Design and Synthesis of 99mTcN-Labeled Dextran-Mannose Derivatives for Sentinel Lymph Node Detection
Abstract
:1. Introduction
2. Results and Discussion
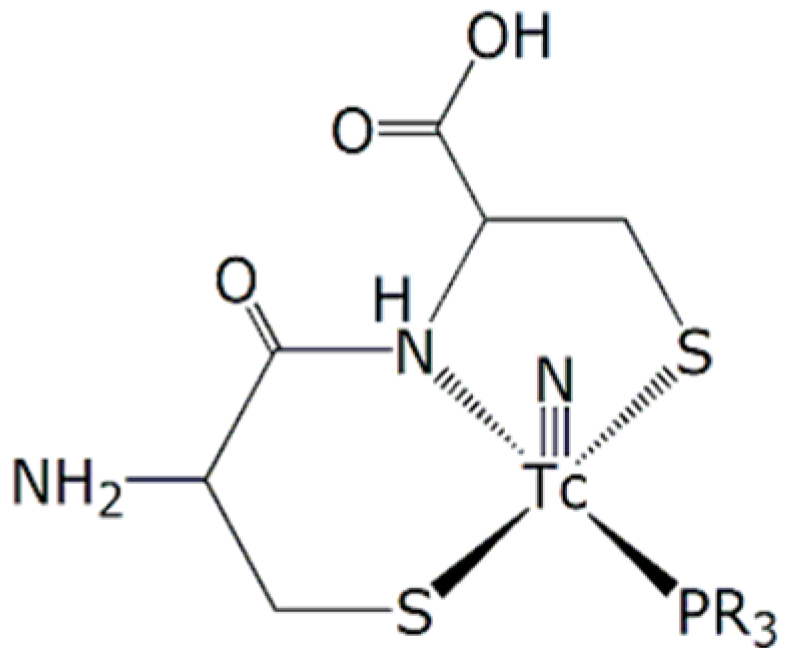
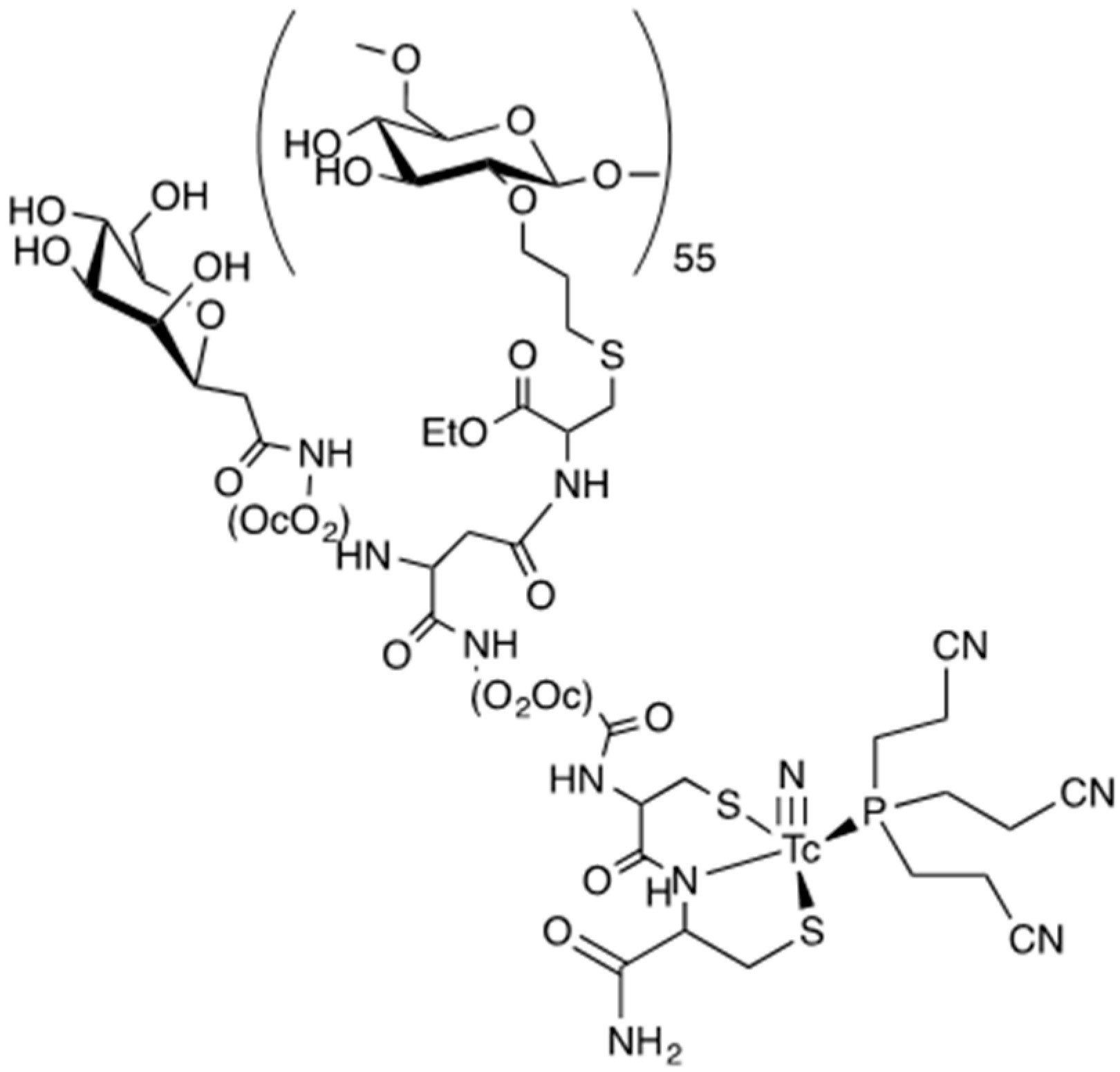
2.1. Design of a Dextran-Mannose Multifunctional Ligand for Coordination to the [99mTc≡N]2+ Core
2.2. Synthesis of Dextran-Mannosyl Multifunctional Ligands for the [99mTc≡N]2+ Core
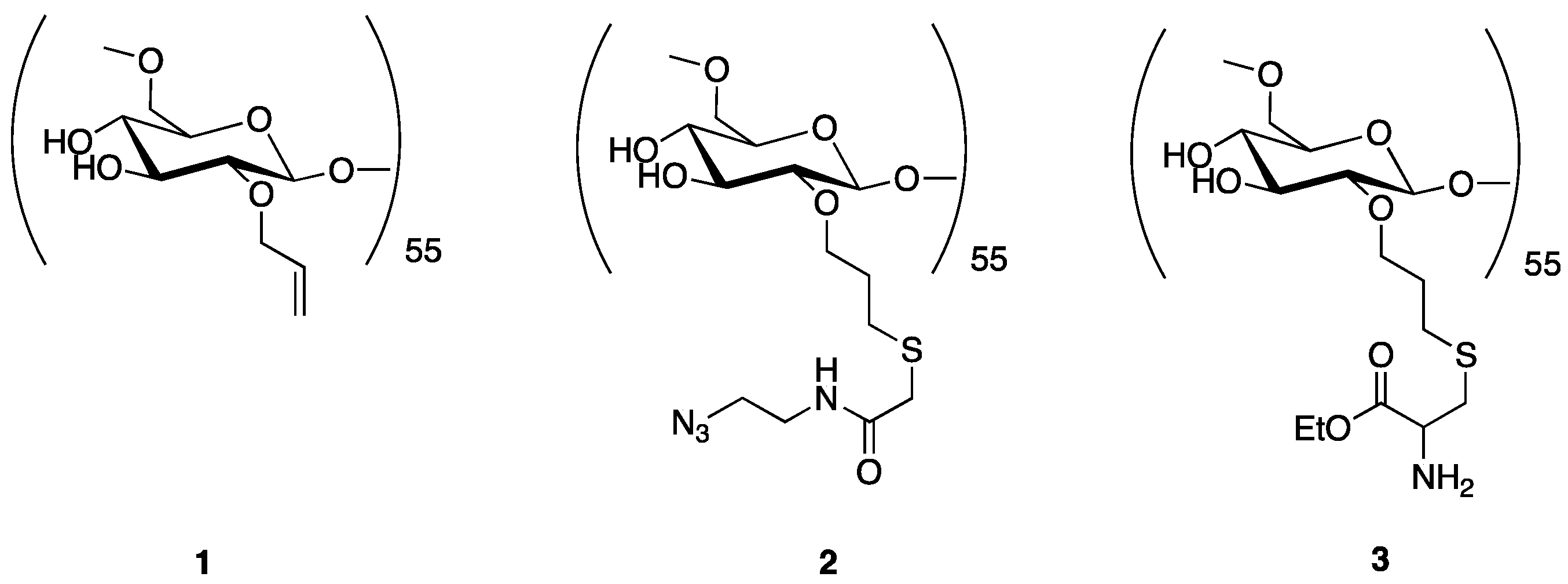
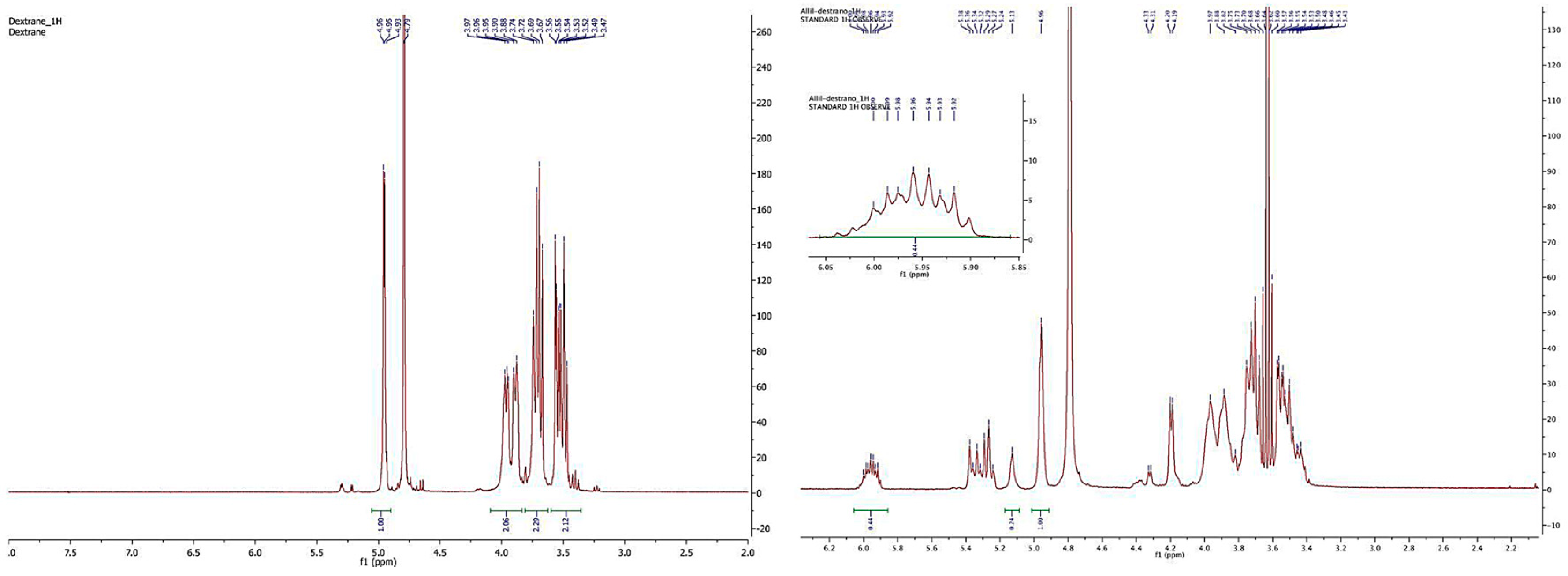
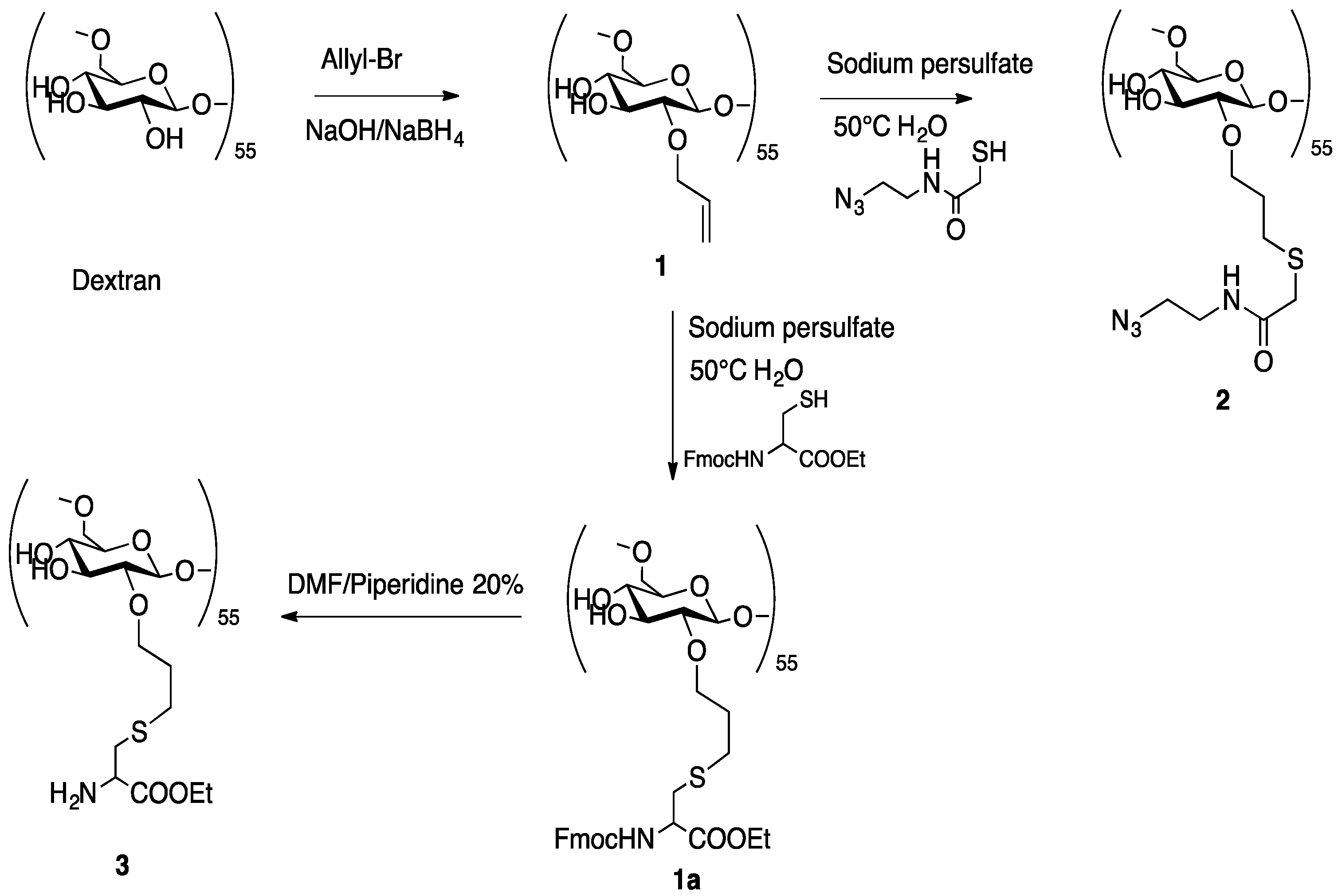
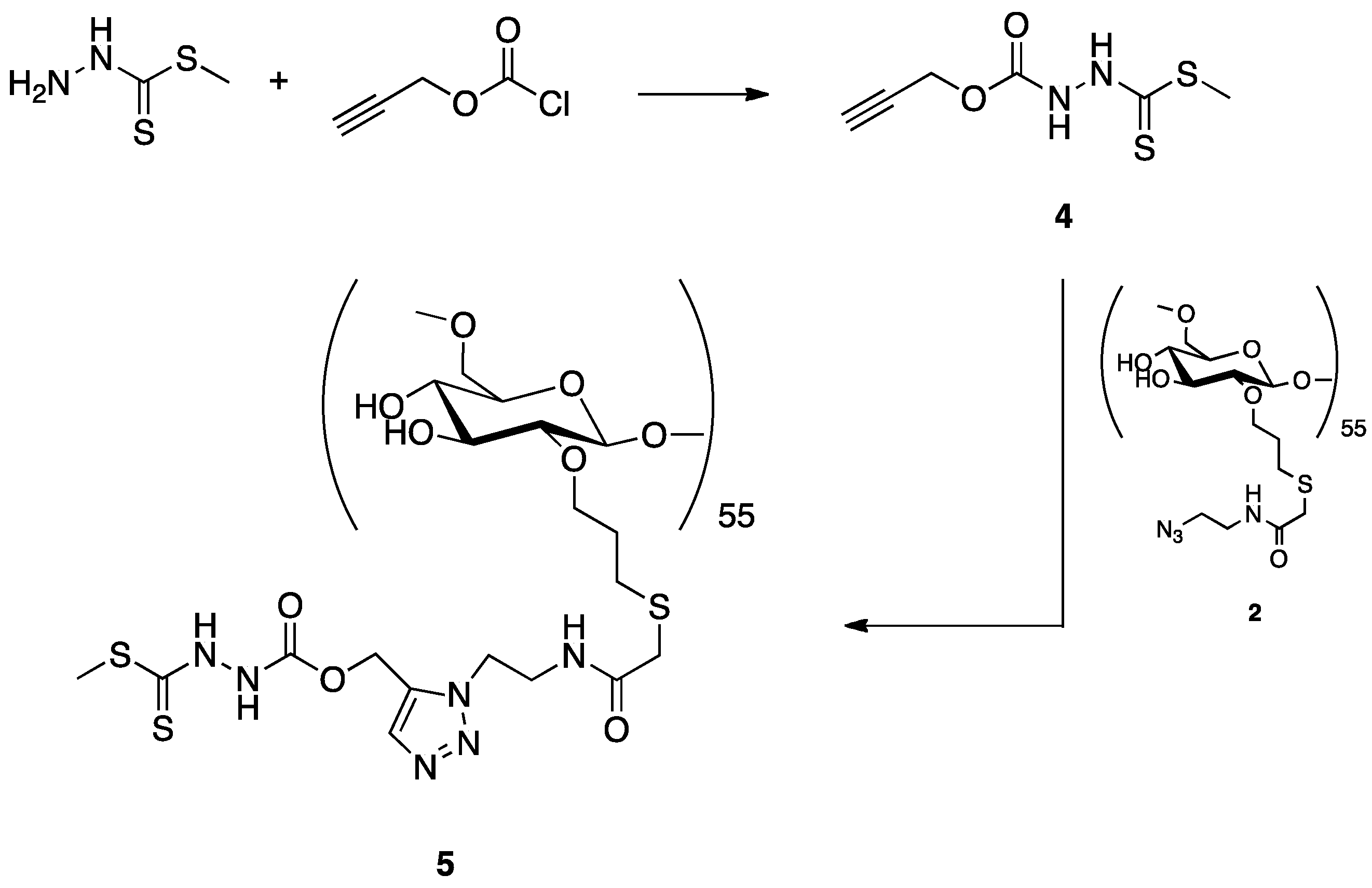
2.2.1. Synthesis of Dextran Derivatives
2.2.2. Synthesis of a Dextran-DTCZ Multifunctional Ligand
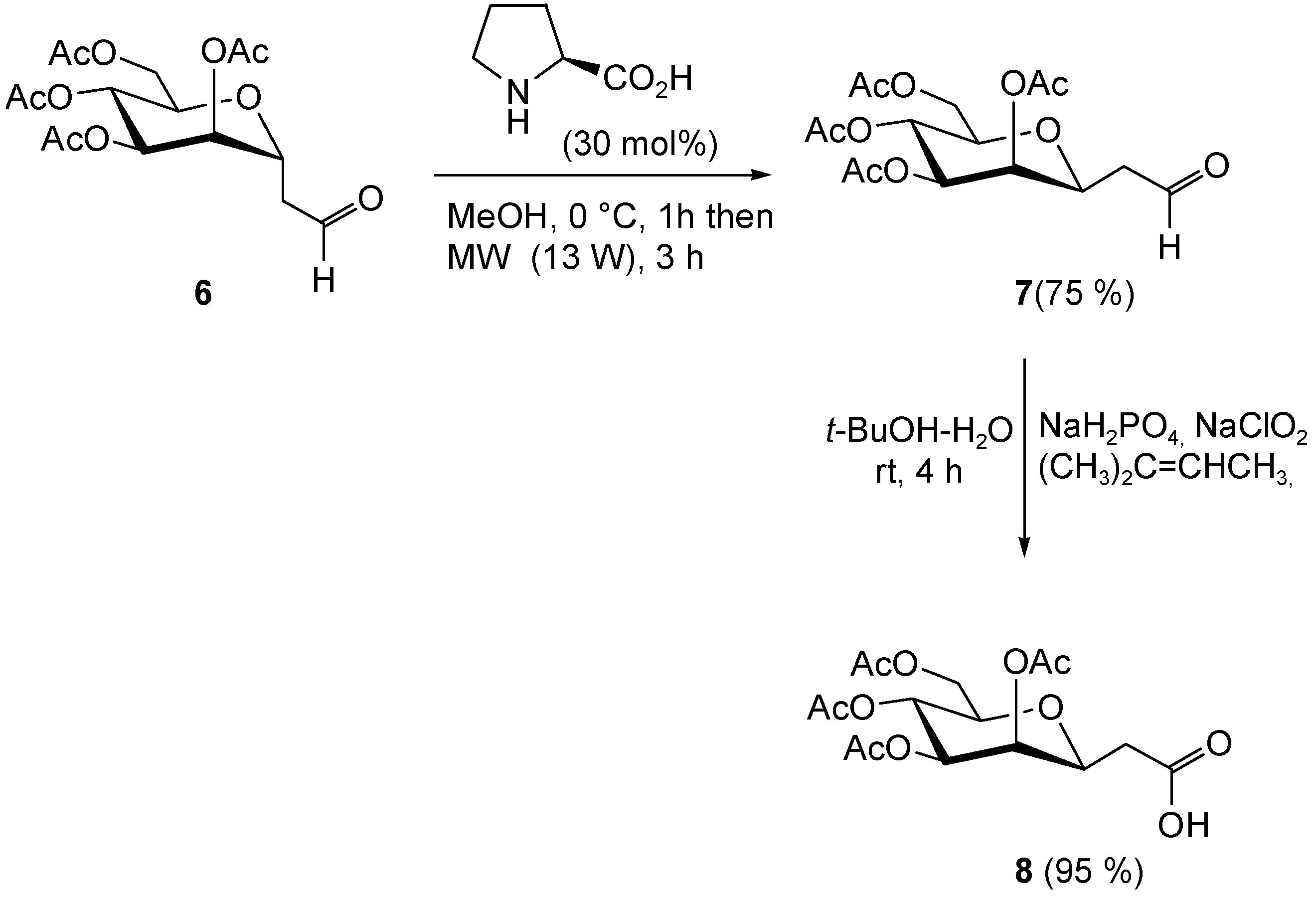
2.3. Synthesis of 2-(2,3,4,6-tetra-O-acetyl-β-d-mannopyranosyl)-Acetic Acid
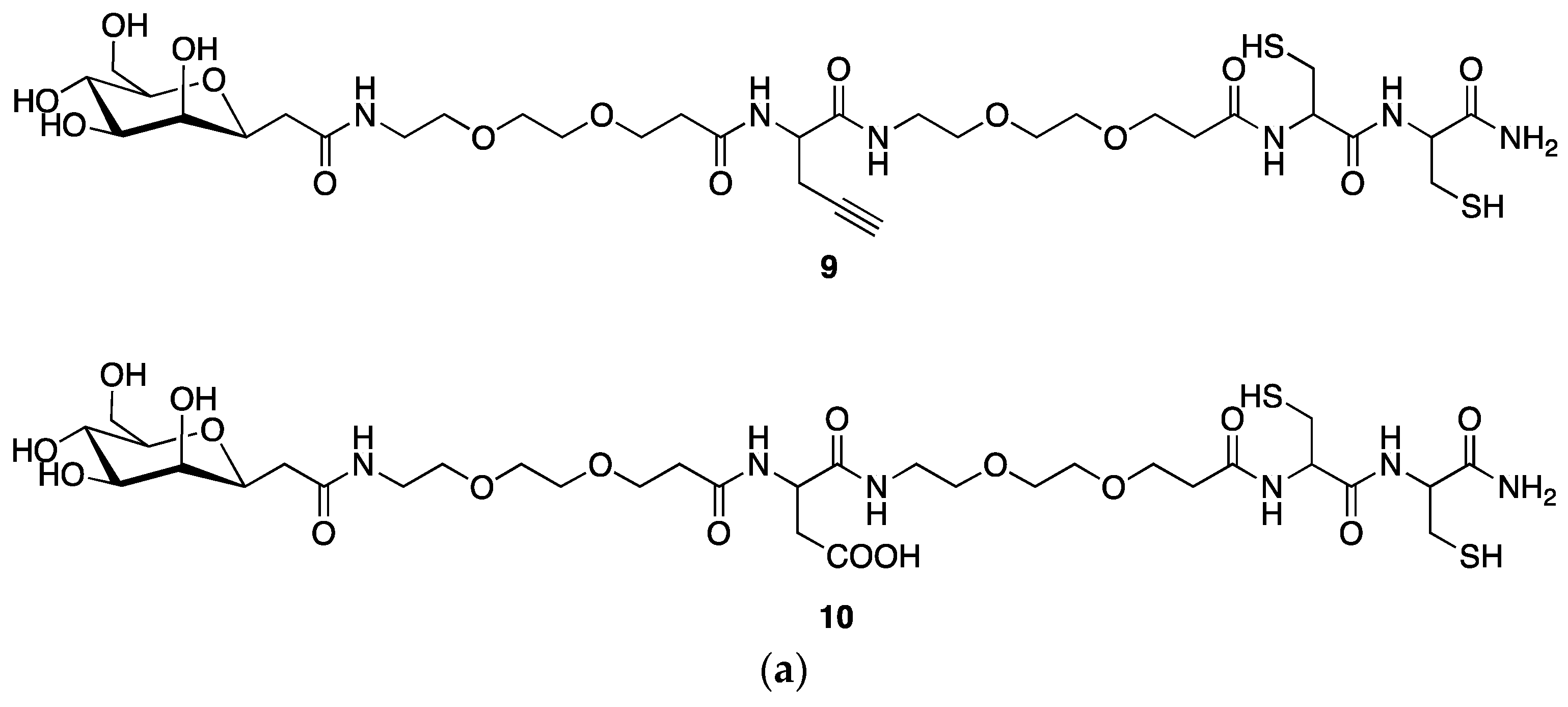
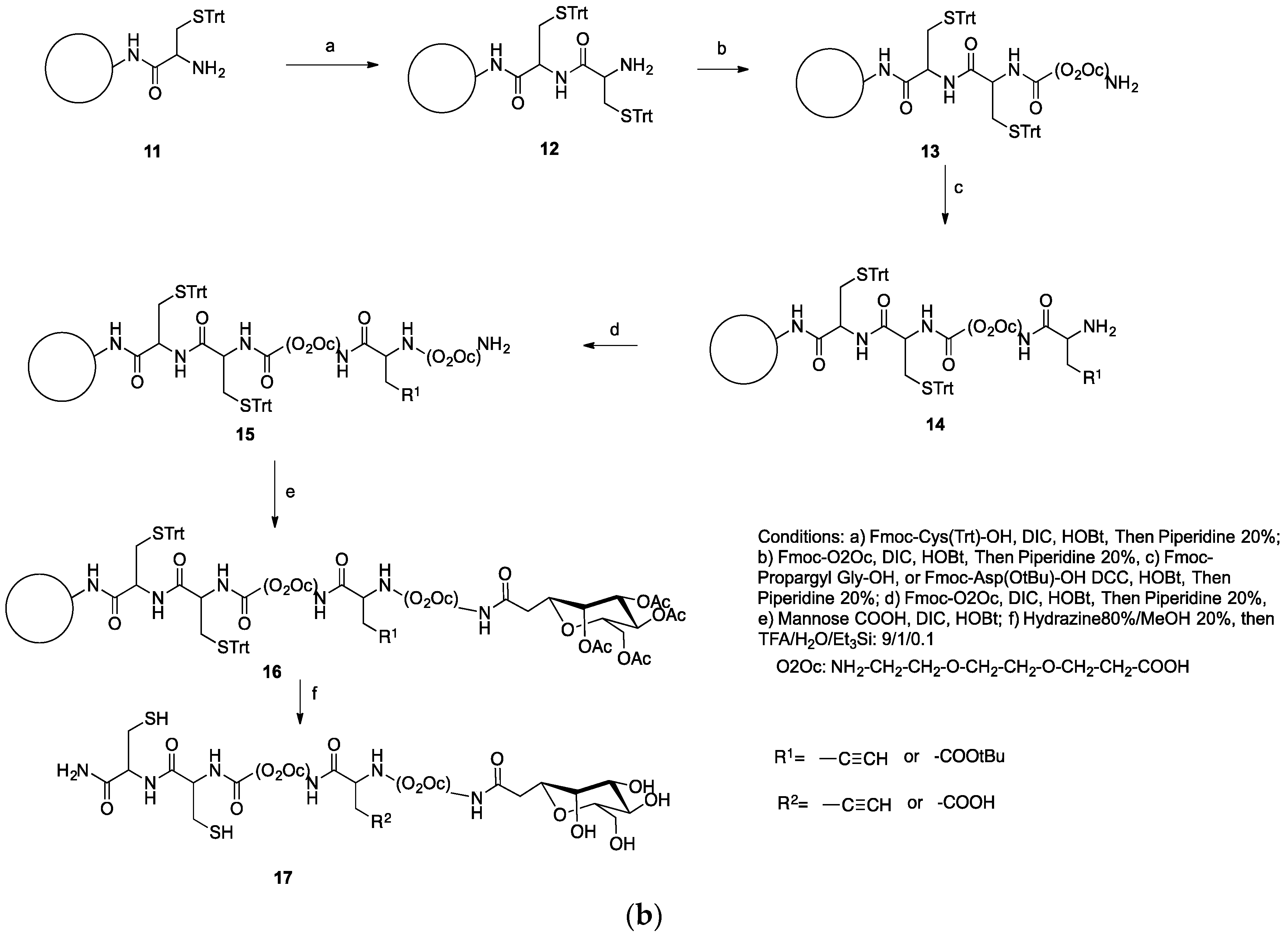
2.4. Synthesis of a Dextran-Mannose-CysCys Multifunctional Ligand
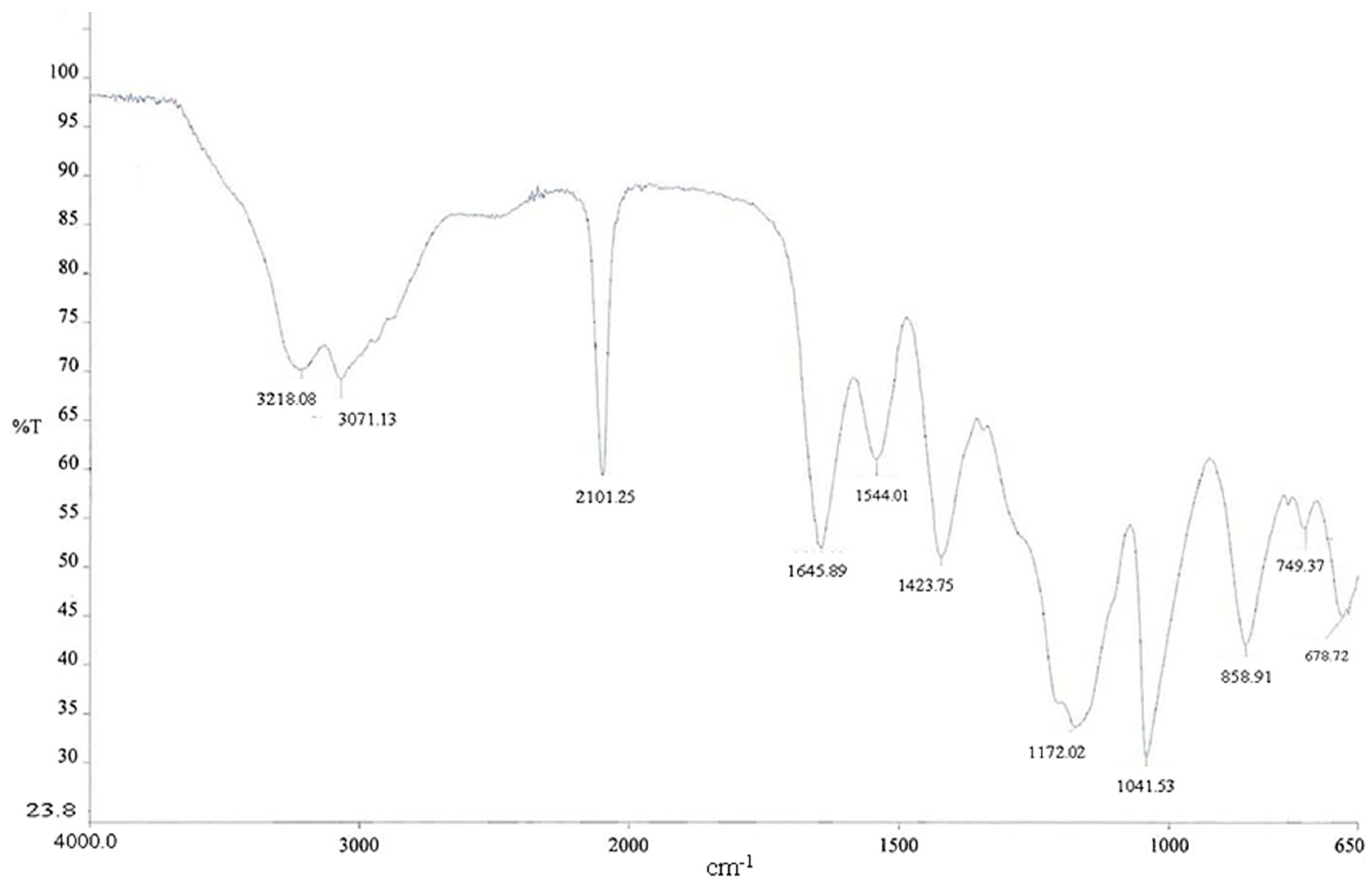
2.5. Preparation of 99mTcN-“3 + 1” Labeled Dextran-Mannose Derivates
2.5.1. Stability Studies
2.5.2. Cysteine and Glutathione (GSH) Challenge
3. Materials and Methods
3.1. General
3.1.1. Synthesis of 1
3.1.2. Synthesis of Thioglycol Amide
3.1.3. Synthesis of 3
3.1.4. Synthesis of 4
3.1.5. Synthesis of 5
3.1.6. Synthesis of 2-(2,3,4,6-Tetra-O-acetyl-β-d-Mannopyranosyl)-Acetaldehyde (7)
3.1.7. Synthesis of 2-(2,3,4,6-Tetra-O-Acetyl-β-d-Mannopyranosyl)-Acetic Acid (8)
3.1.8. Synthesis of Pseudopeptide 9
3.1.9. Synthesis of Pseudopeptide 10
3.1.10. Synthesis of 18
3.1.11. Synthesis of 19
3.2. Preparation of 99mTcN-“3 + 1” Labeled Dextran-Mannose Derivate
3.3. Chromatography
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buscombe, J.; Paganelli, G.; Burak, Z.E.; Waddington, W.; Maublant, J.; Prats, E.; Palmedo, H.; Schillaci, O.; Maffioli, L.; Lassmann, M.; et al. Sentinel node in breast cancer procedural guidelines. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Alazraki, N.; Aarsvold, J.N.; Audisio, R.A.; Glass, E.; Grant, S.F.; Kunikowska, J.; Leidenius, M.; Moncayo, V.M.; Uren, R.F.; et al. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1932–1947. [Google Scholar] [CrossRef] [PubMed]
- Alazraki, N.; Glass, E.C.; Castronovo, F.; Olmos, R.A.; Podoloff, D. Society of Nuclear Medicine procedure guideline for lymphoscintigraphy and the use of intraoperative gamma probe for sentinel lymph node localization in melanoma of intermediate thickness. J. Nucl. Med. 2002, 43, 1414–1418. [Google Scholar] [PubMed]
- Wong, S.L.; Balch, C.M.; Hurley, P.; Agarwala, S.S.; Akhurst, T.J.; Cochran, A.; Cormier, J.N.; Gorman, M.; Kim, T.Y.; McMasters, K.M.; et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J. Clin. Oncol. 2012, 30, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Alkureishi, L.W.; Burak, Z.; Alvarez, J.A.; Ballinger, J.; Bilde, A.; Britten, A.J.; Calabrese, L.; Chiesa, C.; Chiti, A.; de Bree, R.; et al. Joint practice guidelines for radionuclide lymphoscintigraphy for sentinel node localization in oral/oropharyngeal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1915–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, A.; Mijnhout, G.S.; Franssen, E.J.D. Radiopharmaceuticals in sentinel lymph node detection: An overview. Eur. J. Nucl. Med. 1999, 26, 36–42. [Google Scholar] [CrossRef]
- Eshima, D.; Fauconnier, T.; Eshima, L.; Thornback, J.R. Radiopharmaceuticals for lymphoscintigraphy: Including dosimetry and radiation considerations. Semin. Nucl. Med. 2000, 1, 25–32. [Google Scholar] [CrossRef]
- Hoh, C.K.; Wallace, A.M.; Vera, D.R. Preclinical studies of [99mTc] DTPA-mannosyl-dextran. Nucl. Med. Biol. 2003, 30, 457–464. [Google Scholar] [CrossRef]
- Sharma, R.; Wendt, J.A.; Rsmussen, J.C.; Adams, K.E.; Marshall, M.V.; Secick-Muraka, E.M. New horizons for imaging lymphatic function. Ann. N. Y. Acad. Sci. 2008, 1131, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Ravizzini, G.; Turkbey, B.; Barrett, T.; Kobayashi, H.; Choyke, P.L. Nanoparticles in sentinel lymph node mapping. WIREs Nanomed. Nonobiotechnol. 2009, 1, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Subramanian, S.; Pandey, U.; Samuel, G.; Venkatesh, M.; Martins, M.; Pereira, S.; Correia, J.D.G.; Santos, I. Mannosylated dextranderivatives labeled with fac-[M(CO)3]+ (M = Tc-99m, Re) for specific targeting of sentinel lymph node. Mol. Pharm. 2011, 8, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Pirmettis, I.; Arano, Y.; Tsotakos, T.; Okada, K.; Yamaguchi, A.; Uehara, T.; Morais, M.; Correia, J.D.G.; Santos, I.; Martins, M.; et al. New Tc-99m (CO)3 mannosilated dextran bearing s-derivatized cysteine chelator for sentinel lymph node detection. Mol. Pharm. 2012, 9, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.V.; Makinen, T.P.; Saarela, J.; Virtanen, J.; Ferrell, R.E.; Finegold, D.N.; Kerjaschki, D.; Yla-Herttuala, S.; Alitalo, K. Lymphatic endothelial reprogramming of vascular endotelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002, 21, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Martilla-Ichihara, F.; Turia, R.; Miiluniemi, M.; Karikoski, M.; Maksimow, M.; Niemela, J.; Martinez-Pomares, L.; Salmi, M.; Jalkanen, S. Macrophages mannose receptor on lymphatics controls cell trafficking. Blood 2008, 112, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.M.; Hoh, C.K.; Limmer, K.K.; Darrah, D.D.; Schulteis, G.; Vera, D.R. Sentinel lymph node accumulation of Lymphoseek and Tc-99m-sulfur colloid using a “2-day” protocol. Nucl. Med. Biol. 2009, 36, 687–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Hnatowich, D.J. Labeling Biomolecules with Radiorhenium—A Review of the Bifunctional Chelators. Anticancer Agents Med. Chem. 2007, 7, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Fernández, S.; Jentschel, C.; Pietzsch, H.-J.; Papadopoulos, M.; Pelecanou, M.; Pirmettis, I.; Paolino, A.; Rey, A. Design and Development of 99mTc-‘4+1’-Labeled Dextran-Mannose Derivatives as Potential Radiopharmaceuticals for Sentinel Lymph Node Detection. Cancer Biother. Radiopharm. 2013, 28. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Cazzola, E.; Uccelli, L.; Pasquali, M.; Ferretti, V.; Bertolasi, V.; Duatti, A. Rhenium(V) and technetium(V) nitrido complexes with mixed tridentate p-donor and monodentate p-acceptor ligands. Inorg. Chem. 2012, 51, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Uccelli, L.; Pasquali, M.; Pasqualini, R.; Guerrini, R.; Duatti, A. Mixed tridentate p-donor and monodentate p-acceptor ligands as chelating systems for rhenium-188 and technetium-99m nitride radiopharmaceuticals. Curr. Radiopharm. 2013, 6, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Smilkov, K.; Janevik, E.; Guerrini, R.; Pasquali, M.; Boschi, A.; Uccelli, L.; Di Domenico, G.; Duatti, A. Preparation and first biological evaluation of novel Re-188/Tc-99m peptide conjugates with substance-P. Appl. Radiat. Isot. 2014, 92, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boschi, A.; Massi, A.; Uccelli, L.; Pasquali, M.; Duatti, A. PEGylated N-methyl-S-methyl dithiocarbazate as a new reagent for the high-yield preparation of nitrido Tc-99m and Re-188 radiopharmaceuticals. Nucl. Med. Biol. 2010, 37, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Duatti, A.; Uccelli, L. Development of Technetium-99m and Rhenium-188 Radiopharmaceuticals Containing a Terminal Metal-Nitrido Multiple Bond for Diagnosis and Theraphy. Top. Curr. Chem. 2005, 252, 85–115. [Google Scholar] [CrossRef]
- Holmberg, A.; Meurling, L. Preparation of Sulfhydrylborane-Dextran Conjugates for Boron Neutron Capture Therapy. Bioconjugate Chem. 1993, 4, 570–573. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective Ligation∫of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Hamzavi, R.; Dolle, F.; Tavitian, B.; Dahl, O.; Nielsen, P.E. Modulation of the Pharmacokinetic Properties of PNA: Preparation of Galactosyl, Mannosyl, Fucosyl, N-Acetylgalactosaminyl, and N-Acetylglucosaminyl Derivatives of Aminoethylglycine Peptide Nucleic Acid Monomers and Their Incorporation into PNA Oligomers. Bioconjugate Chem. 2003, 14, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Massi, A.; Nuzzi, A.; Dondoni, A.J. Microwave-Assisted Organocatalytic Anomerization of α-C-Glycosylmethyl Aldehydes and Ketones. Org. Chem. 2007, 72, 10279–10282. [Google Scholar] [CrossRef] [PubMed]
- Postema, M.H.D.; Calimente, D. Glycochemistry: Principles, Synthesis and Applications; Wang, P.G., Bertozzi, C., Eds.; Marcel Dekker: New York, NY, USA, 2000; Chapter 4; pp. 77–131. [Google Scholar]
- Kosmol, R.; Hennig, L.; Welzel, P.; Findeisen, M.; Müller, D.; Markus, A.; van Heijenoort, J.J. A Moenomycin-type Structural Analogue of Lipid II some possible mechanisms of the mode of action of transglycosylase inhibitors can be discarded. J. Prakt. Chem. 1997, 339, 340–358. [Google Scholar] [CrossRef]
- Ossipov, D.A.; Hilborn, J. Poly(vinyl alcohol)-Based Hydrogels Formed by “Click Chemistry”. Macromolecules 2006, 39, 1709–1718. [Google Scholar] [CrossRef]
- Gude, M.; Ryf, J.; White, P.D. An accurate method for the quantitation of Fmoc-derivatized solid phase supports. Lett. Pept. Sci. 2002, 9, 203–206. [Google Scholar] [CrossRef]


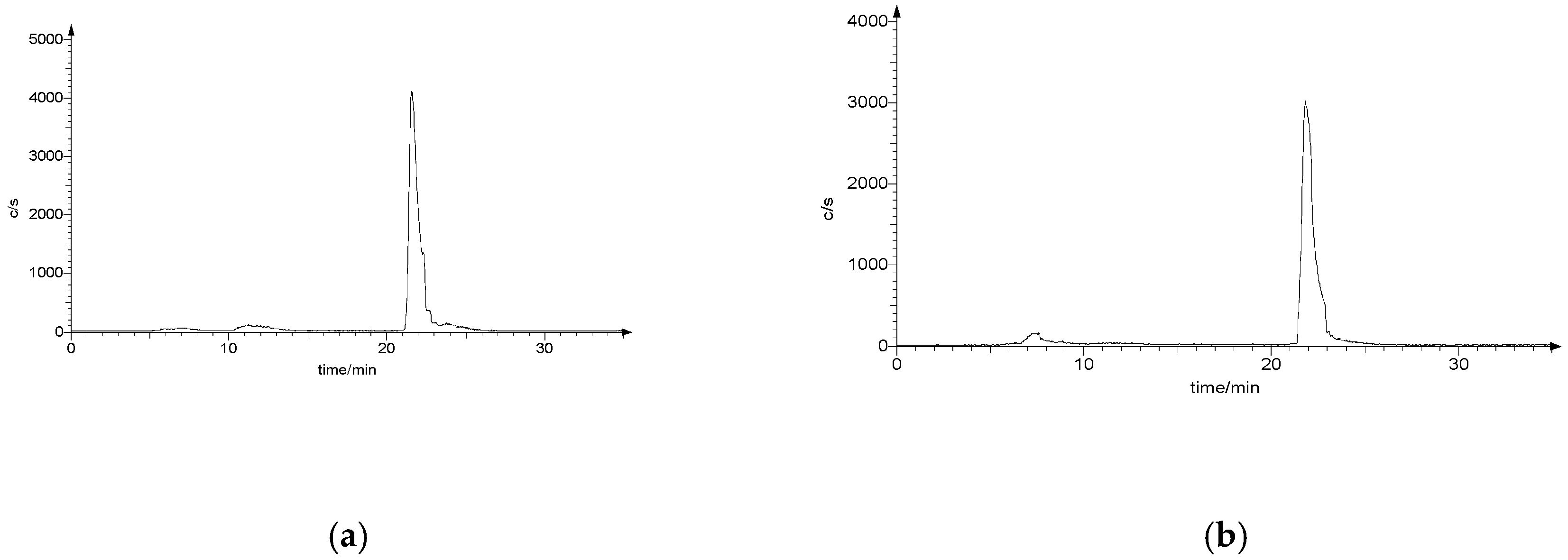

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschi, A.; Pasquali, M.; Trapella, C.; Massi, A.; Martini, P.; Duatti, A.; Guerrini, R.; Zanirato, V.; Fantinati, A.; Marzola, E.; et al. Design and Synthesis of 99mTcN-Labeled Dextran-Mannose Derivatives for Sentinel Lymph Node Detection. Pharmaceuticals 2018, 11, 70. https://doi.org/10.3390/ph11030070
Boschi A, Pasquali M, Trapella C, Massi A, Martini P, Duatti A, Guerrini R, Zanirato V, Fantinati A, Marzola E, et al. Design and Synthesis of 99mTcN-Labeled Dextran-Mannose Derivatives for Sentinel Lymph Node Detection. Pharmaceuticals. 2018; 11(3):70. https://doi.org/10.3390/ph11030070
Chicago/Turabian StyleBoschi, Alessandra, Micòl Pasquali, Claudio Trapella, Alessandro Massi, Petra Martini, Adriano Duatti, Remo Guerrini, Vinicio Zanirato, Anna Fantinati, Erika Marzola, and et al. 2018. "Design and Synthesis of 99mTcN-Labeled Dextran-Mannose Derivatives for Sentinel Lymph Node Detection" Pharmaceuticals 11, no. 3: 70. https://doi.org/10.3390/ph11030070










