppm; 1 min, pH 8, at ambient temp. | Quaternary ammonium | Plastic | <2.3 * | − | − | − | [75] |
| 1000 ppm; 1 min, pH 12, at ambient temp. | Quaternary ammonium | Plastic | >3 * | − | − | − | [75] |
| Solution studies | |||||||
| Formaldehyde; 0.7%, 30 min at 20° C | Aldehyde | Virus stock + disinfectant | ≥2.3 * | − | − | − | [112] |
| Formaldehyde; 0.7%, 60 min at 20° C | Aldehyde | Virus stock + disinfectant | 4.0 * | − | − | − | [113] |
| Formaldehyde; 0.7%, 30 min at 20 °C | Aldehyde | Virus stock + disinfectant | >4.0 | − | − | − | [114] |
| Formaldehyde; 1%, 20 min at 25° C | Aldehyde | Virus stock + disinfectant | − | − | 1.9 | 4 | [79] |
| Glutaraldehyde; 0.5%, 1 min at ambient temp. | Aldehyde | Virus stock + disinfectant | ≥5 * | − | − | − | [8] |
| Venno FF Super; glutaraldehyde, 0.1%, 15 min at 20 °C | Aldehyde | Virus stock + disinfectant + 40% FBS | ≥3 * | − | − | − | [88] |
| Coupon studies | |||||||
| Sodium hydroxide; 2%, 2 min at 4 °C | Base | Virus stock + disinfectant | − | − | ≥6.3 | ≥6.3 | [79] |
| Benzalkonium chloride; 5%, 20 min at 25 °C | Quaternary ammonium | Virus stock + disinfectant | − | − | <1 | <1 | [79] |
| Benzalkonium chloride; 5%, 20 min at 37 °C | Quaternary ammonium | Virus stock + disinfectant | − | − | ≥6.3 | ≥6.3 | [79] |
| Bacoban WB; 2%; 240 min at 20° C | Quaternary ammonium | Virus stock + disinfectant | ≥4.0 * | − | − | − | [113] |
| A33® Dry; 0.39%; 10 min at ambient temp. | Quaternary ammonium | Virus stock + disinfectant | 1.0 | − | − | − | [82] |
| Pine O Cleen; 0.15%; 1 min at ambient temp. | Quaternary ammonium | Virus stock + disinfectant | 0 * | − | − | − | [8] |
| BARDAC® 208M Blend; 1X, 30 s, pH 6.5, at ambient temp. | Quaternary ammonium | Virus stock + disinfectant | <0.5 * | <0.5 | − | − | [77] |
| Asphène381®; 0.25%; 30 min at ambient temp. | Quaternary ammonium + surfactant | Virus stock + disinfectant | − | 0.35 | − | − | [69] |
| STERiZAR® ; 80%; 5 min at 20° C | Surfactant | Virus stock + disinfectant | >4.0 | − | − | − | [114] |
| Eradic8® A2Z; 4%, 30 min at 20 °C | Surfactant | Virus stock + disinfectant | ≥4.3 * | − | − | − | [112] |
| β-Propiolactone; 0.1%, 60 min at 22 °C | Lactone | IgG | 5.2 | − | − | − | [115] |
| β-Propiolactone; 0.1%, 300 min at 22 °C | Lactone | Cryo poor plasma | 1.9 | − | − | − | [115] |
| INACTINE™ PEN 110; 0.1%, 3 hr at 22 °C | Alkylating agent | RBC concentrates | − | − | ≥7.5 | − | [116] |
| pH | Acid, Temperature, Time | Coupon Material/ Test Matrix | Log10 reduction in infectivity titer | Ref. | ||
|---|---|---|---|---|---|---|
| FeCV | MNV | CaCV | ||||
| Coupon Studies | ||||||
| <1.0 | 0.38% Hydrochloric acid; 1 min at ambient temp. | Plastic | >5.0 * | − | − | [75] |
| 1.6 | 0.38% Hydrochloric acid; 1 min at ambient temp. | Plastic | >3.2 * | − | − | [75] |
| 2.5 | 0.25% Citric acid; 1 min, at ambient temp. | Plastic | >5.0 * | − | − | [75] |
| Solution Studies | ||||||
| 2 | 0.1 M Phosphoric acid; 15 min at ambient temp. | Virus in phosphate buffer | ~4 † | − | − | [6] |
| 0.1 M Citric acid; 30 min at 37 °C | Virus stock in DMEM | 4.4 * | 0.6 | − | [12] | |
| Citric acid; 30 min at 37 °C | Virus stock in DMEM | >5 * | − | >5 | [9] | |
| 0.1 M Citric acid; 8.6 hr at 37 °C | Virus in PBS | - | 1.0 | − | [22] | |
| 2.5 | 0.1 M Phosphoric acid; 1 min at ambient temp. | Virus in phosphate buffer | >4 ‡ | − | − | [6] |
| 0.1 M Phosphoric acid; 60 min at ambient temp. | Virus in phosphate buffer | ~1.3 † | − | − | [6] | |
| 3 | 0.1 M Citric acid; 15 min at ambient temp. | Virus in citrate buffer | ~3.8 ‡ | − | − | [6] |
| Citric acid; 30 min at 37 °C | Virus stock in DMEM | ~4.7 * | − | >5 | [9] | |
| 0.1 M Citric acid; 30 min at 37 °C | Virus stock in DMEM | 3.7 * | 0.6 | − | [12] | |
| 4 | 0.1 M Citric acid; 30 min at 37 °C | Virus stock in DMEM | 2.3 * | 0.5 | − | [12] |
| 0.1 M Citric acid, 22 hr at 37 °C | Virus in PBS | − | 1.0 | − | [22] | |
3.10. Appropriateness of Calicivirus Surrogates for Studying Human Norovirus Inactivation
| Inactivation approach and conditions | Log10 reduction in infectivity titer | Ref. | |
|---|---|---|---|
| FeCV | MNV | ||
| Wet heat; 50 °C, 30 min | 0.6 * | 0.3 | [20] |
| Wet heat; 55 °C, 3 min | 0.5 * | 0.8 | [24] |
| Wet heat; 60 °C, 30 min | 2.1 * | 2.2 | [20] |
| Wet heat; 65 °C, 2 min | >6.7 * | >6.7 | [24] |
| Wet heat; 72 °C, 1 min | >6.7 * | >6.7 | [24] |
| UV-C; in low protein virus stock, ambient temp. , 30 mJ/cm2 | 4.8 * | 4.1 | [44] |
| 50% Ethanol; 5 min at ambient temp. | 2.2 * | 0.4 | [74] |
| 70% Ethanol; 30 s at ambient temp. | <0.5 * | ~3.5 | [77] |
| 70% Ethanol; 5 min at ambient temp. | 2.6 * | >3.6 | [74] |
| Purell® VF447; 70% ethanol, 30 s at ambient temp. | ≥4.8 * | ≥3.7 | [71] |
| 75% Ethanol; 20 s, on skin at ambient temp. | ~0.8 * | ~3 | [78] |
| 75% Ethanol; 30 s, on skin at ambient temp. | ~2.4 * | ~2.7 | [78] |
| 90% Ethanol; 30 s at ambient temp. | <0.5 * | ~3.6 | [77] |
| 90% Ethanol; 5 min at ambient temp. | 0.3 * | >3.6 | [74] |
| Total chlorine; 75 ppm, 0.5 min at ambient temp. | ~1 * | ~2.6 | [77] |
| Total chlorine; 250 ppm, 0.5 min at ambient temp. | ~2.5 * | ~3.9 | [77] |
| Free chlorine; 500 ppm, 10 min at ambient temp. | 1.9* | 1.0 | [100] |
| Free chlorine; 5000 ppm, 3 min at ambient temp. | 4.5 * | 2.8 | [100] |
| Ozone; 6.3 ppm, 1 min at ambient temp. | 2.7 ‡ | 3.9 | [99] |
| Ozone; 6.3 ppm, 5 min at ambient temp. | ≥6.8 ‡ | 4.7 | [99] |
| Benzalkonium chloride; 0.1 mg/mL, 2 hr at ambient temp. | 2.9 * | 1.6 | [103] |
| Benzalkonium chloride; 0.25 mg/mL, 2 hr at ambient temp. | 3.1 * | 2.3 | [103] |
| Benzalkonium chloride; 0.5 mg/mL, 2 hr at ambient temp. | 3.3 * | 2.8 | [103] |
| Potassium peroxymonosulfate; 2.5 mg/mL, 2 hr at ambient | ≥7.1 * | 0.9 | [103] |
| Potassium peroxymonosulfate; 5 mg/mL, 2 hr at ambient | ≥7.1 * | 3.4 | [103] |
| Potassium peroxymonosulfate; 10 mg/mL, 2 hr at ambient | ≥7.1 * | ≥6.6 | [103] |
| pH 3; 30 min at 37 °C | 3.7 * | 0.6 | [12] |
| pH 4; 30 min at 37 °C | 2.3 * | 0.5 | [12] |
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Plavsic, M.; Qiu, Y.; Jones, N.; Keegan, J.; Woodcock, D.; Morris, J.; Davies, C.; Palerno, A.; Pomponio, R.; Scaria, A. Caliciviridae and vesivirus 2117. BioProcess. J. 2011, 9, 6–12. [Google Scholar]
- Oehmig, A.; Büttner, M.; Weiland, F.; Werz, W.; Bergemann, K.; Pfaff, E. Identification of a calicivirus isolate of unknown origin. J. Gen. Virol. 2003, 84, 2837–2845. [Google Scholar] [CrossRef]
- Genzyme Press Release. 2009. Available online: http://www.businesswire.com/news/genzyme/20090616005692/en (accessed on 07 March 2013).
- Nims, R.W. Adventitious viral contamination of biopharmaceuticals: Who is at risk? BioProcess. J. 2011, 10, 4–10. [Google Scholar]
- Zee, Y.C.; Hackett, A.J. The influence of cations on the thermal inactivation of vesicular exanthema of swine virus. Arch. Virol. 1967, 20, 473–476. [Google Scholar]
- Lee, K.M.; Gillespie, J.H. Thermal and pH stability of feline calicivirus. Infect. Immunity 1973, 7, 678–679. [Google Scholar]
- Slomka, M.J.; Appleton, H. Feline calicivirus as a model system for heat inactivation studies of small round structured viruses in shellfish. Epidemiol. Infect. 1998, 121, 401–407. [Google Scholar] [CrossRef]
- Doultree, J.C.; Druce, J.D.; Birch, C.J.; Bowden, D.S.; Marshall, J.A. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 1999, 41, 51–57. [Google Scholar] [CrossRef]
- Duizer, E.; Bijkerk, P.; Rockx, B.; de Groot, A.; Twisk, F.; Koopmans, M. Inactivation of caliciviruses. Appl. Environ. Microbiol. 2004, 70, 4538–4543. [Google Scholar] [CrossRef]
- Allwood, P.B.; Malik, Y.S.; Maherchandani, S.; Hedberg, C.W.; Goyal, S.M. Effect of temperature on the survival of F-specific RNA coliphage, feline calicivirus, and Esherichia. coli in chlorinated water. Int. J. Environ. Res. Public Health 2005, 2, 442–446. [Google Scholar] [CrossRef]
- Chen, H.; Hoover, D.G.; Kingsley, D.H. Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. J. Food Prot. 2005, 68, 2389–2394. [Google Scholar]
- Cannon, J.L.; Papafragkou, E.; Park, G.W.; Osborne, J.; Jaykus, L.-A.; Vinjé, J. Surrogates for the study of norovirus stability and inactivation in the environment: A comparison of murine norovirus and feline calicivirus. J. Food Prot. 2006, 69, 2761–2765. [Google Scholar]
- Ossiboff, R.J.; Sheh, A.; Shotton, J.; Pesavento, P.A.; Parker, J.S.L. Feline caliciviruses (FCVs) isolated from cats with virulent systemic disease possess in vitro phenotypes distinct from those of other FCV isolates. J. Gen. Virol. 2007, 88, 506–517. [Google Scholar] [CrossRef]
- Baert, L.; Wobus, C.E.; Van Coillie, E.; Thackray, L.B.; Debevere, J.; Uyttendaele, M. Detection of murine norovirus 1 by using plaque assay, transfection assay, and real-time reverse transcription-PCR before and after heat exposure. Appl. Environ. Microbiol. 2008, 74, 543–546. [Google Scholar] [CrossRef]
- Buckow, R.; Isbarn, S.; Knorr, D.; Heinz, V.; Lehmacher, A. Predictive model for inactivation of feline calicivirus, a norovirus surrogate, by heat and high hydrostatic pressure. Appl. Environ. Microbiol. 2008, 74, 1030–1038. [Google Scholar] [CrossRef]
- Hewitt, J.; Rivera-Aban, M.; Greening, GE. Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. J. Appl. Microbiol. 2009, 107, 65–71. [Google Scholar] [CrossRef]
- Topping, J.R.; Schnerr, H.; Haines, J.; Scott, M.; Carter, M.J.; Willcocks, M.M.; Bellamy, K.; Brown, D.W.; Gray, J.J.; Gallimore, C.I.; Knight, A.I. Temperature inactivation of feline calicivirus vaccine strain FCV F-9 in comparison with human noroviruses using an RNA exposure assay and reverse transcribed quantitative real-time polymerase chain-reaction—A novel method for predicting virus infectivity. J. Virol. Meth. 2009, 156, 89–95. [Google Scholar] [CrossRef]
- Wolf, S.; Rivera-Aban, M.; Greening, G.E. Long-range reverse transcription as a useful tool to assess the genomic integrity of norovirus. Food Environ. Virol. 2009, 1, 129–136. [Google Scholar] [CrossRef]
- Takahashi, H.; Ohuchi, A.; Miya, S.; Izawa, Y.; Kimura, B. Effect of food residue on norovirus survival on stainless steel surfaces. PLoS ONE 2011, 6, e21951. [Google Scholar]
- Gibson, K.E.; Schwab, K.J. Thermal inactivation of human norovirus surrogates. Food Environ. Virol. 2011, 3, 74–77. [Google Scholar] [CrossRef]
- Sow, H.; Desbiens, M.; Morales-Rayas, R.; Ngazoa, S.E.; Jean, J. Heat inactivation of hepatitis A virus and a norovirus surrogate in soft-shell clams (Mya. arenaria). Foodborne. Pathog. Dis. 2011, 8, 387–393. [Google Scholar]
- Seo, K.; Lee, J.E.; Lim, M.Y.; Ko, G. Effect of temperature, pH, and NaCl on the inactivation kinetics of murine norovirus. J. Food Prot. 2012, 75, 533–540. [Google Scholar] [CrossRef]
- Tuladhar, E.; Bouwknegt, M.; Zwietering, M.H.; Koopmans, M.; Duizer, E. Thermal stability of structurally different viruses with proven or potential relevance to food safety. J. Appl. Microbiol. 2012, 112, 1050–1057. [Google Scholar] [CrossRef]
- Bozkurt, H.; D’Souza, D.; Davidson, P.M. Determination of the thermal inactivation kinetics of the human norovirus surrogates, murine norovirus and feline calicivirus. J. Food Prot. 2013, 76, 79–84. [Google Scholar] [CrossRef]
- Richards, G.P.; Watson, M.A.; Meade, G.K.; Hovan, G.L.; Kingsley, D.H. Resilience of norovirus GII.4 to freezing and thawing: Implications for virus infectivity. Food Environ. Virol. 2012, 4, 192–197. [Google Scholar]
- Ginoza, W.; Hoelle, C.J.; Vessey, K.B.; Carmack, C. Mechanisms of inactivation of single-stranded virus nucleic acids by heat. Nature 1964, 203, 606–609. [Google Scholar] [CrossRef]
- Nuanualsuwan, S.; Cliver, D.O. Inactivation of picornaviruses and caliciviruses; Part 2: Inactivation. Thai J. Vet. Med. 2003, 33, 19–33. [Google Scholar]
- Hirneisen, K.A.; Black, E.P.; Cascarino, J.L.; Fino, V.R.; Hoover, D.G.; Kniel, K.E. Viral inactivation in foods: A review of traditional and novel food-processing technologies. Comp. Rev. Food Sci. Food Safety 2010, 9, 3–20. [Google Scholar] [CrossRef]
- Hiatt, C.W. Kinetics of the inactivation of viruses. Bacteriol. Rev. 1964, 28, 150–163. [Google Scholar]
- Lamhoujeb, S.; Fliss, I.; Ngazoa, S.E.; Jean, J. Molecular study of the persistence of infectious human norovirus on food contact surfaces. Food Environ. Virol. 2009, 1, 51–56. [Google Scholar] [CrossRef]
- Górski, J.; Mizak, B.; Chrobocińska, M. Control of viral haemorrhagic disease of rabbits in Poland. Rev. Sci. Tech. Off. Int. Epiz. 1994, 13, 881–891. [Google Scholar]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Fino, V.R.; Kniel, K.E. UV light inactivation of hepatitis A virus, Aichi virus, and feline calicivirus on strawberries, green onions, and lettuce. J. Food Prot. 2008, 71, 908–913. [Google Scholar]
- Jean, J.; Morales-Rayas, R.; Anoman, M.-N.; Lamhoujeb, S. Inactivation of hepatitis A virus and norovirus surrogate in suspension and on food-contact surfaces using pulsed UV light (pulsed light inactivation of food-borne viruses). Food Microbiol. 2011, 28, 568–572. [Google Scholar] [CrossRef]
- Schleh, M.; Lawrence, B.; Park, T.; Rosenthal, S.; Hart, R.; Dehghani, H. Effectiveness of upstream barrier technologies for inactivation of adventitious contaminants of cell culture. Am. Pharm. Rev. 2010, 13, 72–76. [Google Scholar]
- Weaver, B.; Rosenthal, S. Viral risk mitigation for mammalian cell culture media. PDA J. Pharm. Sci. Technol. 2010, 64, 436–439. [Google Scholar]
- Kowalski, W.J.; Bahnfleth, W.P.; Hernandez, M.T. A genomic model for predicting the ultraviolet susceptibility of viruses. IUVA News 2009, 11, 15–28. [Google Scholar]
- Nuanualsuwan, S.; Mariam, T.; Himathongkham, S.; Cliver, D.O. Ultraviolet inactivation of feline calicivirus, human enteric viruses and coliphages. Photochem. Photobiol. 2002, 76, 406–410. [Google Scholar] [CrossRef]
- Thurston-Enriquez, J.A.; Haas, C.N.; Jacangelo, J.; Riley, K.; Gerba, C.P. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 2003, 69, 577–582. [Google Scholar] [CrossRef]
- De Roda Husman, A.M.; Bijkerk, P.; Lodder, W.; van den Berg, H.; Pribil, W.; Cabaj, A.; Gehringer, P.; Sommer, R.; Duizer, E. Calicivirus inactivation by nonionizing (253.7-nanometer-wavelength [UV]) and ionizing (gamma) radiation. Appl. Environ. Microbiol. 2004, 70, 5089–5093. [Google Scholar]
- Malley, J.P.; Ballester, N.A.; Margolin, A.B.; Linden, K.G.; Mofidi, A.; Bolton, J.R.; Crozes, G.; Laine, J.M.; Janex, M.L. Inactivation of Pathogens with Innovative UV Technologies; American Research Foundation and American Water Works Association: Denver, USA, 2004. [Google Scholar]
- Tree, J.A.; Adams, M.R.; Lees, D.N. Disinfection of feline calicivirus (a surrogate for norovirus) in wastewaters. J. Appl. Microbiol. 2005, 98, 155–162. [Google Scholar] [CrossRef]
- Lee, J.E.; Zoh, K.D.; Ko, G.P. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl. Environ. Microbiol. 2008, 74, 2111–2117. [Google Scholar] [CrossRef]
- Park, G.W.; Linden, K.G.; Sobsey, M.D. Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Lett. Appl. Microbiol. 2011, 52, 162–167. [Google Scholar]
- Lytle, C.D.; Sagripanti, J.-L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef]
- Nims, R.; Plavsic, M. Circovirus inactivation: a literature review. BioProcess. J. 2012, 11, 4–10. [Google Scholar]
- Nims, R.W.; Plavsic, M. Polyomavirus inactivation—A review. Biologicals. 2013, 41, 63–70. [Google Scholar] [CrossRef]
- Bryant, B.J.; Klein, H.G. Pathogen inactivation. The definitive safeguard for the blood supply. Arch. Pathol. Lab. Med. 2007, 131, 719–733. [Google Scholar]
- Mohr, H.; Lambrecht, B.; Selz, A. Photodynamic virus inactivation of blood components. Immunol. Invest. 1995, 24, 73–85. [Google Scholar] [CrossRef]
- Kato, T.; Tohma, H.; Miki, O.; Shibata, T.; Tamura, M. Degradation of Norovirus in Sewage Treatment Water by Photocatalytic Ultraviolet Disinfection; Nippon Steel Technical Report 92; Nippon Steel Corporation: Tokyo, Japan, 2005; pp. 41–44. [Google Scholar]
- Sang, X.; Phan, T.G.; Sugihara, S.; Yagyu, F.; Okitsu, S.; Maneekarn, N.; Müller, W.E.G.; Ushijima, H. Photocatalytic inactivation of diarrheal viruses by visible-light-catalytic titanium dioxide. Clin. Lab. 2007, 53, 413–421. [Google Scholar]
- Ginoza, W. Inactivation of viruses by ionizing radiation and heat. Meth. Virol. 1968, 4, 139–209. [Google Scholar]
- Feng, K.; Divers, E.; Ma, Y.; Li, J. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl. Environ. Microbiol. 2011, 77, 3507–3517. [Google Scholar] [CrossRef]
- Sanglay, G.C.; Li, J.; Uribe, R.M.; Lee, K. Electron-beam inactivation of a norovirus surrogate in fresh produce and model systems. J. Food Prot. 2011, 74, 1155–1160. [Google Scholar] [CrossRef]
- Zhou, F.; Harmon, K.M.; Yoon, K.-J.; Olson, D.G.; Dickson, J.S. Inactivation of feline calicivirus as a surrogate for norovirus on lettuce by electron beam irradiation. J. Food Prot. 2011, 74, 1500–1503. [Google Scholar] [CrossRef]
- Nair, C.; Pillai, S. Sensitivity of murine norovirus and Hepatitis A virus to E-beam irradiation in whole oyster and oyster homogenate. In Proceedings of International Association Food Protection Conference in Providence, Rhode Island, 22–25 July, 2012; Available online: https://iafp.confex.com/iafp/2012/webprogram/Paper2796.html/ (accessed on 07 March 2013).
- Nims, R.W.; Gauvin, G.; Plavsic, M. Gamma irradiation of animal sera for inactivation of viruses and mollicutes—A review. Biologicals 2011, 39, 370–377. [Google Scholar] [CrossRef]
- Richards, G.P.; McLeod, C.; Le Guyader, F.S. Processing strategies to inactivate enteric viruses in shellfish. Food Environ. Virol. 2010, 2, 183–193. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Chen, H. Aqueous matrix compositions and pH influence feline calicivirus inactivation by high pressure processing. J. Food Prot. 2008, 71, 1598–1603. [Google Scholar]
- Kingsley, D.H.; Holliman, D.R.; Calci, K.R.; Chen, H.; Flick, G.J. Inactivation of norovirus by high-pressure processing. Appl. Environ. Microbiol. 2007, 73, 581–585. [Google Scholar]
- Tang, Q.; Li, D.; Xu, J.; Wang, J.; Zhao, Y.; Li, Z.; Xue, C. Mechanism of inactivation of murine norovirus-1 by high pressure processing. Int. J. Food Microbiol. 2010, 137, 186–189. [Google Scholar] [CrossRef]
- Lou, F.; Neetoo, H.; Chen, H.; Li, J. Inactivation of a human norovirus surrogate by high-pressure processing: Effectiveness, mechanism, and potential application in the fresh produce industry. Appl. Environ. Microbiol. 2011, 77, 1862–1871. [Google Scholar] [CrossRef]
- Grove, S.F.; Forsyth, S.; Wan, J.; Coventry, J.; Cole, M.; Stewart, C.M.; Lewis, T.; Ross, T.; Lee, A. Inactivation of hepatitis A virus, poliovirus and a norovirus surrogate by high pressure processing. Innovat. Food Sci. Emerg. Technol. 2008, 9, 206–210. [Google Scholar] [CrossRef]
- Leon, J.S.; Kingsley, D.H.; Montes, J.S.; Richards, G.P.; Lyon, G.M.; Abdulhafid, G.M.; Seitz, S.R.; Fernandez, M.L.; Teunis, P.F.; Flick, G.J.; Moe, C.L. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl. Environ. Microbiol. 2011, 77, 5476–5482. [Google Scholar] [CrossRef]
- Seefeldt, M.B.; Rosendahl, M.S.; Cleland, J.L.; Hesterberg, L.K. Application of high hydrostatic pressure to dissociate aggregates and refold proteins. Curr. Pharm. Biotechnol. 2009, 10, 447–455. [Google Scholar] [CrossRef]
- Seefeldt, M.B.; Ouyang, J.; Froland, W.A.; Carpenter, J.F.; Randolph, T.W. High-pressure refolding of bikunin: Efficacy and thermodynamics. Protein Sci. 2004, 13, 2639–2650. [Google Scholar]
- Gehrke, C.; Steinmann, J.; Goroncy-Bermes, P. Inactivation of feline calicivirus, a surrogate of norovirus (formerly Norwalk-like viruses), by different types of alcohol in vitro and in vivo. J. Hosp. Infect. 2004, 56, 49–55. [Google Scholar]
- Malik, Y.S.; Maherchandani, S.; Goyal, S.M. Comparative efficacy of ethanol and isopropanol against feline calicivirus, a norovirus surrogate. Am. J. Infect. Control. 2006, 34, 31–35. [Google Scholar] [CrossRef]
- Belliot, G.; Lavaux, A.; Souihel, D.; Agnello, D.; Pothier, P. Use of murine norovirus as a surrogate to evaluate resistance of human norovirus to disinfectants. Appl. Environ. Microbiol. 2008, 74, 3315–3318. [Google Scholar] [CrossRef]
- Lages, S.LS.; Ramakrishnan, M.A.; Goyal, S.M. In-vivo efficacy of hand sanitizers against feline calicivirus: a surrogate for norovirus. J. Hosp. Infect. 2008, 68, 159–163. [Google Scholar] [CrossRef]
- Macinga, D.R.; Sattar, S.A.; Jaykus, L.-A.; Arbogast, J.W. Improved inactivation of nonenveloped enteric viruses and their surrogates by a novel alcohol-based hand sanitizer. Appl. Environ. Microbiol. 2008, 74, 5047–5052. [Google Scholar] [CrossRef]
- Magulski, T.; Paulmann, D.; Bischoff, B.; Becker, B.; Steinmann, E.; Steinmann, J.; Goroncy-Bermes, P.; Steinmann, J. Inactivation of murine norovirus by chemical biocides on stainless steel. BMC Infect. Dis. 2009, 9, 107–114. [Google Scholar] [CrossRef]
- Liu, P.; Yuen, Y.; Hsiao, H.-M.; Jaykus, L.-A.; Moe, C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl. Environ. Microbiol. 2010, 76, 394–399. [Google Scholar] [CrossRef]
- Park, G.W.; Barclay, L.; Macinga, D.; Charbonneau, D.; Pettigrew, C.A.; Vinjé, J. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J. Food Prot. 2010, 73, 2232–2238. [Google Scholar]
- Whitehead, K.; McCue, K.A. Virucidal efficacy of disinfectant actives against feline calicivirus, a surrogate for norovirus, in a short contact time. Am. J. Infect. Control. 2010, 38, 26–30. [Google Scholar] [CrossRef]
- Beekes, M.; Lemmer, K.; Thomzig, A.; Joncic, M.; Tintelnot, K.; Mielke, M. Fast, broad-range disinfection of bacteria, fungi, viruses and prions. J. Gen. Virol. 2010, 91, 580–589. [Google Scholar] [CrossRef]
- Tung, G. Efficacy of Commonly Used Disinfectants for Inactivation of Human Noroviruses and Its Surrogates; North Carolina State University: Raleigh, NC, USA, 2011. [Google Scholar]
- Sattar, S.A.; Ali, M.; Tetro, J.A. In vivo comparison of two human norovirus surrogates for testing ethanol-based handrubs: The mouse chasing the cat! PLoS One 2011, 6, e17340. [Google Scholar] [CrossRef]
- Blackwell, J.H. Comparative resistance of San Miguel sea lion virus and vesicular exanthema of swine virus to chemical disinfectants. Res. Vet. Sci. 1978, 25, 25–28. [Google Scholar]
- Heckert, R.A.; Best, M.; Jordan, L.T.; Dulac, G.C.; Eddington, D.L.; Sterritt, W.G. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl. Environ. Microbiol. 1997, 63, 3916–3918. [Google Scholar]
- Gulati, B.R.; Allwood, P.B.; Hedberg, C.W.; Goyal, S.M. Efficacy of commonly used disinfectants for the inactivation of calicivirus on strawberry, lettuce, and a food contact surface. J. Food Prot. 2001, 64, 1430–1434. [Google Scholar]
- Eleraky, N.Z.; Potgieter, L.N.D.; Kennedy, M.A. Virucidal efficacy of four new disinfectants. J. Am. Anim. Hosp. Assoc. 2002, 38, 231–234. [Google Scholar]
- Thurston-Enriquez, J.A.; Haas, C.N.; Jacangelo, J.; Gerba, C.P. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl. Environ. Microbiol. 2003, 69, 3979–3985. [Google Scholar] [CrossRef]
- Thurston-Enriquez, J.A.; Haas, C.N.; Jacangelo, J.; Gerba, C.P. Inactivation of enteric adenovirus and feline calicivirus by ozone. Water Res. 2005, 39, 3650–3656. [Google Scholar] [CrossRef]
- Jimenez, L.; Chiang, M. Virucidal activity of a quaternary ammonium compound disinfectant against feline calicivirus: A surrogate for norovirus. Am. J. Infect. Control. 2006, 34, 269–273. [Google Scholar] [CrossRef]
- Hudson, J.B.; Sharma, M.; Petric, M. Inactivation of norovirus by ozone gas in conditions relevant to healthcare. J. Hosp. Infect. 2007, 66, 40–45. [Google Scholar] [CrossRef]
- Park, G.W.; Boston, D.M.; Kase, J.A.; Sampson, M.N.; Sobsey, M.D. Evaluation of liquid- and fog-based application of Sterilox hypochlorous acid solution for surface inactivation of human norovirus. Appl. Environ. Microbiol. 2007, 73, 4463–4468. [Google Scholar]
- Poschetto, L.F.; Ike, A.; Papp, T.; Mohn, U.; Böhm, R.; Marschang, R.E. Comparison of the sensitivities of noroviruses and feline calicivirus to chemical disinfection under field-like conditions. Appl. Environ. Microbiol. 2007, 73, 5494–5500. [Google Scholar] [CrossRef]
- Urakami, H.; Ikarashi, K.; Okamoto, K.; Abe, Y.; Ikarashi, T.; Kono, T.; Konagaya, Y.; Yanaka, N. Chlorine sensitivity of feline calicivirus, a norovirus surrogate. Appl. Environ. Microbiol. 2007, 73, 5679–5682. [Google Scholar] [CrossRef]
- Zoni, R.; Zanelli, R.; Riboldi, E.; Bigliardi, L.; Sansebastiano, G. Investigation on virucidal activity of chlorine dioxide. Experimental data on feline calicivirus, HAV and coxsackie B5. J. Prev. Med. 2007, 48, 91–95. [Google Scholar]
- Morino, H.; Fukuda, T.; Miura, T.; Lee, C.; Shibata, T.; Sanekata, T. Inactivation of feline calicivirus, a norovirus surrogate, by chlorine dioxide gas. Biocontrol. Sci. 2009, 14, 147–153. [Google Scholar] [CrossRef]
- Cromeans, T.L.; Kahler, A.M.; Hill, V.R. Inactivation of adenoviruses, enteroviruses, and murine norovirus in water by free chlorine and monochloramine. Appl. Environ. Microbiol. 2010, 76, 1028–1033. [Google Scholar] [CrossRef]
- Girard, M.; Ngazoa, S.; Mattison, K.; Jean, J. Attachment of noroviruses to stainless steel and their inactivation, using household disinfectants. J. Food Prot. 2010, 73, 400–404. [Google Scholar]
- Kahler, A.M.; Cromeans, T.L.; Roberts, J.M.; Hill, V.R. Effects of source water quality on chlorine inactivation of adenovirus, coxsackievirus, echovirus, and murine norovirus. Appl. Environ. Microbiol. 2010, 76, 5159–5164. [Google Scholar] [CrossRef]
- Kitajima, M.; Tohya, Y.; Matsubara, K.; Haramoto, E.; Utagawa, E.; Katayama, H. Chlorine inactivation of human norovirus, murine norovirus and poliovirus in drinking water. Lett. Appl. Microbiol. 2010, 51, 119–121. [Google Scholar]
- Lim, M.Y.; Kim, J.-M.; Lee, J.E.; Ko, G. Characterization of ozone disinfection of murine norovirus. Appl. Environ. Microbiol. 2010, 76, 1120–1124. [Google Scholar] [CrossRef]
- Lim, M.Y.; Kim, J.-M.; Ko, G. Disinfection kinetics of murine norovirus using chlorine and chlorine dioxide. Water Res. 2010, 44, 3243–3251. [Google Scholar] [CrossRef]
- Fraisse, A.; Temmam, S.; Deboosere, N.; Guillier, L.; Delobel, A.; Maris, P.; Vialette, M.; Morin, T.; Perelle, S. Comparison of chlorine and peroxyacetic-based disinfectant to inactivate feline calicivirus, murine norovirus and hepatitis A virus on lettuce. Int. J. Food Microbiol. 2011, 151, 98–104. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Markland, S.M.; Kniel, K.E. Ozone inactivation of norovirus surrogates on fresh produce. J. Food Prot. 2011, 74, 836–839. [Google Scholar] [CrossRef]
- Park, G.W.; Sobsey, M.D. Simultaneous comparison of murine norovirus, feline calicivirus, coliphage MS2, and GII.4 norovirus to evaluate the efficacy of sodium hypochlorite against human norovirus on a fecally soiled stainless steel surface. Foodborne. Pathog. Dis. 2011, 8, 1005–1010. [Google Scholar] [CrossRef]
- Bentley, K.; Dove, B.K.; Parks, S.R.; Walker, J.T.; Bennett, A.M. Hydrogen peroxide vapour decontamination of surfaces artificially contaminated with norovirus surrogate feline calicivirus. J. Hosp. Inf. 2012, 80, 116–121. [Google Scholar] [CrossRef]
- Corrêa, A.A.; Carratala, A.; Monte Barardi, C.R.; Calvo, M.; Girones, R.; Bofill-Mas, S. Comparative inactivation of murine norovirus, human adenovirus and human JC polyomavirus by chlorine in seawater. Appl. Environ. Microbiol. 2012, 78, 6450–6457. [Google Scholar] [CrossRef]
- Su, X.; D’Souza, D.H. Inactivation of human norovirus surrogates by benzalkonium chloride, potassium peroxymonosulfate, tannic acid, and gallic acid. Foodborne. Pathog. Dis. 2012, 9, 829–834. [Google Scholar] [CrossRef]
- Gutzmann, K. Virucidal activity of a disinfectant for use on inanimate environmental surfaces utilizing feline calicivirus as a surrogate for norovirus. ATS Labs Project A04151. 2006, pp. 1–14. Available online: http://www.bioprotectionservices.com/wp-content/uploads/2012/04/NorovirusTestReport.pdf (accessed on 07 March 2013).
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 2012, 113, 580–586. [Google Scholar] [CrossRef]
- Keswick, B.H.; Satterwhite, T.K.; Johnson, P.C.; DuPont, H.L.; Secor, S.L.; Bitsura, J.A.; Gary, G.W.; Hoff, J.C. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 1985, 50, 261–264. [Google Scholar]
- Kennedy, M.A.; Mellon, V.S.; Caldwell, G.; Potgieter, L.N.D. Virucidal efficacy of the newer quaternary ammonium compounds. J. Am. Anim. Hosp. Assoc. 1995, 31, 254–258. [Google Scholar]
- Tuladhar, E.; Terpstra, P.; Koopmans, M.; Duizer, E. Virucidal efficacy of hydrogen peroxide vapour disinfection. J. Hosp. Inf. 2012, 80, 110–115. [Google Scholar] [CrossRef]
- Genzyme press release. 25 June 2009. Available online: http://www.businesswire.com/news/home/20090625005653/en/Genzyme-Reports-Progress-Related-Allston-Plant (accessed on 07 March 2013).
- Lutgen, M. Chlorine dioxide remediation of a virus-contaminated manufacturing facility. PDA J. Pharm. Sci. Technol. 2011, 65, 620–624. [Google Scholar] [CrossRef]
- Moody, M.; Alves, W.; Varghese, J.; Khan, F. Mouse minute virus (MMV) contamination—A case study: Detection, root cause determination, and corrective actions. PDA J. Pharm. Sci. Technol. 2011, 65, 580–588. [Google Scholar] [CrossRef]
- Steinmann, J. Evaluation of the effectiveness of eradic8 A2Z against feline calicivirus (surrogate of human norovirus). MikroLab GmbH Test. Report ML167/05. 2005, pp. 1–6. Available online: http://www.ebiox.co.uk/images/uploads/eradic8_A2Z_DVV_FCV_19_06_2009.pdf (accessed on 07 March 2013).
- Steinmann, J. Evaluation of effectiveness of Bacoban WB against feline calicivirus as surrogate for human norovirus. MikroLab GmbH Test. Report S07ML416F. 2008, pp. 1–14. Available online: http://www.bacoban.de/downloads/zertifikate/Bacoban%20WB/Norovirus_EN14476_EN.pdf (accessed on 20 February 2013).
- Woodall, C. Standard test method for efficacy of antimicrobial agents against viruses in suspension modified for feline calicivirus (human norovirus surrogate). BlueTest Test. Report EN 14476. 2011, pp. 1–4. Available online: http://www.hiclean.co/42.pdf (accessed on 07 March 2013).
- Scheidler, A.; Rokos, K.; Reuter, T.; Ebermann, R.; Pauli, G. Inactivation of viruses by β-propiolactone in human cryo poor plasma and IgG concentrates. Biologicals 1998, 26, 135–144. [Google Scholar] [CrossRef]
- Lazo, A.; Tasselo, J.; Jayarama, V.; Ohagen, A.; Gibaja, V.; Kramer, E.; Marmorato, A.; Billia-Shaveet, D.; Purmal, A.; Brown, F.; Chapman, J. Broad-spectrum virus reduction in red cell concentrates using INACTINE™ PEN110 chemistry. Vox. Sanguinis. 2002, 83, 313–323. [Google Scholar] [CrossRef]
- Martella, V.; Lorusso, E.; DeCaro, N.; Elia, G.; Radogna, A.; D’Abramo, M.; Desario, C.; Cavalli, A.; Corrente, M.; Camero, M.; et al. Detection and molecular characterization of a canine norovirus. Emerg. Inf. Dis. 2008, 14, 1306–1308. [Google Scholar] [CrossRef]
- Dolin, R.; Blacklow, N.R.; DuPont, H.; Buscho, R.F.; Wyatt, R.G.; Kasel, J.A.; Hornick, R.; Dhanock, R.M. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc. Soc. Exp. Biol. Med. 1972, 140, 578–583. [Google Scholar]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W.IV. Murine norovirus: A model system to study norovirus biology and pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef]
- Richards, G.P. Critical review of norovirus surrogates in food safety research: Rationale for considering volunteer studies. Food Environ. Virol. 2012, 4, 6–13. [Google Scholar] [CrossRef]
- Rodríguez, R.A.; Pepper, I.L.; Gerba, C.P. Application of PCR-based methods to assess the infectivity of enteric viruses in environmental samples. Appl. Environ. Microbiol. 2009, 75, 297–307. [Google Scholar] [CrossRef]
- Nuanualsuwan, S.; Cliver, D.O. Pretreatment to avoid positive RT-PCR results with inactivated viruses. J. Virol. Meth. 2002, 104, 217–225. [Google Scholar] [CrossRef]
- Noda, M.; Uema, M. Current topics on inactivation of norovirus. Kokuritsu. Iyakuhin. Shokuhin. Eisei. Kenkyusho. Hokoku. 2011, 129, 37–54. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nims, R.; Plavsic, M. Inactivation of Caliciviruses. Pharmaceuticals 2013, 6, 358-392. https://doi.org/10.3390/ph6030358
Nims R, Plavsic M. Inactivation of Caliciviruses. Pharmaceuticals. 2013; 6(3):358-392. https://doi.org/10.3390/ph6030358
Chicago/Turabian StyleNims, Raymond, and Mark Plavsic. 2013. "Inactivation of Caliciviruses" Pharmaceuticals 6, no. 3: 358-392. https://doi.org/10.3390/ph6030358




