Cell Penetration Properties of a Highly Efficient Mini Maurocalcine Peptide
Abstract
:1. Introduction

2. Results and Discussion
2.1. A Peptide Derived from the Hydrophobic Face of MCa Behaves as a Highly Competitive CPP
2.2. Randomly Defined Control Peptides Delimit the Threshold Level of an Acceptable Cell Penetration
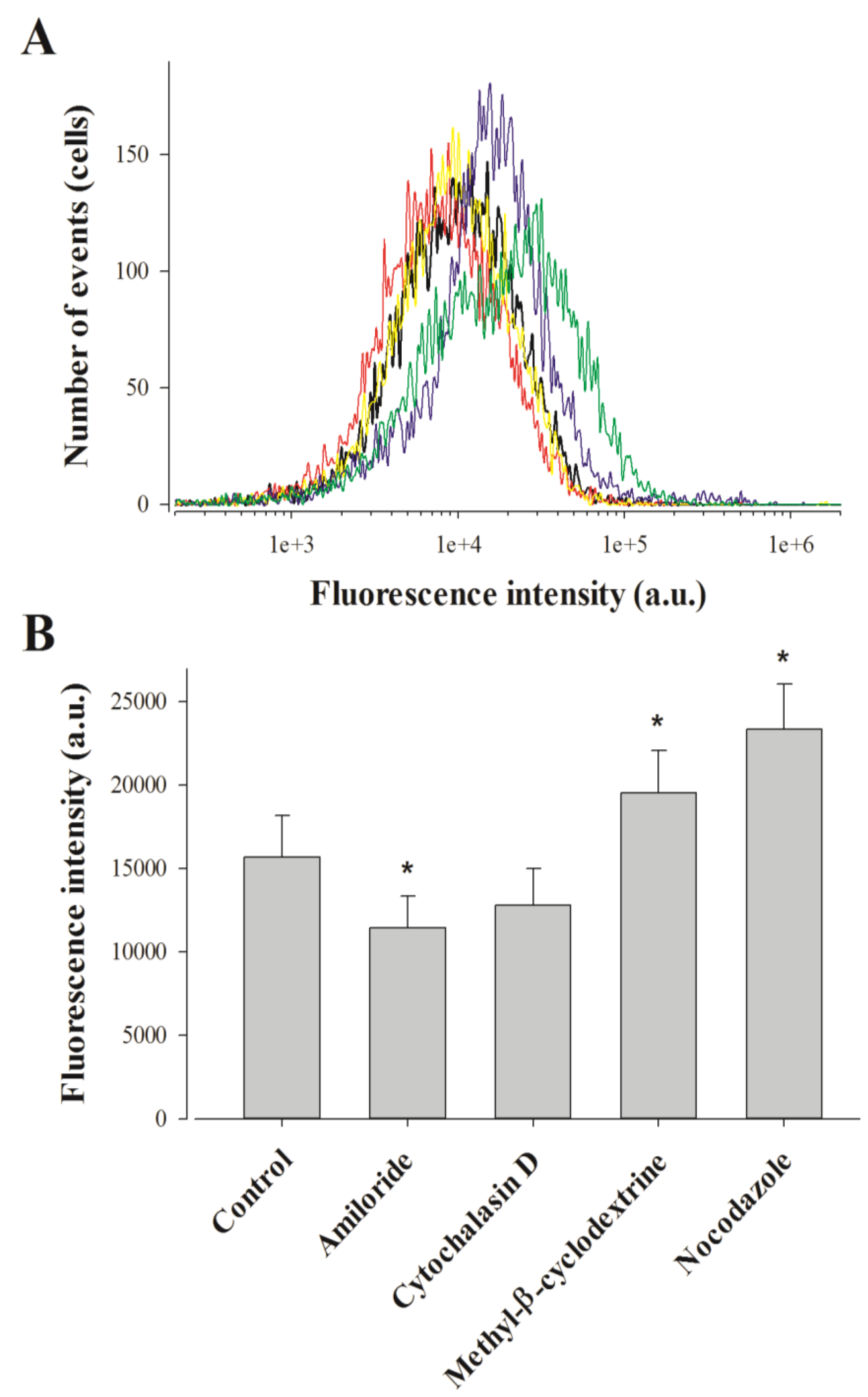
2.3. Pharmacological Blockade of Endocytosis in F98 Cells Affects Poorly MCaUF1-9 Cell Entry
2.4. Point Mutation of MCaUF1-9 Fails to Optimize The Cell Penetrating Properties of This Peptide
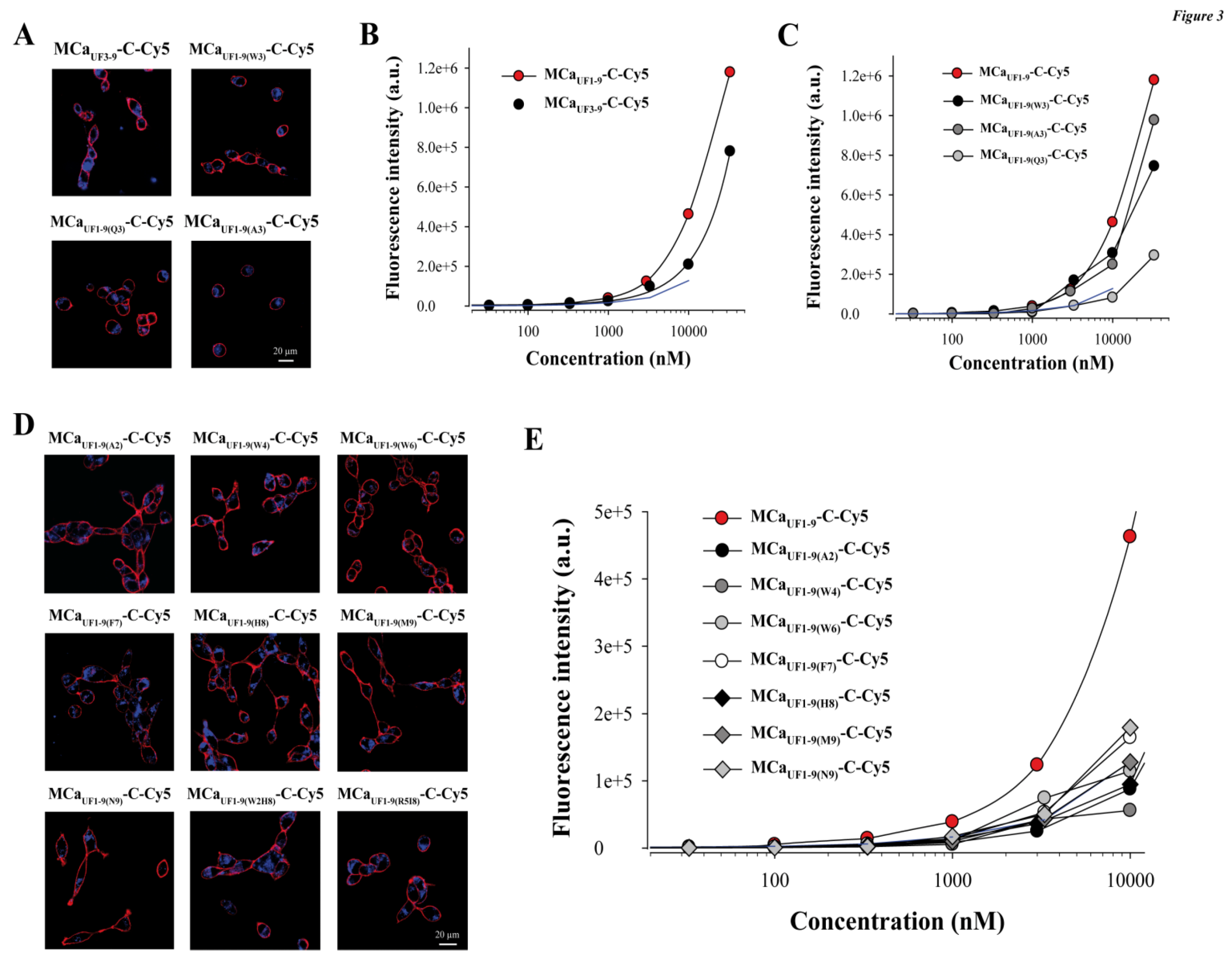
2.5. Analogous Hydrophobic Domains of Other Toxin Members of the Calcin Family Are Also Excellent CPP

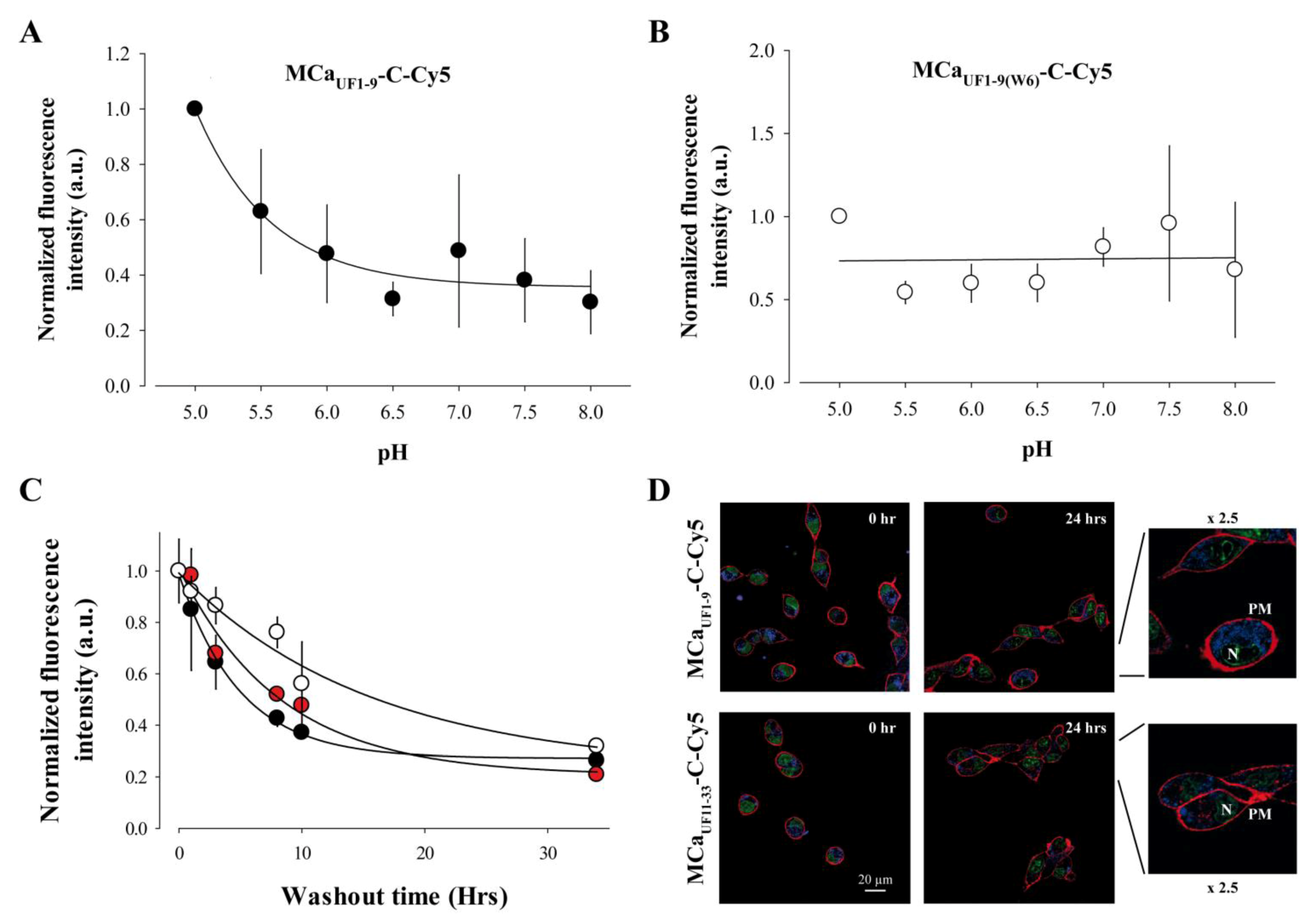
2.6. Cell Penetration of MCaUF1-9 is pH-sensitive Owing to the Presence of an His Residue in its Amino Acid Sequence
2.7. Long-Lasting Cell Retention of MCaUF1-9
2.8. Discussion
3. Experimental
3.1. Reagents
3.2. Peptide Syntheses
3.3. Peptide Labeling With Cy5
3.4. Cell Culture
3.5. Confocal Microscopy
3.6. Fluorescence Activated Cell Sorting Analyses
3.7. Molecular Modeling
4. Conclusions
Acknowledgements
References
- Fajloun, Z.; Kharrat, R.; Chen, L.; Lecomte, C.; di Luccio, E.; Bichet, D.; El Ayeb, M.; Rochat, H.; Allen, P.D.; Pessah, I.N.; et al. Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca2+ release channel/ryanodine receptors. FEBS Lett. 2000, 469, 179–185. [Google Scholar] [CrossRef]
- Mouhat, S.; Jouirou, B.; Mosbah, A.; de Waard, M.; Sabatier, J.M. Diversity of folds in animal toxins acting on ion channels. Biochem J. 2004, 378, 717–726. [Google Scholar] [CrossRef]
- Mosbah, A.; Kharrat, R.; Fajloun, Z.; Renisio, J.G.; Blanc, E.; Sabatier, J.M.; El Ayeb, M.; Darbon, H. A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel. Proteins 2000, 40, 436–442. [Google Scholar] [CrossRef]
- Zamudio, F.Z.; Gurrola, G.B.; Arevalo, C.; Sreekumar, R.; Walker, J.W.; Valdivia, H.H.; Possani, L.D. Primary structure and synthesis of imperatoxin A (iptx(a)), a peptide activator of Ca2+ release channels/ryanodine receptors. FEBS Lett. 1997, 405, 385–389. [Google Scholar] [CrossRef]
- Zhu, S.; Darbon, H.; Dyason, K.; Verdonck, F.; Tytgat, J. Evolutionary origin of inhibitor cystine knot peptides. FASEB J. 2003, 17, 1765–1767. [Google Scholar]
- Shahbazzadeh, D.; Srairi-Abid, N.; Feng, W.; Ram, N.; Borchani, L.; Ronjat, M.; Akbari, A.; Pessah, I.N.; de Waard, M.; El Ayeb, M. Hemicalcin, a new toxin from the iranian scorpion hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem. J. 2007, 404, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, E.F.; Capes, E.M.; Diego-Garcia, E.; Zamudio, F.Z.; Fuentes, O.; Possani, L.D.; Valdivia, H.H. Characterization of hadrucalcin, a peptide from hadrurus gertschi scorpion venom with pharmacological activity on ryanodine receptors. Br. J. Pharmacol. 2009, 157, 392–403. [Google Scholar] [CrossRef]
- Altafaj, X.; France, J.; Almassy, J.; Jona, I.; Rossi, D.; Sorrentino, V.; Mabrouk, K.; de Waard, M.; Ronjat, M. Maurocalcine interacts with the cardiac ryanodine receptor without inducing channel modification. Biochem. J. 2007, 406, 309–315. [Google Scholar] [CrossRef]
- Szappanos, H.; Smida-Rezgui, S.; Cseri, J.; Simut, C.; Sabatier, J.M.; de Waard, M.; Kovacs, L.; Csernoch, L.; Ronjat, M. Differential effects of maurocalcine on Ca2+ release events and depolarization-induced Ca2+ release in rat skeletal muscle. J. Physiol. 2005, 565, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Gurrola, G.B.; Arevalo, C.; Sreekumar, R.; Lokuta, A.J.; Walker, J.W.; Valdivia, H.H. Activation of ryanodine receptors by imperatoxin A and a peptide segment of the II-III loop of the dihydropyridine receptor. J. Biol. Chem. 1999, 274, 7879–7886. [Google Scholar]
- Esteve, E.; Mabrouk, K.; Dupuis, A.; Smida-Rezgui, S.; Altafaj, X.; Grunwald, D.; Platel, J.C.; Andreotti, N.; Marty, I.; Sabatier, J.M.; et al. Transduction of the scorpion toxin maurocalcine into cells. Evidence that the toxin crosses the plasma membrane. J. Biol. Chem. 2005, 280, 12833–12839. [Google Scholar]
- Tanabe, T.; Beam, K.G.; Adams, B.A.; Niidome, T.; Numa, S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature 1990, 346, 567–569. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Powell, J.A.; Numa, S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 1988, 336, 134–139. [Google Scholar] [CrossRef]
- Altafaj, X.; Cheng, W.; Esteve, E.; Urbani, J.; Grunwald, D.; Sabatier, J.M.; Coronado, R.; de Waard, M.; Ronjat, M. Maurocalcine and domain a of the II-III loop of the dihydropyridine receptor Cav1.1 subunit share common binding sites on the skeletal ryanodine receptor. J. Biol. Chem. 2005, 280, 4013–4016. [Google Scholar]
- Chen, L.; Esteve, E.; Sabatier, J.M.; Ronjat, M.; de Waard, M.; Allen, P.D.; Pessah, I.N. Maurocalcine and peptide a stabilize distinct subconductance states of ryanodine receptor type 1, revealing a proportional gating mechanism. J. Biol. Chem. 2003, 278, 16095–16106. [Google Scholar]
- Poillot, C.; Dridi, K.; Bichraoui, H.; Pecher, J.; Alphonse, S.; Douzi, B.; Ronjat, M.; Darbon, H.; de Waard, M. D-maurocalcine, a pharmacologically inert efficient cell-penetrating peptide analogue. J. Biol. Chem. 2010, 285, 34168–34180. [Google Scholar] [CrossRef]
- Mabrouk, K.; Ram, N.; Boisseau, S.; Strappazzon, F.; Rehaim, A.; Sadoul, R.; Darbon, H.; Ronjat, M.; de Waard, M. Critical amino acid residues of maurocalcine involved in pharmacology, lipid interaction and cell penetration. Biochim. Biophys. Acta 2007, 1768, 2528–2540. [Google Scholar] [CrossRef] [Green Version]
- Ram, N.; Weiss, N.; Texier-Nogues, I.; Aroui, S.; Andreotti, N.; Pirollet, F.; Ronjat, M.; Sabatier, J.M.; Darbon, H.; Jacquemond, V.; et al. Design of a disulfide-less, pharmacologically-inert and chemically-competent analog of maurocalcine for the efficient transport of impermeant compounds into cells. J. Biol. Chem. 2008, 283, 27048–27056. [Google Scholar] [CrossRef] [Green Version]
- Poillot, C.; Bichraoui, H.; Tisseyre, C.; Bahemberae, E.; Andreotti, N.; Sabatier, J.M.; Ronjat, M.; de Waard, M. Small efficient cell-penetrating peptides derived from scorpion toxin maurocalcine. J. Biol. Chem. 2012, 287, 17331–17342. [Google Scholar] [CrossRef] [Green Version]
- Aroui, S.; Brahim, S.; de Waard, M.; Breard, J.; Kenani, A. Efficient induction of apoptosis by doxorubicin coupled to cell-penetrating peptides compared to unconjugated doxorubicin in the human breast cancer cell line MDA-MB 231. Cancer Lett. 2009, 285, 28–38. [Google Scholar] [CrossRef]
- Aroui, S.; Brahim, S.; de Waard, M.; Kenani, A. Cytotoxicity, intracellular distribution and uptake of doxorubicin and doxorubicin coupled to cell-penetrating peptides in different cell lines: A comparative study. Biochem. Biophys. Res. Commun. 2010, 391, 419–425. [Google Scholar] [CrossRef]
- Aroui, S.; Brahim, S.; Hamelin, J.; de Waard, M.; Breard, J.; Kenani, A. Conjugation of doxorubicin to cell penetrating peptides sensitizes human breast MDA-MB 231 cancer cells to endogenous trail-induced apoptosis. Apoptosis 2009, 14, 1352–1365. [Google Scholar] [CrossRef]
- Aroui, S.; Ram, N.; Appaix, F.; Ronjat, M.; Kenani, A.; Pirollet, F.; de Waard, M. Maurocalcine as a non-toxic drug carrier overcomes doxorubicin resistance in the cancer cell line MDA-MB 231. Pharm. Res. 2009, 26, 836–845. [Google Scholar] [CrossRef]
- Ram, N.; Texier-Nogues, I.; Pernet-Gallay, K.; Poillot, C.; Ronjat, M.; Andrieux, A.; Arnoult, C.; Daou, J.; de Waard, M. In vitro and in vivo cell delivery of quantum dots by the cell penetrating peptide maurocalcine. Int. J. Biomed. Nanosci. Nanotechnol. 2011, 2, 12–32. [Google Scholar] [CrossRef]
- Stasiuk, G.J.; Tamang, S.; Imbert, D.; Poillot, C.; Giardiello, M.; Tisseyre, C.; Barbider, E.L.; Fries, P.H.; de Waard, M.; Reiss, P.; Mazzanti, M. Cell-permeable ln(III) chelate-functionalized InP quantum dots as multimodal imaging agents. ACS Nano 2011, 5, 8193–8201. [Google Scholar] [CrossRef]
- Ram, N.; Aroui, S.; Jaumain, E.; Bichraoui, H.; Mabrouk, K.; Ronjat, M.; Lortat-Jacob, H.; de Waard, M. Direct peptide interaction with surface glycosaminoglycans contributes to the cell penetration of maurocalcine. J. Biol. Chem. 2008, 283, 24274–24284. [Google Scholar] [CrossRef] [Green Version]
- Boisseau, S.; Mabrouk, K.; Ram, N.; Garmy, N.; Collin, V.; Tadmouri, A.; Mikati, M.; Sabatier, J.M.; Ronjat, M.; Fantini, J.; et al. Cell penetration properties of maurocalcine, a natural venom peptide active on the intracellular ryanodine receptor. Biochim. Biophys. Acta 2006, 1758, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.T.; Sayers, E.J. Cell entry of cell penetrating peptides: Tales of tails wagging dogs. J. Control. Release 2012, 161, 582–591. [Google Scholar] [CrossRef]
- Lindgren, M.; Hallbrink, M.; Prochiantz, A.; Langel, U. Cell-penetrating peptides. Trends Pharmacol. Sci. 2000, 21, 99–103. [Google Scholar] [CrossRef]
- Lundberg, P.; Langel, U. A brief introduction to cell-penetrating peptides. J. Mol. Recognit. 2003, 16, 227–233. [Google Scholar] [CrossRef]
- Mano, M.; Teodosio, C.; Paiva, A.; Simoes, S.; Pedroso de Lima, M.C. On the mechanisms of the internalization of s4(13)-pv cell-penetrating peptide. Biochem. J. 2005, 390, 603–612. [Google Scholar] [CrossRef]
- Gurrola, G.B.; Capes, E.M.; Zamudio, F.Z.; Possani, L.D.; Valdivia, H.H. Imperatoxin A, a cell-penetrating peptide from scorpion venom, as a probe of Ca-release channels/ryanodine receptors. Pharmaceuticals (Basel) 2010, 3, 1093–1107. [Google Scholar]
- El-Hayek, R.; Lokuta, A.J.; Arevalo, C.; Valdivia, H.H. Peptide probe of ryanodine receptor function. Imperatoxin A, a peptide from the venom of the scorpion pandinus imperator, selectively activates skeletal-type ryanodine receptor isoforms. J. Biol. Chem. 1995, 270, 28696–28704. [Google Scholar]
- Garcia-Martin, M.L.; Herigault, G.; Remy, C.; Farion, R.; Ballesteros, P.; Coles, J.A.; Cerdan, S.; Ziegler, A. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: Comparison with maps of metabolites. Cancer Res. 2001, 61, 6524–6531. [Google Scholar]
- Merrifield, R.B. Solid-phase peptide synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969, 32, 221–296. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tisseyre, C.; Bahembera, E.; Dardevet, L.; Sabatier, J.-M.; Ronjat, M.; De Waard, M. Cell Penetration Properties of a Highly Efficient Mini Maurocalcine Peptide. Pharmaceuticals 2013, 6, 320-339. https://doi.org/10.3390/ph6030320
Tisseyre C, Bahembera E, Dardevet L, Sabatier J-M, Ronjat M, De Waard M. Cell Penetration Properties of a Highly Efficient Mini Maurocalcine Peptide. Pharmaceuticals. 2013; 6(3):320-339. https://doi.org/10.3390/ph6030320
Chicago/Turabian StyleTisseyre, Céline, Eloi Bahembera, Lucie Dardevet, Jean-Marc Sabatier, Michel Ronjat, and Michel De Waard. 2013. "Cell Penetration Properties of a Highly Efficient Mini Maurocalcine Peptide" Pharmaceuticals 6, no. 3: 320-339. https://doi.org/10.3390/ph6030320





