Potential Use of Polyamidoamine Dendrimer Conjugates with Cyclodextrins as Novel Carriers for siRNA
Abstract
:1. Introduction
2. Dendrimers for siRNA Carriers
3. Cyclodextrins for siRNA Carriers
4. Cyclodextrin Conjugates with Dendrimer (CDE) as siRNA Carrier

| Conjugate | Abbreviation | G | DS of CyD | DS of ligand | DNA or RNA | Ref. |
| α-Cyclodextrin/dendrimer | α-CDE | 2 | 1.0 | 0 | pDNA | [57] |
| α-Cyclodextrin/dendrimer | α-CDE | 3 | 1.0 | 0 | pDNA | [58, 59] |
| α-Cyclodextrin/dendrimer | α-CDE | 4 | 1.0 | 0 | pDNA | [58] |
| α-Cyclodextrin/dendrimer | α-CDE | 3 | 2.4 | 0 | pDNA, siRNA, shRNA | [59, 62,63,64] |
| Mannosylated α-CDE | Man-α-CDE | 2 | 1.0 | 3.3 | pDNA | [70] |
| Mannosylated α-CDE | Man-α-CDE | 3 | 2.2 | 10 | pDNA | [61] |
| Galactosylated α-CDE | Gal-α-CDE | 2 | 1.0 | 4 | pDNA | [72] |
| Lactosylated α-CDE | Lac-α-CDE | 2 | 1.1 | 2.6 | pDNA, siRNA | [44, 73] |
| Lactosylated α-CDE | Lac-α-CDE | 3 | 2.4 | 1.2 | pDNA, siRNA | [44, 74] |
| Folated α-CDE | Fol-α-CDE | 3 | 2.4 | 5 | pDNA | [44] |
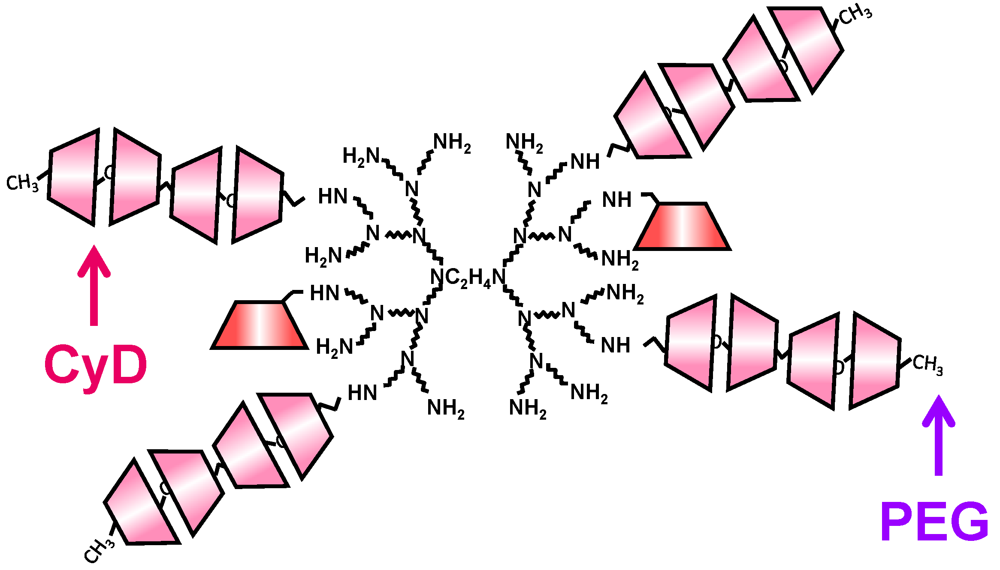
| Fol-pegylated α-CDE | Fol-PαC | 3 | 2.4 | 5 | pDNA, siRNA | [44] |
| β-Cyclodextrin/dendrimer | β-CDE | 2 | 1.0 | 0 | pDNA | [57, 60, 61] |
| Glucuronylglucosyl-β-CDE | GUG-β-CDE | 2 | 1.8 | 0 | pDNA | [60, 61] |
| γ-Cyclodextrin/dendrimer | γ-CDE | 2 | 1.0 | 0 | pDNA | [57] |

5. Sugar-Appended α-CDEs as siRNA Carriers

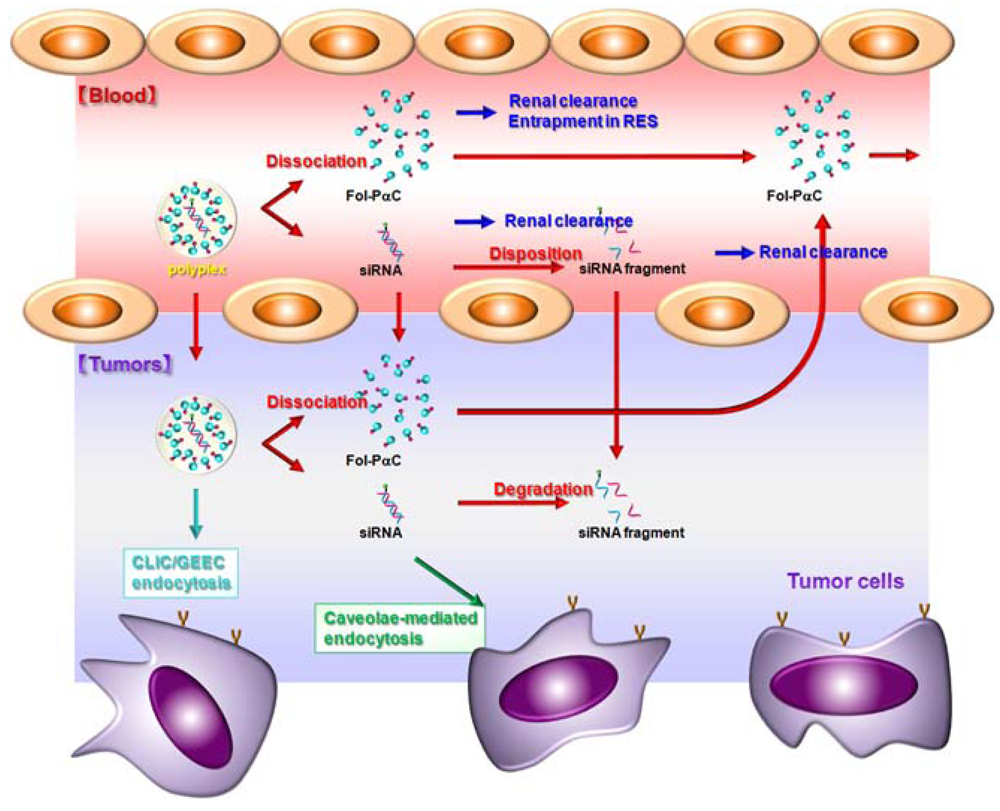
6. Folate-Appended α-CDEs as siRNA Carriers
7. Sustained Release System of pDNA Using CyD Polypseudorotaxane
8. Conclusions
Acknowledgments
Conflict of Interest
References
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L. Delivery of sirna therapeutics: Barriers and carriers. AAPS J. 2010, 12, 492–503. [Google Scholar] [CrossRef]
- Castanotto, D.; Rossi, J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009, 457, 426–433. [Google Scholar]
- Kim, D.H.; Rossi, J.J. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 2007, 8, 173–184. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J.J. Aptamer-targeted cell-specific RNA interference. Silence 2010, 1, 4. [Google Scholar] [CrossRef]
- Grimm, D.; Streetz, K.L.; Jopling, C.L.; Storm, T.A.; Pandey, K.; Davis, C.R.; Marion, P.; Salazar, F.; Kay, M.A. Fatality in mice due to oversaturation of cellular microrna/short hairpin RNA pathways. Nature 2006, 441, 537–541. [Google Scholar]
- Grimm, D.; Kay, M.A. Therapeutic short hairpin RNA expression in the liver: Viral targets and vectors. Gene Ther. 2006, 13, 563–575. [Google Scholar] [CrossRef]
- Aagaard, L.; Rossi, J.J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar]
- Tseng, Y.C.; Mozumdar, S.; Huang, L. Lipid-based systemic delivery of siRNA. Adv. Drug Deliv. Rev. 2009, 61, 721–731. [Google Scholar] [CrossRef]
- Elouahabi, A.; Ruysschaert, J.M. Formation and intracellular trafficking of lipoplexes and polyplexes. Mol. Ther. 2005, 11, 336–347. [Google Scholar]
- Rudzinski, W.E.; Aminabhavi, T.M. Chitosan as a carrier for targeted delivery of small interfering RNA. Int. J. Pharm. 2010, 399, 1–11. [Google Scholar] [CrossRef]
- Moschos, S.A.; Williams, A.E.; Lindsay, M.A. Cell-penetrating-peptide-mediated siRNA lung delivery. Biochem. Soc. Trans. 2007, 35, 807–810. [Google Scholar] [CrossRef]
- de Paula, D.; Bentley, M.V.; Mahato, R.I. Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting. RNA 2007, 13, 431–456. [Google Scholar] [CrossRef]
- de Martimprey, H.; Vauthier, C.; Malvy, C.; Couvreur, P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: Oligonucleotides and siRNA. Eur. J. Pharm. Biopharm. 2009, 71, 490–504. [Google Scholar]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roek, J.; Ryder, J.; Smith, P. A new class of polymers: Starburt-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tomalia, D.A. Dendrimer research. Science 1991, 252, 1231. [Google Scholar]
- Boas, U.; Heegaard, P.M. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (pamam) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Braun, C.S.; Vetro, J.A.; Tomalia, D.A.; Koe, G.S.; Koe, J.G.; Middaugh, C.R. Structure/function relationships of polyamidoamine/DNA dendrimers as gene delivery vehicles. J. Pharm. Sci. 2005, 94, 423–436. [Google Scholar] [CrossRef]
- Fant, K.; Esbjorner, E.K.; Lincoln, P.; Norden, B. DNA condensation by pamam dendrimers: Self-assembly characteristics and effect on transcription. Biochemistry 2008, 47, 1732–1740. [Google Scholar]
- Dutta, T.; Jain, N.K.; McMillan, N.A.; Parekh, H.S. Dendrimer nanocarriers as versatile vectors in gene delivery. Nanomedicine 2010, 6, 25–34. [Google Scholar] [CrossRef]
- Hui, Z.; He, Z.G.; Zheng, L.; Li, G.Y.; Shen, S.R.; Li, X.L. Studies on polyamidoamine dendrimers as efficient gene delivery vector. J. Biomater. Appl. 2008, 22, 527–544. [Google Scholar]
- Klajnert, B.; Bryszewska, M. Dendrimers: Properties and applications. Acta Biochim. Pol. 2001, 48, 199–208. [Google Scholar]
- Braun, C.S.; Fisher, M.T.; Tomalia, D.A.; Koe, G.S.; Koe, J.G.; Middaugh, C.R. A stopped-flow kinetic study of the assembly of nonviral gene delivery complexes. Biophys. J. 2005, 88, 4146–4158. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker, J.R., Jr. Efficient transfer of genetic material into mammalian cells using starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar]
- Shah, N.; Steptoe, R.J.; Parekh, H.S. Low-generation asymmetric dendrimers exhibit minimal toxicity and effectively complex DNA. J. Pept. Sci. 2011, 17, 470–478. [Google Scholar] [CrossRef] [Green Version]
- Merkel, O.M.; Mintzer, M.A.; Simanek, E.E.; Keissel, T. Perfectly shaped siRNA delivery. Ther. Deliv. 2010, 1, 737–742. [Google Scholar] [CrossRef]
- Perez, A.P.; Romero, E.L.; Morilla, M.J. Ethylendiamine core pamam dendrimers/siRNA complexes as in vitro silencing agents. Int. J. Pharm. 2009, 380, 189–200. [Google Scholar] [CrossRef]
- Morgan, D.M.; Larvin, V.L.; Pearson, J.D. Biochemical characterisation of polycation-induced cytotoxicity to human vascular endothelial cells. J. Cell Sci. 1989, 94, 553–559. [Google Scholar]
- Ohsaki, M.; Okuda, T.; Wada, A.; Hirayama, T.; Niidome, T.; Aoyagi, H. In vitro gene transfection using dendritic poly(L-lysine). Bioconjug. Chem. 2002, 13, 510–517. [Google Scholar] [CrossRef]
- Bermejo, J.F.; Ortega, P.; Chonco, L.; Eritja, R.; Samaniego, R.; Mullner, M.; de Jesus, E.; de la Mata, F.J.; Flores, J.C.; Gomez, R.; Munoz-Fernandez, A. Water-soluble carbosilane dendrimers: Synthesis biocompatibility and complexation with oligonucleotides; evaluation for medical applications. Chemistry 2007, 13, 483–495. [Google Scholar] [CrossRef]
- Merkel, O.M.; Mintzer, M.A.; Librizzi, D.; Samsonova, O.; Dicke, T.; Sproat, B.; Garn, H.; Barth, P.J.; Simanek, E.E.; Kissel, T. Triazine dendrimers as nonviral vectors for in vitro and in vivo RNAi: The effects of peripheral groups and core structure on biological activity. Mol. Pharm. 2010, 7, 969–983. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Singha, K.; Namgung, R.; Kim, W.J. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011, 21, 133–147. [Google Scholar]
- Yuan, X.; Naguib, S.; Wu, Z. Recent advances of siRNA delivery by nanoparticles. Expert Opin. Drug Deliv. 2011, 8, 521–536. [Google Scholar] [CrossRef]
- Challa, T.; Agaiah Goud, B.; Baskar, S.; Chandra Mouli, G.; Jukuri, R. Dendrimers: A novel polymer for drug delivery. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 88–99. [Google Scholar]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z.; Tziveleka, L.A. Drug delivery using multifunctional dendrimers and hyperbranched polymers. Expert Opin. Drug Deliv. 2010, 7, 1387–1398. [Google Scholar] [CrossRef]
- Shcharbin, D.G.; Klajnert, B.; Bryszewska, M. Dendrimers in gene transfection. Biochemistry 2009, 74, 1070–1079. [Google Scholar]
- Szejtli, J. Medicinal applications of cyclodextrins. Med. Res. Rev. 1994, 14, 353–386. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem Rev. 1998, 98, 2045–2076. [Google Scholar]
- Uekama, K. Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. (Tokyo) 2004, 52, 900–915. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Irie, T.; Otagiri, M.; Sunada, M.; Uekama, K.; Ohtani, Y.; Yamada, Y.; Sugiyama, Y. Cyclodextrin-induced hemolysis and shape changes of human erythrocytes in vitro. J. Pharmacobio-dyn. 1982, 5. [Google Scholar]
- Ohtani, Y.; Irie, T.; Uekama, K.; Fukunaga, K.; Pitha, J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 1989, 186, 17–22. [Google Scholar] [CrossRef]
- Fauvelle, F.; Debouzy, J.C.; Crouzy, S.; Goschl, M.; Chapron, Y. Mechanism of alpha-cyclodextrin-induced hemolysis. 1. The two-step extraction of phosphatidylinositol from the membrane. J. Pharm. Sci. 1997, 86, 935–943. [Google Scholar] [CrossRef]
- Arima, H.; Motoyama, K. Recent findings concerning pamam dendrimer conjugates with cyclodextrins as carriers of DNA and RNA. Sensors 2009, 9, 6364–6361. [Google Scholar]
- Gonzalez, H.; Hwang, S.J.; Davis, M.E. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem. 1999, 10, 1068–1074. [Google Scholar] [CrossRef]
- Hwang, S.J.; Bellocq, N.C.; Davis, M.E. Effects of structure of beta-cyclodextrin-containing polymers on gene delivery. Bioconjug. Chem. 2001, 12, 280–290. [Google Scholar] [CrossRef]
- Pun, S.H.; Tack, F.; Bellocq, N.C.; Cheng, J.; Grubbs, B.H.; Jensen, G.S.; Davis, M.E.; Brewster, M.; Janicot, M.; Janssens, B.; Floren, W.; Bakker, A. Targeted delivery of RNA-cleaving DNA enzyme (dnazyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol. Ther. 2004, 3, 641–650. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Davis, M.E. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol. Bioeng. 2008, 99, 975–985. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Ganguly, K.; Kulkarni, A.R.; Kulkarni, V.H.; Nadagouda, M.N.; Rudzinski, W.E.; Aminabhavi, T.M. Cyclodextrin-based siRNA delivery nanocarriers: A state-of-the-art review. Expert Opin. Drug Deliv. 2011, 8, 1455–1468. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Davis, M.E. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug. Chem. 2007, 18, 456–468. [Google Scholar] [CrossRef]
- Davis, M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar]
- Heidel, J.D.; Yu, Z.; Liu, J.Y.; Rele, S.M.; Liang, Y.; Zeidan, R.K.; Kornbrust, D.J.; Davis, M.E. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc. Natl. Acad. Sci. USA 2007, 104, 5715–5721. [Google Scholar]
- Boe, S.L.; Longva, A.S.; Hovig, E. Cyclodextrin-containing polymer delivery system for light-directed siRNA gene silencing. Oligonucleotides 2010, 20, 175–182. [Google Scholar] [CrossRef]
- Redenti, E.; Pietra, C.; Gerloczy, A.; Szente, L. Cyclodextrins in oligonucleotide delivery. Adv. Drug Deliv. Rev. 2001, 53, 235–244. [Google Scholar] [CrossRef]
- Ortiz Mellet, C.; Garcia Fernandez, J.M.; Benito, J.M. Cyclodextrin-based gene delivery systems. Chem. Soc. Rev. 2011, 40, 1586–1608. [Google Scholar]
- Arima, H.; Kihara, F.; Hirayama, F.; Uekama, K. Enhancement of gene expression by polyamidoamine dendrimer conjugates with α-, β-, and γ-cyclodextrins. Bioconjug. Chem. 2001, 12, 476–484. [Google Scholar] [CrossRef]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Effects of structure of polyamidoamine dendrimer on gene transfer efficiency of the dendrimer conjugate with α-cyclodextrin. Bioconjug. Chem. 2002, 13, 1211–1219. [Google Scholar] [CrossRef]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. In vitro and in vivo gene transfer by an optimized α-cyclodextrin conjugate with polyamidoamine dendrimer. Bioconjug. Chem. 2003, 14, 342–350. [Google Scholar] [CrossRef]
- Anno, T.; Motoyama, K.; Higashi, T.; Hirayama, F.; Uekama, K.; Arima, H. Preparation and evaluation of polyamidoamine dendrimer (g2)/branched-β-cyclodextrin conjugate as a novel gene transfer carrier. J. Incl. Phenom. Macro. Chem. 2011, 70, 339–344. [Google Scholar] [CrossRef]
- Anno, T.; Higashi, T.; Motoyama, K.; Hirayama, F.; Uekama, K.; Arima, H. Potential use of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate (G2) as a DNA carrier in vitro and in vivo. J. Drug. Target. 2011, in press. [Google Scholar]
- Tsutsumi, T.; Hirayama, F.; Uekama, K.; Arima, H. Evaluation of polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, g3) as a novel carrier for small interfering RNA (siRNA). J. Control. Release 2007, 119, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, T.; Hirayama, F.; Uekama, K.; Arima, H. Potential use of polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, g3) as a novel carrier for short hairpin RNA-expressing plasmid DNA. J. Pharm. Sci. 2008, 97, 3022–3034. [Google Scholar] [CrossRef]
- Arima, H.; Tsutsumi, T.; Yoshimatsu, A.; Ikeda, H.; Motoyama, K.; Higashi, T.; Hirayama, F.; Uekama, K. Inhibitory effect of siRNA complexes with polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, g3) on endogenous gene expression. Eur. J. Pharm. Sci. 2011, 44, 375–384. [Google Scholar] [CrossRef]
- Rao, D.D.; Vorhies, J.S.; Senzer, N.; Nemunaitis, J. siRNA vs. shRNA: Similarities and differences. Adv. Drug Deliv. Rev. 2009, 61, 746–759. [Google Scholar] [CrossRef]
- Roche, A.C.; Fajac, I.; Grosse, S.; Frison, N.; Rondanino, C.; Mayer, R.; Monsigny, M. Glycofection: Facilitated gene transfer by cationic glycopolymers. Cell. Mol. Life Sci. 2003, 60, 288–297. [Google Scholar] [CrossRef]
- Monsigny, M.; Rondanino, C.; Duverger, E.; Fajac, I.; Roche, A.C. Glyco-dependent nuclear import of glycoproteins, glycoplexes and glycosylated plasmids. Biochim. Biophys. Acta 2004, 1673, 94–103. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kursa, M.; Wagner, E.; Cotten, M.; Zenke, M. Mannose polyethylenimine conjugates for targeted DNA delivery into dendritic cells. J. Biol. Chem. 1999, 274, 19087–19094. [Google Scholar]
- Zanta, M.A.; Boussif, O.; Adib, A.; Behr, J.P. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjug. Chem. 1997, 8, 839–844. [Google Scholar] [CrossRef]
- Wada, K.; Arima, H.; Tsutsumi, T.; Chihara, Y.; Hattori, K.; Hirayama, F.; Uekama, K. Improvement of gene delivery mediated by mannosylated dendrimer/α-cyclodextrin conjugates. J. Control. Release 2005, 104, 397–413. [Google Scholar] [CrossRef]
- Arima, H.; Chihara, Y.; Arizono, M.; Yamashita, S.; Wada, K.; Hirayama, F.; Uekama, K. Enhancement of gene transfer activity mediated by mannosylated dendrimer/α-cyclodextrin conjugate (generation 3, g3). J. Control. Release 2006, 116, 64–74. [Google Scholar] [CrossRef]
- Wada, K.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Enhancing effects of galactosylated dendrimer/α-cyclodextrin conjugates on gene transfer efficiency. Biol. Pharm. Bull. 2005, 28, 500–505. [Google Scholar] [CrossRef]
- Arima, H.; Yamashita, S.; Mori, Y.; Hayashi, Y.; Motoyama, K.; Hattori, K.; Takeuchi, T.; Jono, H.; Ando, Y.; Hirayama, F.; Uekama, K. In vitro and in vivo gene delivery mediated by lactosylated dendrimer/α-cyclodextrin conjugates (g2) into hepatocytes. J. Control. Release 2010, 146, 106–117. [Google Scholar] [CrossRef]
- Motoyama, K.; Mori, Y.; Yamashita, S.; Hayashi, Y.; Jono, H.; Ando, Y.; Hirayama, F.; Uekama, K.; Arima, H. In vitro gene delivery mediated by lactosylated dendrimer (generation 3, g3)/α-cyclodextrin conjugates into hepatocytes. J. Incl. Phenom. Macro. Chem. 2011, 70, 333–338. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar]
- Harata, M.; Soda, Y.; Tani, K.; Ooi, J.; Takizawa, T.; Chen, M.; Bai, Y.; Izawa, K.; Kobayashi, S.; Tomonari, A.; et al. Cd19-targeting liposomes containing imatinib efficiently kill philadelphia chromosome-positive acute lymphoblastic leukemia cells. Blood 2004, 104, 1442–1449. [Google Scholar]
- Chen, H.; Ahn, R.; van den Bossche, J.; Thompson, D.H.; O'Halloran, T.V. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol. Cancer Ther. 2009, 8, 1955–1963. [Google Scholar] [CrossRef]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef]
- Talekar, M.; Kendall, J.; Denny, W.; Garg, S. Targeting of nanoparticles in cancer: Drug delivery and diagnostics. Anticancer Drugs 2011, 22, 949–962. [Google Scholar]
- Kolhatkar, R.; Lote, A.; Khambati, H. Active tumor targeting of nanomaterials using folic acid, transferrin and integrin receptors. Curr. Drug Discov. Technol. 2011, 8, 197–206. [Google Scholar] [CrossRef]
- Yuan, Q.; Lee, E.; Yeudall, W.A.; Yang, H. Dendrimer-triglycine-EGF nanoparticles for tumor imaging and targeted nucleic acid and drug delivery. Oral Oncol. 2010, 46, 698–704. [Google Scholar] [CrossRef]
- Temming, K.; Schiffelers, R.M.; Molema, G.; Kok, R.J. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Updat. 2005, 8, 381–402. [Google Scholar] [CrossRef]
- Jackman, A.L.; Theti, D.S.; Gibbs, D.D. Antifolates targeted specifically to the folate receptor. Adv. Drug Deliv. Rev. 2004, 56, 1111–1125. [Google Scholar] [CrossRef]
- Konda, S.D.; Aref, M.; Wang, S.; Brechbiel, M.; Wiener, E.C. Specific targeting of folatedendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magn. Reson. Mater. Phys. Bio. Med. 2001, 12, 104–113. [Google Scholar] [CrossRef]
- Shukla, R.; Thomas, T.P.; Desai, A.M.; Kotlyar, A.; Park, S.J.; Baker, J.R. HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology 2008, 19, 295102. [Google Scholar]
- Singh, I.; Rehni, A.K.; Kalra, R.; Joshi, G.; Kumar, M. Dendrimers and their pharmaceutical applications—a review. Pharmazie 2008, 63, 491–496. [Google Scholar]
- Wang, P.; Zhao, X.H.; Wang, Z.Y.; Meng, M.; Li, X.; Ning, Q. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Lett. 2010, 298, 34–49. [Google Scholar] [CrossRef]
- Harada, A. Cyclodextrin-based molecular machines. Acc. Chem. Res. 2001, 34, 456–464. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar]
- Motoyama, K.; Hayashida, K.; Arima, H. Potential use of polypseudorotaxanes of pegylated polyamidoamine dendrimer with cyclodextrins as novel sustained release systems for DNA. Chem. Pharm. Bull. 2011, 59, 476–479. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arima, H.; Motoyama, K.; Higashi, T. Potential Use of Polyamidoamine Dendrimer Conjugates with Cyclodextrins as Novel Carriers for siRNA. Pharmaceuticals 2012, 5, 61-78. https://doi.org/10.3390/ph5010061
Arima H, Motoyama K, Higashi T. Potential Use of Polyamidoamine Dendrimer Conjugates with Cyclodextrins as Novel Carriers for siRNA. Pharmaceuticals. 2012; 5(1):61-78. https://doi.org/10.3390/ph5010061
Chicago/Turabian StyleArima, Hidetoshi, Keiichi Motoyama, and Taishi Higashi. 2012. "Potential Use of Polyamidoamine Dendrimer Conjugates with Cyclodextrins as Novel Carriers for siRNA" Pharmaceuticals 5, no. 1: 61-78. https://doi.org/10.3390/ph5010061