Phytochemical Study of the Ecuadorian Species Lepechinia mutica (Benth.) Epling and High Antifungal Activity of Carnosol against Pyricularia oryzae
Abstract
:1. Introduction
2. Results
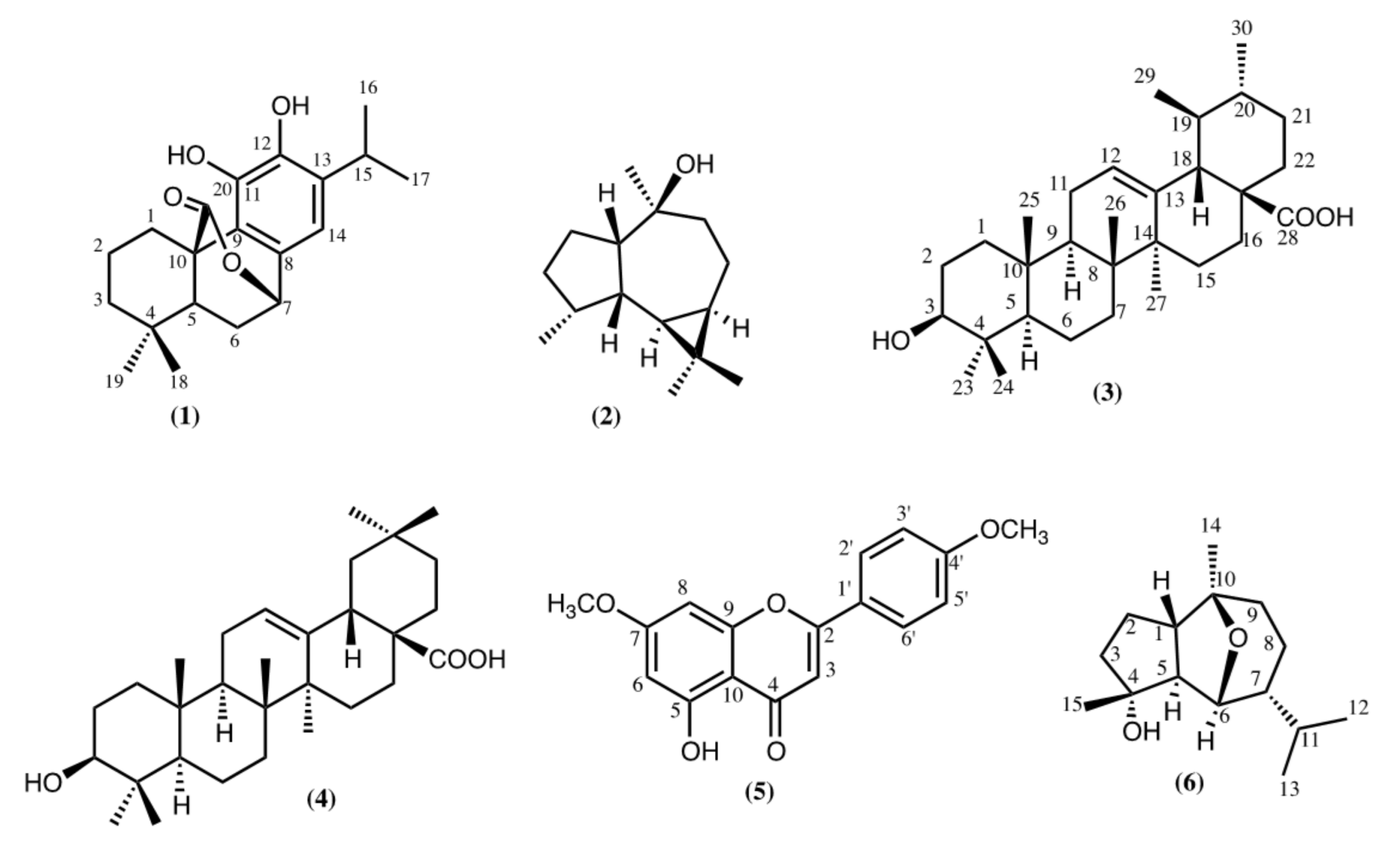
2.1. Non-Volatile Fraction
2.2. Chemical Analysis of the Volatile Fraction from Flowers
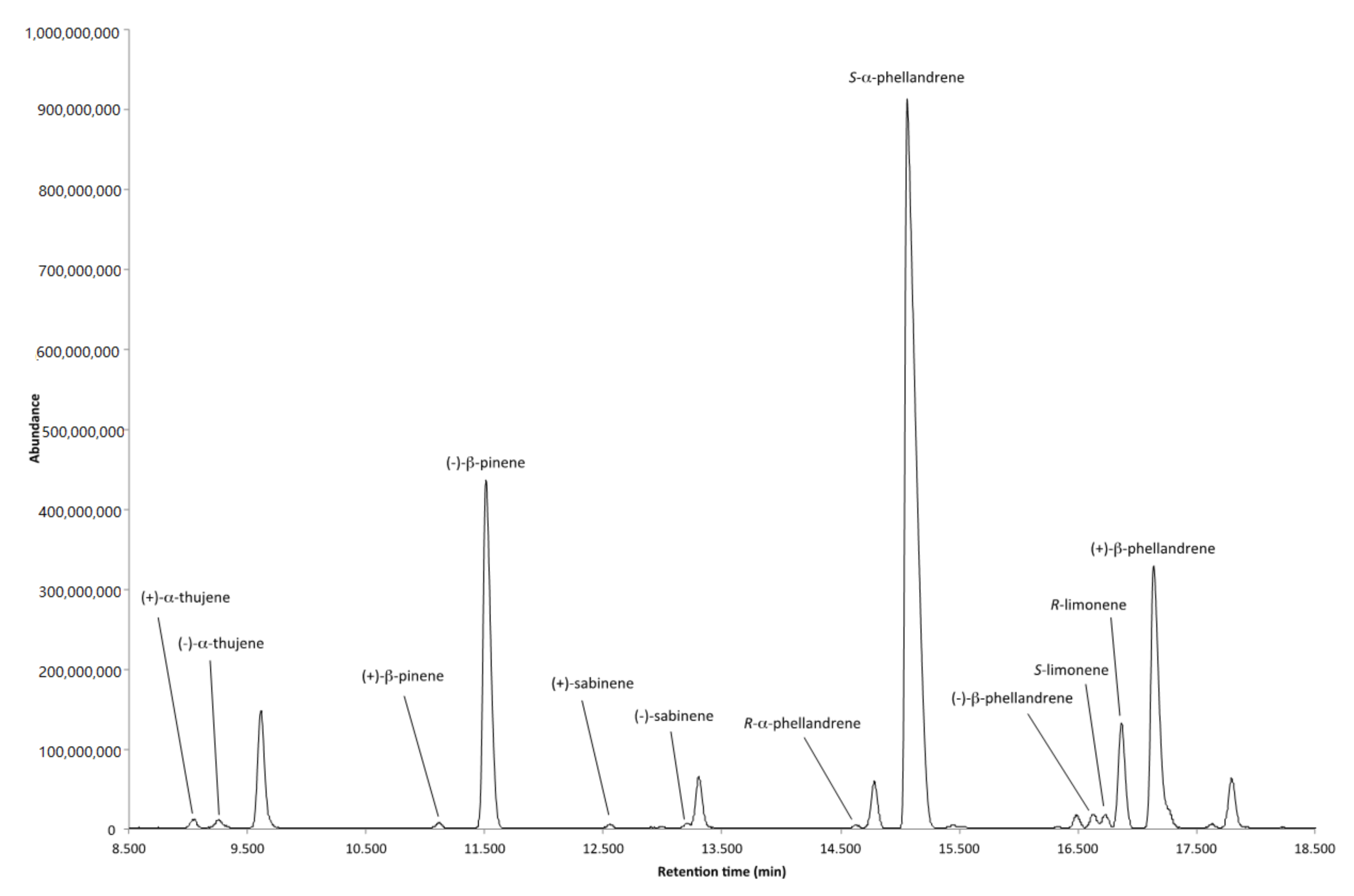
2.3. Enantiomeric Analysis of the Volatile Fraction from the Flowers
2.4. Biological Activity
3. Discussion
3.1. Non-Volatile Fraction
3.2. Volatile Fraction
4. Materials and Methods
4.1. Instruments and Disposables
4.2. Plant Material
4.3. Extraction and Purification of Non-Volatile Metabolites
4.4. Distillation of the Volatile Fraction
4.5. Qualitative Analysis of the Essential Oil
4.6. Quantitative Analysis of the Essential Oil
4.7. Enantioselective Analysis of the Essential Oil
4.8. Antifungal Activity
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drew, B.T.; Sytsma, K.J. The South American radiation of Lepechinia (Lamiaceae): Phylogenetics. divergence times and evolution of dioecy. Bot. J. Linn. Soc. 2013, 171, 171–190. [Google Scholar] [CrossRef]
- Epling, C. A Synopsis of the Tribe Lepechinieae (Labiatae). Brittonia 1948, 6, 352–364. [Google Scholar] [CrossRef]
- Esteves, P.F.; Kuster, R.M.; Barbi, N.S.; Menezes, F.d.S. Chemical Composition and Cytotoxic Activity of Lepechinia speciosa (St. Hill) Epling. Lat. Am. J. Pharm. 2010, 29, 38–44. [Google Scholar]
- Delgado, G.; Hernández, J.; Chávez, M.I.; Alvarez, L.; Gonzaga, V.; Martínez, E. Di- and triterpenpoid acids from Lepechinia caulescens. Phytochemistry 1994, 37, 1119–1121. [Google Scholar] [CrossRef]
- Jorgensen, P.; León-Yánez, S. Catalogue of the Vascular Plants of Ecuador; Monographs in Systematic Botany; Missouri Botanical Garden: St. Louis, MO, USA, 1999; pp. 1–1182. [Google Scholar]
- Naranjo, P.; Escaleras, R. La Medicina Tradicional en el Ecuador: Memorias de las Primeras Jornadas Ecuatorianas de Etnomedicina Andina, 1st ed.; Universidad Andina Simón Bolívar-Corporación Editora Nacional: Quito, Ecuador, 1995; pp. 1–192. [Google Scholar]
- Tene, V.; Malagón, O.; Vita Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Pecher, V.; Leplanquais, V.; Lazou, K.; Dumas, M.C. Use of a Lepechinia caulescens Extract as a Cosmetic Agent and Cosmetic Composition Containing Same. U.S. Patent 2009/0304829 A1, 3 June 2009. [Google Scholar]
- Roderick, J.H. Fungal infections. Clin. Dermatol. 2006, 24, 201–212. [Google Scholar] [CrossRef]
- Larypoor, M.; Akhavansepahy, A.; Rahimifard, N.; Rashedi, H. Antidermatophyte activity of the essential oil of Hypericum perforatum of North of Iran. J. Med. Plants 2009, 8, 110–117. [Google Scholar]
- Couch, B.C.; Kohn, L.M. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 2002, 94, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, R.S.; Leong, S.A.; Teeng, P.S. Rice Blast Disease; CAB International: Wallingford, UK; International Rice Research Institute: Los Banos, Philippines, 1994; pp. 1–626. [Google Scholar]
- Khush, G.S.; Jena, K.K. Current status and future prospects for research on blast resistance in rice (Oryza sativa L.). In Advances in Genetics, Genomics and Control of Rice Blast Disease; Wang, X., Valent, B., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–10. [Google Scholar]
- Suriani, N.; Suprapta, D.; Sudana, I.; Temaja, I.G. Antigungal Activity of Piper caninum against Pyricularia oryzae Cav. the Cause of Rice Blast Disease on Rice. J. Biol. Agric. Healthc. 2015, 5, 72–78. [Google Scholar]
- Yolanda, K. Penyakit Blas Pada Padi; BPTP Bangka Balitung, Balitbang Pertanian RI: Pangkalpinang, Indonesia, 2013. [Google Scholar]
- Ramirez, J.; Cartuche, L.; Morocho, V.; Aguilar, S.; Malagon, O. Antifungal activity of raw extract and flavanons isolated from Piper ecuadorense from Ecuador. Braz. J. Pharm. 2013, 23, 370–373. [Google Scholar] [CrossRef]
- Ramírez, J.Y.; Gilardoni, G.; Jácome, M.J.; Montesinos, J.V.; Rodolfi, M.; Guglielminetti, M.L.; Cagliero, C.; Bicchi, C.; Vidari, G. Chemical composition, enantiomeric analysis, AEDA sensorial evaluation and antifungal activity of the essential oil from the Ecuadorian plant Lepechinia mutica Benth (Lamiaceae). Chem. Biodivers 2017, 14, e1700292. [Google Scholar] [CrossRef] [PubMed]
- Malagón, O.G.; Vila, R.; Iglesias, J.; Zaragoza, T.; Cañigueral, S. Composition of the essential oils of four medicinal plants from Ecuador. Flavour Fragr. J. 2003, 18, 527–531. [Google Scholar] [CrossRef]
- Gajhede, M.; Anthoni, U.; Nielsen, P.H.; Pedersen, E.J.; Christophersen, C. Carnosol. Crystal structure. absolute configuration. and spectroscopic properties of a diterpene. J. Crystallogr. Spectrosc. Res. 1990, 20, 165–171. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Chebib, M.; Aburjai, T.; Johnston, G.R.; Hanrahan, J.R. GABAa Receptor Modulation by Compounds Isolated from Salvia triloba L. Adv. Biol. Chem. 2014, 4, 148–159. [Google Scholar] [CrossRef]
- Dimayuga, R.; Garcia, S.; Nielsen, P.H.; Christophersen, C. Traditional medicine of Baja California sur (Mexico) III. Carnosol. A diterpene antibiotic from Lepechinia hastata. J. Ethnopharmacol. 1991, 31, 43–48. [Google Scholar] [PubMed]
- Inatani, R.; Fuwa, H.; Seto, H.; Nakatani, N. Structure of a new antioxidative phenolic diterpene isolated from rosemary (Rosmarinus officinalis L.). Agric. Biol. Chem. 1982, 46, 1661–1666. [Google Scholar] [CrossRef]
- Brieskorn, C.; Fuchs, A.; Bredenberg, J.B.; McChesney, J.D.; Wenkert, E. The structure of carnosol. J. Org. Chem. 1964, 29, 2293–2298. [Google Scholar] [CrossRef]
- Bombarda, I.; Raharivelomanana, P.; Ramanoelina, P.R.; Faure, R.; Bianchini, J.P.; Gaydou, E.M. Spectrometric identifications of sesquiterpene alcohols from niaouli (Melaleuca quinquenervia) essential oil. Anal. Chim. Acta 2001, 447, 113–123. [Google Scholar] [CrossRef]
- Faure, R.; Ramanoelina, A.; Rakatonirainy, O.; Jean-Pierre, B.; Gaydou, E.M. Two-Dimensional Nuclear Magnetic Resonance of Sesquiterpenes. 4*-Application to Complete Assignment of 1H and 13C NMR Spectra of Some Aromadendrane Derivatives. Magn. Reson. Chem. 1991, 29, 969–971. [Google Scholar] [CrossRef]
- Gijsen, H.; Wijnberg, J.; Stork, G.; De Groot, A. The Synthesis of Mono- and Dihydroxy Aromadendrane Sesquiterpenes. Starting from Natural (+)-Armomadendrene-III. Tetrahedron 1992, 48, 2465–2476. [Google Scholar] [CrossRef]
- Mariotti, J.P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A.F. Composition of the essential oil of Cistus ladaniferus L. cultivated in Corsica (France). Flavour Fragr. J. 1997, 12, 147–151. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Aguirre-Crespo, F.; Vergara-Galicia, J.; Villalobos-Molina, R.; López-Guerrero, J.J.; Navarrete-Vázquez, G.; Estrada-Soto, S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006, 79, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2011, 28, 1087–1117. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.; Kundu, A. 13C NMR spectra of pentacyclic Triterpenoids—A compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 1143, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Citoglu, G.; Sever, B.; Antus, S.; Baitz-Gacs, E.; Altanlar, N. Antifungal Diterpenoids and Flavonoids from Ballota inaequidens. Pharm. Biol. 2004, 42, 659–663. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Aguiar, Z.E.; Luis, G.; Ravelo, A.G.; Vázquez, J.; Domínguez, X.A. Flavonoids from Salvia texana. Phytochemistry 1989, 28, 2871–2872. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Hegazy, M.F.; Hassan, N.M.; Wojcinska, M.; Karchesy, J.; Pare, P.W.; Mabry, T.J. Constituents of Chrysothamnus viscidiflorus. Phytochemistry 2006, 67, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- El-Askary, H.I. Terpenoids from Cleome droserifolia (Forssk.) Del. Molecules 2005, 10, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Gedara, S.R.; Abdel-halim, O.B.; El-Sharkawy, S.H.; Salama, O.M.; Shier, T.W.; Halim, A.F. New Erythroxane-Type Diterpenoids from Fagonia boveana (Hadidi) Hadidi & Graf. Z. Naturforsch C 2003, 58, 23–32. [Google Scholar] [PubMed]
- Moghaddam, F.; Farimani, M.; Amin, G. Carnosol from Salvia eremophila Boiss. DARU 2000, 8, 45–46. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2009. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Birtić, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; Schmeda-Hirschmann, G.; Theoduloz, C.; Rodríguez, J.A. Gastroprotective effect and cytotoxicity of abietane diterpenes from the Chilean Lamiaceae Sphacele chamaedryoides (Balbis ) Briq. J. Pharm. Pharmacol. 2009, 61, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Almada, G.; Virgen, M. Minimum antimicrobial inhibitory concentration of carnosol and of the ethanol extract from Lepichinia hastata (Lamiaceae). Phytomedicine 1998, 5, 301–305. [Google Scholar] [CrossRef]
- Guerrero, I.; Andrés, L.; León, L.; Machín, R.; Padrón, J.M.; Luis, J.; Delgadillo, J. Abietane Diterpenoids from Salvia pachyphylla and S. clevelandii with Cytotoxic Activity against Human Cancer Cell Lines. J. Nat. Prod. 2006, 69, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.G.; Moujir, L.; Andrés, L.S.; Montaño, N.P.; Araujo, L.; Luis, J.G. Semisynthesis and biological evaluation of abietane-type diterpenes. Revision of the structure of rosmaquinone. J. Nat. Prod. 2009, 72, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.J.; Reyes, C.P.; Jiménez, I.A.; Hayashi, H.; Tokuda, H.; Bazzocchi, I.L. Ent-Rosane and abietane diterpenoids as cancer chemopreventive agents. Phytochemistry 2011, 72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ireland, B.F.; Hibbert, D.B.; Goldsack, R.J.; Doran, J.C.; Brophy, J.J. Chemical variation in the leaf essential oil of Melaleuca quinquenervia (Cav.) S.T. Blake. Biochem. Syst. Ecol. 2002, 30, 457–470. [Google Scholar] [CrossRef]
- Scher, J.M.; Speakman, J.B.; Zapp, J.; Becker, H. Bioactivity guided isolation of antifungal compounds from the liverwort Bazzania trilobata (L.) S.F. Gray. Phytochemistry 2004, 65, 2583–2588. [Google Scholar] [CrossRef] [PubMed]
- Talzhanov, N.A.; Sadyrbekov, D.T.; Smagulova, F.M.; Mukanov, R.M.; Raldugin, V.A.; Shakirov, M.M.; Tkachev, A.V.; Atazhanova, G.A.; Tuleuov, B.I.; Adekenov, S.M. Components of Artemisia pontica. Chem. Nat. Compd. 2005, 41, 143–145. [Google Scholar] [CrossRef]
- Martini, N.D.; Katerere, D.R.P.; Eloff, J.N. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 2004, 93, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Tewtrakul, S.; Subhadhirasakul, S.; Kummee, S. Anti-allergic activity of compounds from Kaempferia parviflora. J. Ethnopharmacol. 2008, 116, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Bianco, A.; Bruno, M.; Jemia, M.; Ben Jemia, M.; Nicoletti, M. Phytochemical study of Cistus libanotis. Nat. Prod. Res. 2015, 29, 189–192. [Google Scholar] [CrossRef] [PubMed]
- De Saint Laumer, J.Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; d’Acampora Zellner, B.; Crupi, M.L.; De Fina, M.R.; Valentino, M.R.; Dugo, P.; Dugo, G.; Mondello, L. GC-MS, GC-O and enantio-GC investigation of the essential oil of Tarchonanthus camphoratus L. Flavour Fragr. J. 2008, 23, 40–48. [Google Scholar] [CrossRef]
| Calculated LRI a | Reference LRI b | Compounds | Flowers | Leaves [17] | ||
|---|---|---|---|---|---|---|
| % | σ | % | σ | |||
| 921 | 926 | Tricyclene | - | - | Trace | - |
| 924 | 924 | Thujene <α-> | Trace | - | Trace | - |
| 931 | 932 | Pinene <α-> | 2.68 | 0.95 | 1.23 | 0.89 |
| 946 | 949 | Camphene | - | - | 0.75 | 0.80 |
| 948 | 945 | Fenchene <α-> | Trace | - | - | - |
| 971 | 969 | Sabinene | Trace | - | 0.24 | 0.15 |
| 974 | 983 | Oct-3-en-1-ol | - | - | Trace | - |
| 976 | 974 | Pinene <β-> | 7.96 | 0.99 | 3.78 | 1.76 |
| 979 | 989 | Hepten-2-ol <6-methyl-5-> | 0.32 | 0.12 | - | - |
| 984 | 979 | Octanone <3-> | 0.16 | 0.17 | - | - |
| 988 | 988 | Myrcene | 1.51 | 0.47 | 0.52 | 0.28 |
| 998 | - | Undetermined (MW 136) | 0.13 | 0.14 | - | - |
| 1003 | 1003 | Mentha-1(7),8-diene <p-> | Trace | - | 0.16 | 0.13 |
| 1006 | 1002 | Phellandrene <α-> | 0.34 | 0.13 | 3.80 | 1.70 |
| 1008 | 1008 | Carene <δ-3-> | 24.23 | 6,00 | 8.69 | 4.24 |
| 1016 | 1014 | Terpinene <α-> | Trace | - | 0.11 | 0.07 |
| 1019 | 1020 | Cymene <p-> | 1.97 | 0.50 | 0.10 | 0.06 |
| 1023 | 1022 | Cymene <o-> | 2.04 | 0.52 | - | - |
| 1025 | 1023 | Sylvestrene | - | - | 0.29 | 0.18 |
| 1029 | 1024 | Limonene | 4.47 | 0.71 | 3.79 | 2.18 |
| 1029 | 1030 | Phellandrene <β-> | ||||
| 1043 | - | Undetermined (MW 98) | 0.23 | 0.46 | - | - |
| 1045 | 1044 | Ocimene <(E)-β-> | 0.35 | 0.42 | - | - |
| 1052 | 1054 | Terpinene <γ-> | Trace | - | 0.23 | 0.12 |
| 1057 | - | Undetermined (MW 136) | 0.52 | 0.20 | - | - |
| 1065 | 1071 | cis-Sabinene hydrate | - | - | Trace | - |
| 1070 | - | Undetermined (MW 136) | 0.20 | 0.40 | - | - |
| 1080 | 1085 | Mentha-2,4(8)-diene <p-> | 0.86 | 0.25 | 0.35 | 0.18 |
| 1084 | 1086 | Terpinolene | 1.78 | 0.40 | 0.60 | 0.33 |
| 1084 | 1086 | trans-Linalool oxide | - | - | Trace | - |
| 1088 | 1082 | Cymenene <m-> | 1.97 | 0.55 | - | - |
| 1095 | 1102 | Linalool | - | - | 0.20 | 0.09 |
| 1108 | - | Undetermined (MW 136) | 3.26 | 0.71 | - | - |
| 1110 | 1109 | Oct-1-en-3-yl acetate | - | - | 1.37 | 0.60 |
| 1120 | - | Undetermined (MW 136) | 0.27 | 0.12 | - | - |
| 1124 | 1117 | Sabina ketone <dehydro-> | Trace | - | - | - |
| 1141 | 1145 | Camphor | - | - | Trace | - |
| 1142 | - | Undetermined (MW 136) | 0.10 | 0.19 | - | - |
| 1165 | 1172 | Borneol | - | - | 0.25 | 0.05 |
| 1174 | 1180 | 4-Terpineol | - | - | 0.14 | 0.02 |
| 1194 | 1186 | Terpineol <α-> | 0.16 | 0.04 | 0.11 | 0.02 |
| 1283 | 1281 | Isobornyl acetato | - | - | 2.20 | 1.04 |
| 1284 | 1284 | Bornyl acetate | Trace | - | - | - |
| 1335 | 1328 | Elemene <δ-> | - | - | Trace | - |
| 1345 | 1348 | Cubebene <α-> | 0.22 | 0.07 | 0.57 | 0.08 |
| 1346 | 1342 | Terpinyl acetate <α-> | ||||
| 1373 | 1373 | Ylangene <α-> | 0.26 | 0.09 | 0.15 | 0.05 |
| 1374 | 1362 | Isoledene | 0.20 | 0.05 | ||
| 1374 | 1367 | Copaene <α-> | - | - | 1.46 | 0.23 |
| 1381 | 1387 | Bourbonene <β-> | 0.20 | 0.05 | 0.47 | 0.25 |
| 1385 | 1382 | Modheph-2-ene | 0.21 | 0.05 | - | - |
| 1392 | 1387 | Cubebene <β-> | 0.20 | 0.05 | 0.15 | 0.04 |
| 1395 | 1398 | Cyperene | 0.20 | 0.05 | - | - |
| 1404 | 1400 | Sibirene | 0.28 | 0.09 | - | - |
| 1407 | 1409 | Gurjunene <α-> | 0.20 | 0.05 | 1.94 | 0.37 |
| 1407 | 1418 | Longifolene | - | - | 0.15 | 0.07 |
| 1417 | 1411 | Funebrene <2-epi-β-> | 0.57 | 0.19 | Trace | - |
| 1417 | 1412 | (E)-Caryophyllene | - | - | 4.55 | 2.16 |
| 1424 | 1431 | Copaene <β-> | 0.20 | 0.05 | 0.50 | 0.08 |
| 1427 | 1421 | Duprezianene <β-> | 0.21 | 0.06 | - | - |
| 1431 | 1431 | Gurjunene <β-> | 0.21 | 0.05 | 1.47 | 0.78 |
| 1435 | 1437 | Guaiene <α-> | 0.25 | 0.07 | - | - |
| 1439 | 1449 | Aromadendrene | - | - | 0.56 | 0.10 |
| 1443 | 1442 | Guaiadiene <6,9-> | 0.20 | 0.05 | - | - |
| 1446 | 1448 | Muurola-3,5-diene <cis-> | 0.21 | 0.06 | 0.45 | 0.36 |
| 1453 | 1452 | Humulene <α-> | 0.29 | 0.08 | 1.20 | 0.47 |
| 1457 | 1452 | Clovene <α-neo-> | 0.22 | 0.06 | - | - |
| 1459 | 1464 | Caryophyllene <9-epi-(E)-> | 0.21 | 0.06 | - | - |
| 1461 | 1452 | cis-Cadina-1(6),4-diene | - | - | 0.99 | 1.36 |
| 1469 | 1465 | Muurola-4(14),5-diene <cis-> | 0.22 | 0.06 | - | - |
| 1471 | 1463 | Dauca-5,8-diene | - | - | 0.38 | 0.09 |
| 1472 | 1469 | Acoradiene <β-> | 0.23 | 0.07 | - | - |
| 1475 | 1466 | trans-Cadina-1(6),4-diene | - | - | 0.99 | 0.12 |
| 1478 | 1479 | Amorpha-4,7(11)-diene | 0.21 | 0.06 | 0.15 | 0.07 |
| 1482 | 1478 | Muurolene <γ-> | 0.21 | 0.06 | 0.92 | 0.23 |
| 1486 | 1489 | Selinene <β-> | 0.23 | 0.06 | - | - |
| 1488 | 1485 | Himachala-1,4-diene <11-αH-> | 0.23 | 0.06 | - | - |
| 1492 | 1481 | cis-b-Guaiene | - | - | 0.71 | 0.11 |
| 1493 | 1486 | Bicyclogermacrene | - | - | 4.62 | 0.58 |
| 1493 | 1489 | epi-Cubebol | ||||
| 1493 | 1489 | Zingiberene <α-> | ||||
| 1493 | 1492 | Selinene <δ-> | 0.24 | 0.07 | 0.81 | 0.08 |
| 1496 | 1493 | Muurola-4(14),5-diene <trans-> | 0.25 | 0.07 | - | - |
| 1500 | 1505 | Cuprenene <α-> | 0.20 | 0.05 | - | - |
| 1503 | 1505 | Farnesene <(E,E)-α-> | 0.20 | 0.05 | 0.83 | 0.25 |
| 1510 | 1500 | Muurolene <α-> | 0.32 | 0.11 | 0.91 | 0.17 |
| 1513 | 1505 | Cadinene <γ-> | - | - | 2.86 | 0.37 |
| 1514 | 1508 | Cubebol | - | - | 0.36 | 0.21 |
| 1516 | 1511 | Amorphene <δ-> | 0.44 | 0.19 | - | - |
| 1521 | 1512 | trans-Calamenene | - | - | 0.15 | 0.04 |
| 1522 | 1511 | Cadinene <δ-> | - | - | 6.96 | 0.99 |
| 1529 | 1528 | Zonarene | 0.21 | 0.06 | - | - |
| 1533 | 1523 | trans-Cadina-1,4-diene | - | - | 0.37 | 0.10 |
| 1534 | 1537 | Cadinene <α-> | 0.21 | 0.06 | 0.39 | 0.12 |
| 1538 | 1545 | Selina-3,7(11)-diene | 0.20 | 0.05 | 0.14 | 0.04 |
| 1548 | - | Undetermined (MW 204) | 0.21 | 0.07 | - | - |
| 1555 | - | Undetermined (MW 204) | 0.21 | 0.06 | - | - |
| 1559 | 1556 | Dauca-4(11),7-diene <trans-> | 0.20 | 0.05 | - | - |
| 1567 | 1559 | Germacrene B | 0.21 | 0.05 | 0.18 | 0.06 |
| 1574 | 1567 | Germacrene D-4-ol | - | - | 1.46 | 0.40 |
| 1574 | - | Undetermined (MW 204) | 0.25 | 0.04 | - | - |
| 1579 | - | Undetermined (MW 204) | 0.21 | 0.05 | - | - |
| 1582 | 1569 | Caryophyllene oxide | - | - | 0.29 | 0.24 |
| 1583 | - | Undetermined (MW 204) | 0.21 | 0.05 | - | - |
| 1590 | 1584 | Globulol | - | - | 5.91 | 2.61 |
| 1591 | 1586 | Thujopsan-2-α-ol | 11.9 | 1.76 | - | - |
| 1592 | 1592 | Viridiflorol | - | - | 1.29 | 0.45 |
| 1601 | 1600 | Guaiol | 0.15 | 0.12 | - | - |
| 1612 | - | Undetermined (MW 204) | 0.22 | 0.06 | - | - |
| 1618 | 1617 | 1,10-di-epi-Cubenol | - | - | 0.27 | 0.11 |
| 1618 | 1623 | Junenol | - | - | 1.39 | 0.42 |
| 1629 | 1622 | Eudesmol <10-epi-γ-> | 0.83 | 0.10 | 0.54 | 0.15 |
| 1634 | 1630 | Eudesmol <γ-> | 2.02 | 1.48 | - | - |
| 1636 | 1639 | Acorenol <β-> | - | - | 0.47 | 0.81 |
| 1639 | 1632 | Acorenol <α-> | 0.60 | 1.20 | Trace | - |
| 1642 | 1635 | Cadin-4-en-7-ol <cis-> | 0.88 | 1.75 | - | - |
| 1649 | 1644 | Eudesmol <β-> | - | - | 4.47 | 1.93 |
| 1652 | 1644 | Eudesmol <α-> | ||||
| 1652 | 1646 | Cadinol <α-> | ||||
| 1652 | 1656 | Valerianol | 5.19 | 0.66 | - | - |
| 1668 | 1658 | Selin-11-en-4-α-ol | Trace | - | - | - |
| 1688 | 1681 | Shyobunol | - | - | 10.80 | 5.91 |
| 1691 | 1700 | Eudesm-7(11)-en-4-ol | 13.02 | 4.25 | - | - |
| Aliphatic monoterpene hydrocarbons | 48.89 | - | 24.54 | - | ||
| Aromatic monoterpene hydrocarbons | 5.98 | - | 0.10 | - | ||
| Monoterpene alcohols | 0.48 | - | 0.70 | - | ||
| Monoterpene ketones | 0.16 | - | Traces | - | ||
| Aliphatic esters | - | - | 3.57 | - | ||
| Aliphatic sesquiterpene hydrocarbons | 9.86 | - | 35.98 | - | ||
| Sesquiterpene alcohols | 34.59 | - | 27.25 | - | ||
| TOTAL | 99.96 | - | 92.14 | - | ||
| Enantiomers | Retention Time (min) | LRI | Enantiomeric Distribution (%) | ee (%) |
|---|---|---|---|---|
| (+)-α-thujene | 9.05 | 924 | 44.19 | 11.62 |
| (−)-α-thujene | 9.26 | 928 | 55.81 | |
| (+)-β-pinene | 11.12 | 962 | 1.48 | 97.04 |
| (−)-β-pinene | 11.51 | 969 | 98.52 | |
| (+)-sabinene | 12.55 | 988 | 46.37 | 7.26 |
| (−)-sabinene | 13.21 | 1000 | 53.63 | |
| (R)-α-phellandrene | 14.64 | 1024 | 7.17 | 85.66 |
| (S)-α-phellandrene | 14.79 | 1027 | 92.83 | |
| (S)-limonene | 16.73 | 1059 | 34.19 | 31.62 |
| (R)-limonene | 16.87 | 1062 | 65.81 | |
| (−)-β-phellandrene | 16.63 | 1058 | 4.34 | 91.32 |
| (+)-β-phellandrene | 17.14 | 1066 | 95.66 |
| Compound | Microsporum canis (CBS 136538) | Pyricularia oryzae (LM120) | ||
|---|---|---|---|---|
| MIC (mg/mL) | MFC (mg/mL) | MIC (mg/mL) | MFC (mg/mL) | |
| Carnosol (1) | 0.0250 < MIC ≤ 0.0500 | MFC > 0.1000 | 0.0125 < MIC ≤ 0.0250 | 0.0500 < MFC ≤ 0.1000 |
| Flutriafol | - | - | 0.0100 | 0.0100 |
| Intraconazol | 0.0005 | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, J.; Gilardoni, G.; Ramón, E.; Tosi, S.; Picco, A.M.; Bicchi, C.; Vidari, G. Phytochemical Study of the Ecuadorian Species Lepechinia mutica (Benth.) Epling and High Antifungal Activity of Carnosol against Pyricularia oryzae. Pharmaceuticals 2018, 11, 33. https://doi.org/10.3390/ph11020033
Ramírez J, Gilardoni G, Ramón E, Tosi S, Picco AM, Bicchi C, Vidari G. Phytochemical Study of the Ecuadorian Species Lepechinia mutica (Benth.) Epling and High Antifungal Activity of Carnosol against Pyricularia oryzae. Pharmaceuticals. 2018; 11(2):33. https://doi.org/10.3390/ph11020033
Chicago/Turabian StyleRamírez, Jorge, Gianluca Gilardoni, Erika Ramón, Solveig Tosi, Anna Maria Picco, Carlo Bicchi, and Giovanni Vidari. 2018. "Phytochemical Study of the Ecuadorian Species Lepechinia mutica (Benth.) Epling and High Antifungal Activity of Carnosol against Pyricularia oryzae" Pharmaceuticals 11, no. 2: 33. https://doi.org/10.3390/ph11020033







