Use of Fe3O4 Nanoparticles for Enhancement of Biosensor Response to the Herbicide 2,4-Dichlorophenoxyacetic Acid
Abstract
:1. Introduction
2. Results and Discussion
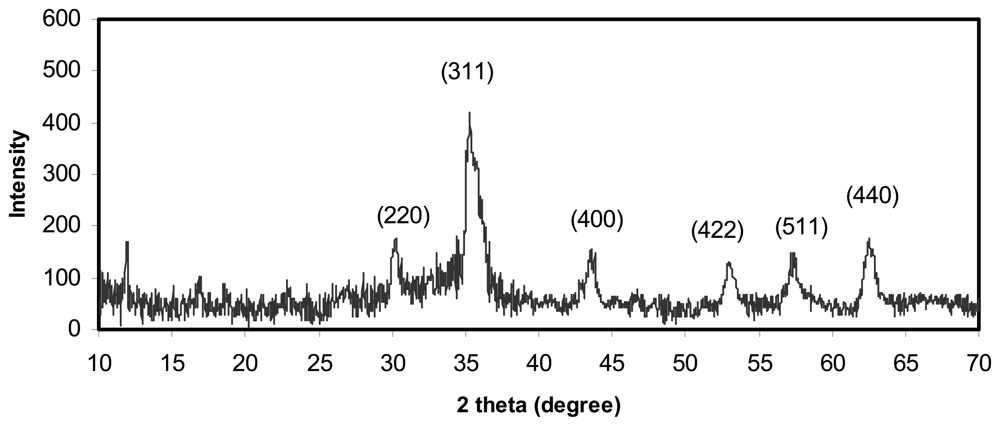
2.1 Characterization of the Fe3O4 magnetic nanoparticles
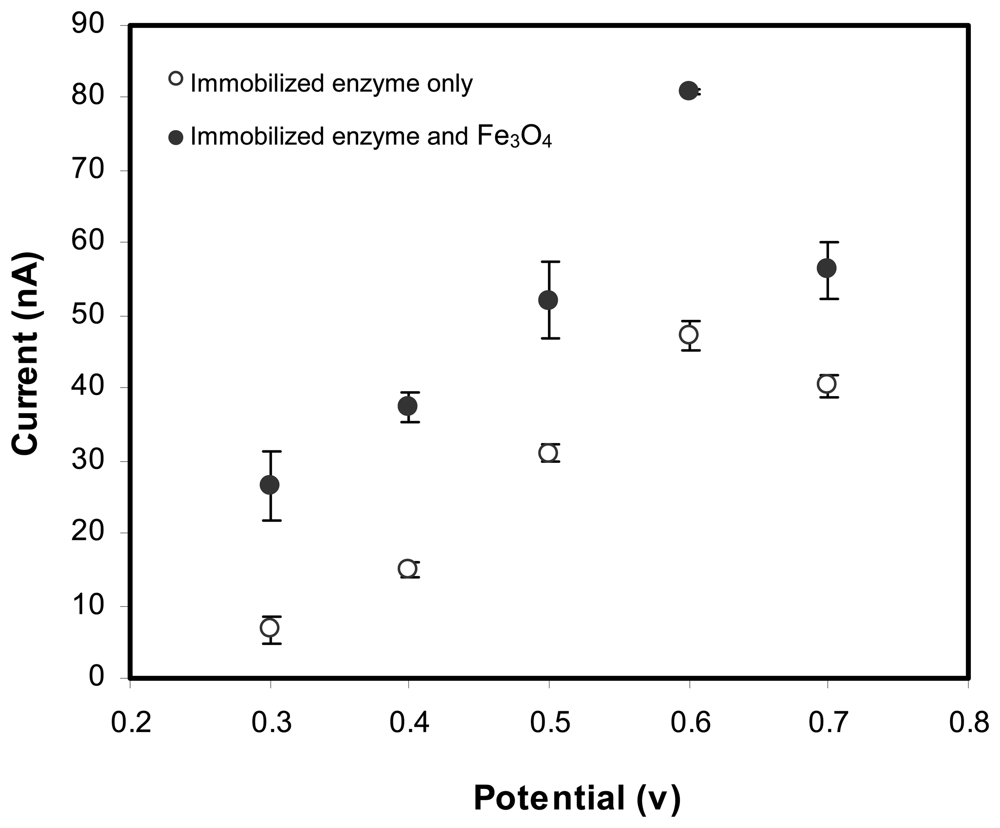
2.2 Optimum conditions for biosensor operation
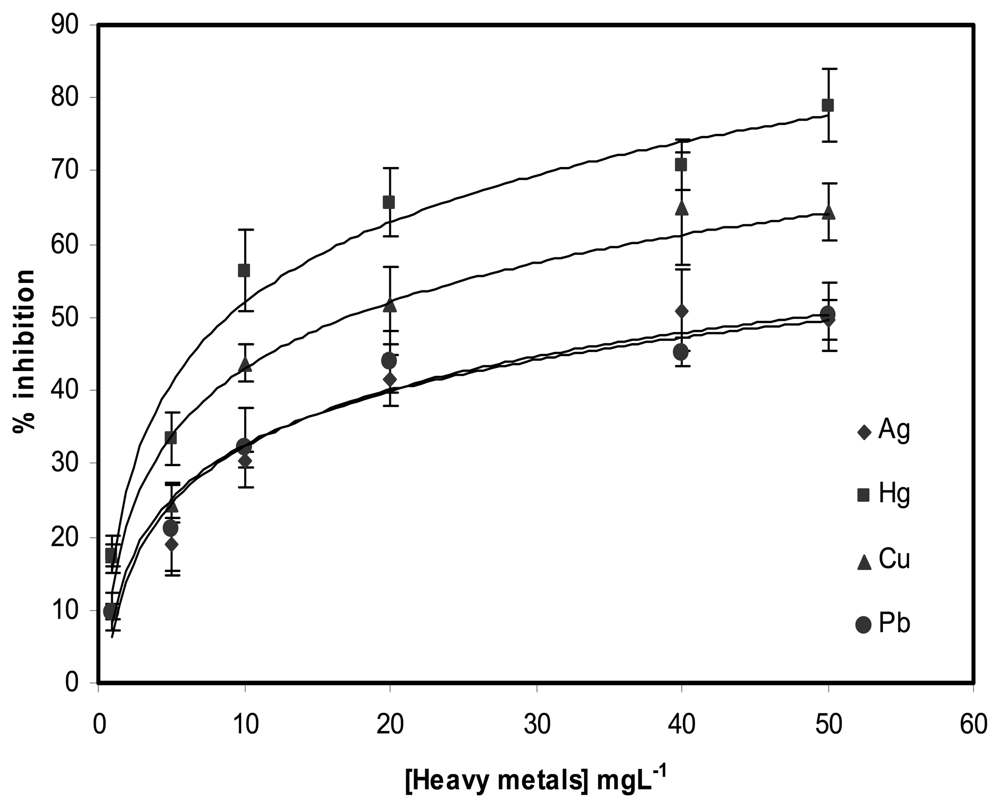
2.2. Determination of 2,4-D and heavy metals with biosensors
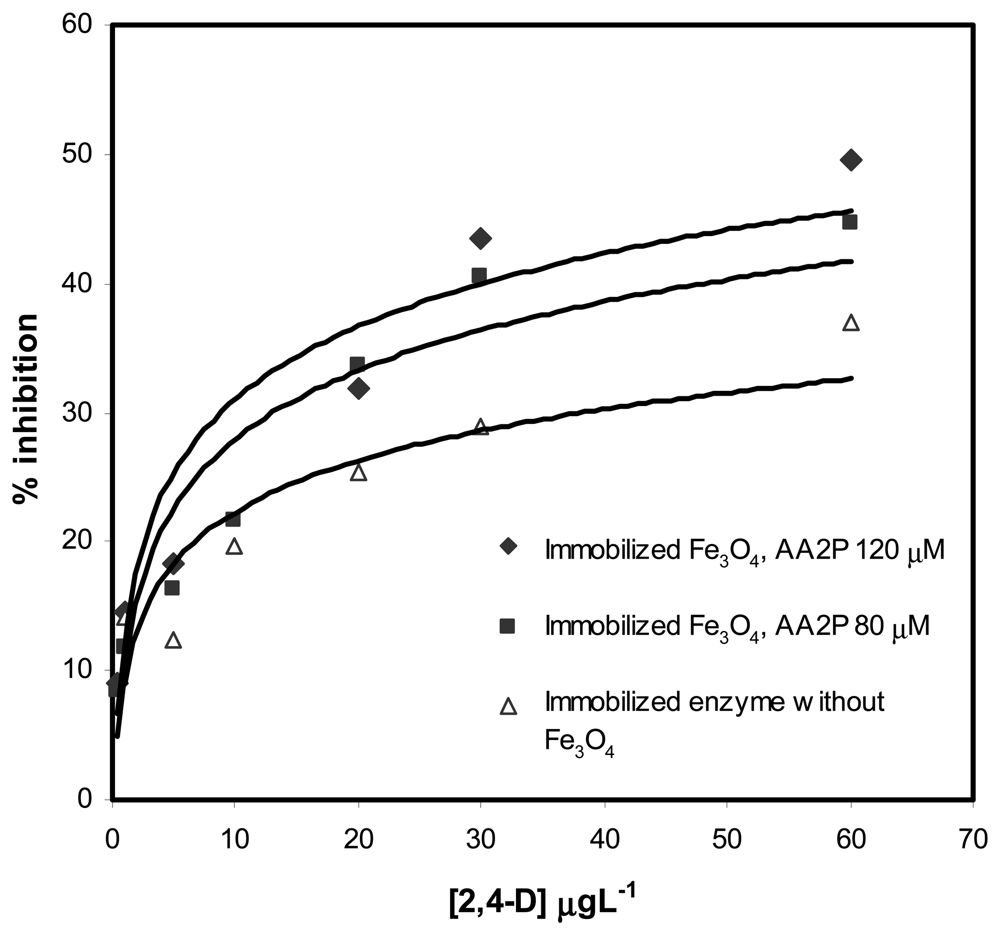
2.3 The analytical performance of biosensor for 2,4-D determination
3. Experimental Section
3.1 Reagents
3.2 Apparatus and measurement
3.3 Preparation and characterization of Fe3O4 nanoparticles
3.4 Preparation of electrodes/biosensor
3.5 Optimization of biosensor response
Optimization of applied potential
Optimization of pH
Optimization of AA2P substrate
3.6 Inhibition of ALP enzyme by 2,4-D and heavy metals
3.7 Sample preparation for recovery studies
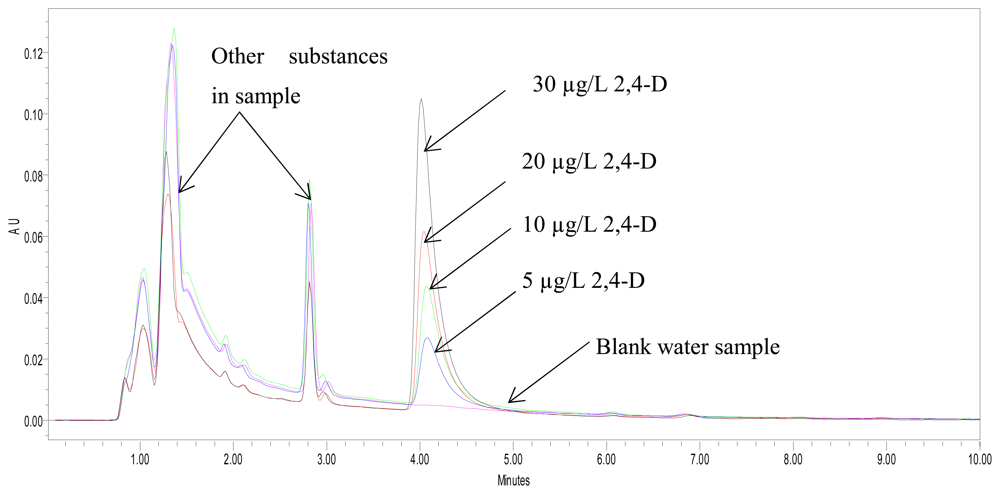
3.8. Comparison of analytical performance of 2,4-D biosensor with HPLC method
4. Conclusions
Acknowledgments
References
- Cao, D.; He, P.; Hu, N. Electrochemical Biosensor utilizing Electron Transfer in Heme Protein Immobilized on Fe3O4 Nanoparticles. Analyst 2003, 128, 1268–1274. [Google Scholar]
- Zhang, Y.; Zeng, G.-M.; Tang, L.; Huang, D.-L.; Jiang, X.-Y.; Chen, Y.-N. A hydroquinone biosensor using modified core-shell magnetic nanoparticles supported on carbon paste electrode. Biosens. Bioelectron. 2007, 22, 2121–2126. [Google Scholar]
- Tang, L.; Zeng, G.; Liu, J.; Xu, X.; Zhang, Y.; Shen, G.; Li, Y.; Liu, C. Catechol determination in compost bioremediation using a laccase sensor and artificial neural networks. Anal. Bioanal. Chem. 2008, 391, 679–685. [Google Scholar]
- Huang, J.; Liu, C.; Xiao, H.; Wang, J.; Jiang, D.; Gu, E. Zinc tetraaminophthalocyanine-Fe3O4 nanoparticle composite for laccase immobilization. Int. J. Nanomed. 2007, 2, 775–784. [Google Scholar]
- Wang, S.; Tan, Y.; Zhao, D.; Liu, G. Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles-chitosan nanocomposite. Biosens. Bioelectron. 2008, 23, 1781–1787. [Google Scholar]
- Liu, Z.; Liu, Y.; Yang, H.; Yang, Y.; Shen, G.; Yu, R. A Phenol Biosensor based on Immobilizing Tyrosinase to Modified Core-Shell Magnetic Nanoparticles Supported at a Carbon Paste Electrode. Anal. Chim. Acta 2005, 533, 3–9. [Google Scholar]
- Rossi, L.M.; Quach, A.D.; Rosenzweig, Z. Glucose oxidase-magnetite nanoparticle bioconjugate for glucose sensing. Anal. Bioanal. Chem. 2004, 380, 606–613. [Google Scholar]
- Qiu, J.; Peng, H.; Liang, R. Ferrocene-modified Fe3O4@SiO2 magnetic nanoparticles as building blocks for construction of reagentless enzyme-based biosensors. Electrochem. Commun. 2007, 9, 2734–2738. [Google Scholar]
- Lai, G.-S.; Han, D.-Y.; Yu, A.-M. An amperometric hydrogen peroxide biosensor based on immobilization of horseradish peroxidase on an electrode modified with magnetic dextran microspheres. Anal. Bioanal. Chem. 2008, 390, 971–977. [Google Scholar]
- Trau, D.; Theuerl, T.; Wilmer, M.; Meusel, M.; Spener, F. Development of an Amperometric Flow Injection Immunoanalysis System for the Determination of the Herbicide 2,4-Dichlorophenoxyacetic Acid in Water. Biosens. Bioelectron. 1997, 12, 499–510. [Google Scholar]
- Medyantseva, E.P.; Vertlib, M.G.; Kutyreva, M.P.; Khaldeeva, E.I.; Budnikov, G.K.; Eremin, S.A. The Specific Immunosensor Detection of 2,4-Dichlorophenoxyacetic Acid and 2,4,5-Trichlorophenoxyacetic Acid Pesticides by Amperometric Cholinesterase Biosensor. Anal. Chim. Acta 1997, 347, 71–78. [Google Scholar]
- Mosiello, L.; Nencini, L.; Segre, L.; Spano, M.A. Fibre-Optic Immunosensor for 2,4-Dichlorophenoxyacetic Acid Detection. Sens. Actuat. B 1997, 38-39, 353–359. [Google Scholar]
- Gobi, K.V.; Tanaka, H.; Shoyama, Y.; Miura, N. Highly Sensitive Regenerable Immnuosensor for Label-free Detection of 2,4-Dichlorophenoxyacetic Acid at ppb Levels by Using Plasma Resonance Imaging. Sens. Actuat. B 2005, 111-112, 562–571. [Google Scholar]
- Bauer, C.G.; Eremenko, A.V.; Ehrentreich-Förster, E.; Bier, F.F.; Makower, A.; Halsall, H.B.; Heineman, W.R.; Scheller, F.W. Zeptomole-detecting biosensor for alkaline phosphatase in an electrochemical immunoassay for 2,4-dichlorophenoxyacetic acid. Anal. Chem. 1996, 68, 2453–2458. [Google Scholar]
- Trau, D.; Theuerl, T.; Wilmer, M.; Meusel, M.; Spener, F. Development of an amperometric flow injection immunoanalysis system for the determination of the herbicide 2,4-dichlorophenoxyacetic acid in water. Biosens. Bioelectron. 1997, 12, 499–510. [Google Scholar]
- Dequaire, M.; Degrand, C.; Limoges, B. An immunomagnetic electrochemical sensor based on a perfluorosulfonate-coated screen-printed electrode for the determination of 2,4-dichlorophenoxyacetic acid. Anal. Chem. 1999, 71, 2571–2577. [Google Scholar]
- Botrè, C.; Botrè, F.; Mazzei, F.; Podestà, E. Inhibition-based biosensors for the detection of environmental contaminants: Determination of 2,4-dichlorophenoxyacetic acid. Environ. Toxic. Chem. 2000, 19, 2876–2881. [Google Scholar]
- Kawakami, M.; Koya, H.; Amada, K.; Shimojo, M. Amperometric 2,4-dichlorophenoxyacetate biosensor system based on a microbial reactor and a tyrosinase-modified electrode. Anal. Lett. 2007, 40, 921–932. [Google Scholar]
- Gong, J.; Lin, X. Facilitated Electron Transfer of Hemoglobin embedded in Nano-sized Fe3O4 Matrix Based on Paraffin Impregnated Graphite Electrode and Electrochemical Catalysis For Trichloroacetic Acid. Microchem. J. 2003, 75, 51–57. [Google Scholar]
- Baraton, M.I.; Chen, X.; Gonsalves, K.E. Application of Fourier Transform Infrared Spectroscopy to Nanostructured Materials Surface Characterization. Study of an Aluminium Nitride Powder Prepared via Chemical Synthesis. In Nanotechnology Moleculary Designed Materials, 1st Ed.; Chow, G. M., Gonsalves, K. E., Eds.; American Chemical Society: Washington DC, 1996; p. 313. [Google Scholar]
- Chen, D.H.; Wu, S.H. Synthesis of Nickel Nanoparticles in Water-in-oil Microemulsions. Chem. Mater. 2000, 12, 1354–1360. [Google Scholar]
- Alonso Sedano, A.B.; Garcia, M.L.T.; Barbado, M.D.V.; Batanero, P.S. Electrochemical Study of Copper and Iron Compounds in the Solid State by using Voltammetry Immobilized Microparticles: Application to Cooper Ferrite Characterization. J. Electroanal. Chem. 2004, 566, 433–441. [Google Scholar]
- Lu, B.W.; Chen, W.C. A Disposable Glucose Biosensor based on Drop-Coating of Screen-Printed Carbon Electrodes with Magnetic Nanoparticle. J. Magn. Magn. Mater. 2006, 304, 400–402. [Google Scholar]
- Qu, S.; Wang, J.; Kong, J.; Yang, P.; Chen, G. Magnetic loading of carbon nanotube/nano-Fe3O4 composite for electrochemical sensing. Talanta 2007, 71, 1096–1102. [Google Scholar]
- Sanchez, F.G.; Diaz, A.N.; Peinado, M.C.R.; Belledone, C. Free and Sol-gel Immobilizd Alkaline Phosphatase-Based Biosensor for the Determination of Pesticides and Inorganic Compound. Anal. Chim. Acta 2003, 484, 45–51. [Google Scholar]
- Ayyagari, M.S.; Kamtekar, S.; Pande, R.; Marx, K.A.; Kumar, J.; Tripathy, S.K.; Kaplan, D.L. Biosensors for Pesticide Detection based on Alkaline Phosphatase Catalyzed Chemiluminescence. Mater. Sci. Eng. C 1995, 2, 191–196. [Google Scholar]
- Diaz, A.N.; Sanchez, F.G.; Ramos, M.C.; Torijas, M.C. Horseradish Peroxidase Sol-gel Immobilized for Chemiluminescence Measurements of Alkaline-phosphatase Activity. Sens. Actuat. B 2002, 82, 176–179. [Google Scholar]
- Cheng, F.Y.; Su, C.H.; Yang, Y.S.; Yeh, C.S.; Tsai, C.Y.; Wu, C.L.; Wu, M.T.; Shieh, D.B. Characterization of Aqueous Dispersion of Fe3O4 Nanoparticles and their Biomedical Applications. Biomaterials 2005, 26, 729–738. [Google Scholar]
- Chan, H.T.; Do, Y.Y.; Huang, P.L.; Chien, P.L.; Chan, T.S.; Liu, R.S.; Yang, C.Y.; Horng, H.E. Preparation and Properties of Bio-compatible Magnetic Fe3O4 Nanoparticles. J. Magn. Magn. Mater. 2006, 304, 415–417. [Google Scholar]
- Liao, M.H.; Chen, D.H. Immobilizing of Yeast Alcohol Dehydrogenase on Magnetic Nanoparticles for Improving its Stability. Biotechnol. Lett. 2001, 23, 1723–1727. [Google Scholar]
- D'Archivio, A.A.; Fanelli, M.; Mazzeo, P.; Ruggieri, F. Comparison of Different Sorbents for Multiresidue Solid-phase Extraction of 16 Pesticides from Groundwater Coupled with High-performance Liquid Chromatograpgy. Talanta 2007, 71, 25–30. [Google Scholar]

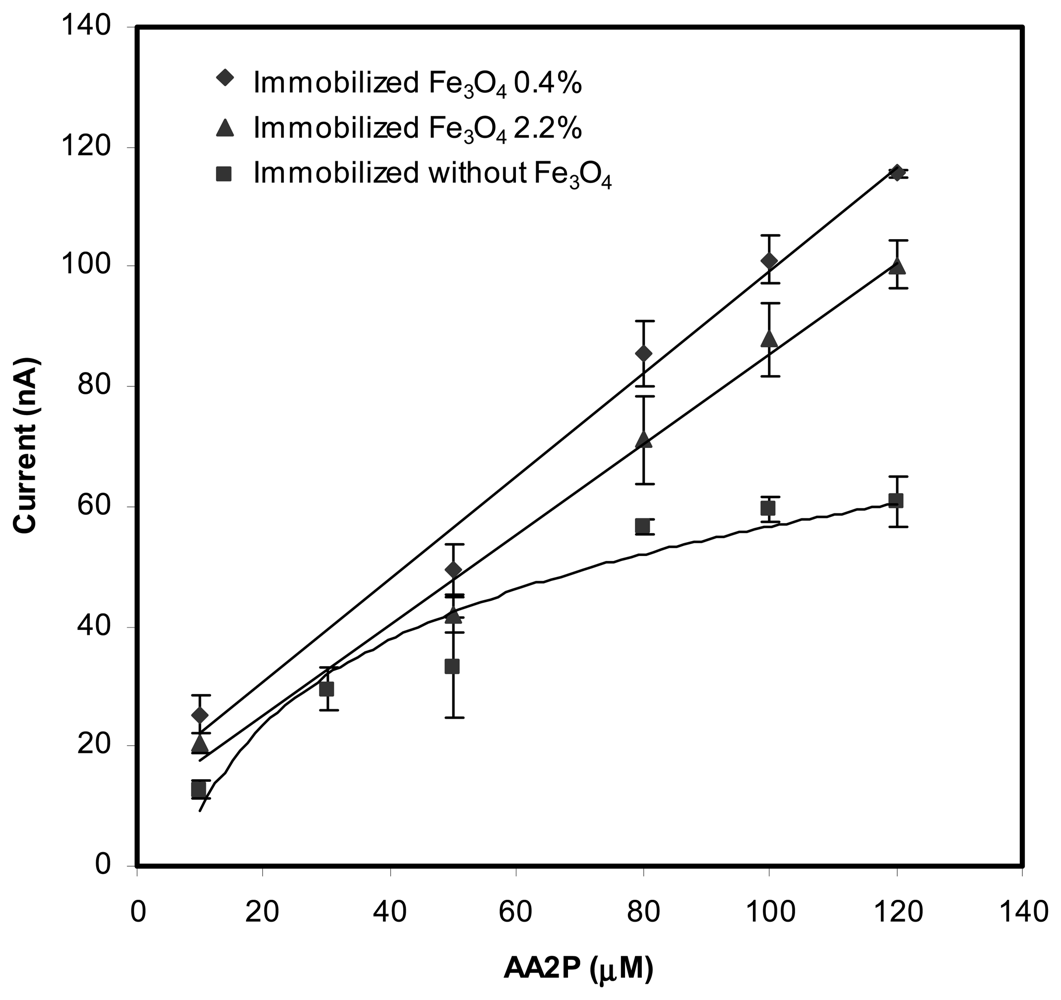

| Amount of Fe3O4(wt %) | Slope (nA mM−1) | Intercept | Correlation coefficient, R2 | Linear range AA2P(μM) |
|---|---|---|---|---|
| 0 | 0.606±0.058 | 6.95±2.58 | 0.9733 | 5 - 80 |
| 0.4 | 0.867±0.036 | 12.86±2.50 | 0.9915 | 5 - 120 |
| 2.2 | 0.742±0.024 | 10.51±2.06 | 0.9935 | 5 – 150 |
| Amount of Fe3O4 (wt %) | Concentration of AA2P(μM) | Slope (% inhibition/μgL−1) | Intercept | Correlation coefficient, R2 | Linear range 2,4-D (μgL−1) |
|---|---|---|---|---|---|
| 0 | 80 | 0.579±0.095 | 12.41±1.60 | 0.9255 | 1 - 30 |
| 0.4 | 80 | 1.067±0.069 | 10.24+1.07 | 0.9834 | 0.5 - 30 |
| 0.4 | 120 | 1.041±0.183 | 13.24±2.83 | 0.8900 | 0.5 – 30 |
| Actual amountof 2,4-D spiked (μgL−1) | Amount 2,4-D recovered (μgL−1) | Recovery (%) | Average recovery (%) (n=5) |
|---|---|---|---|
| 10 | 13.41 | 134.10 | 100.84 ± 20.2 |
| 9.61 | 96.08 | ||
| 9.04 | 90.45 | ||
| 8.09 | 80.85 | ||
| 10.27 | 102.73 | ||
| 20 | 19.11 | 95.55 | 95.41 ± 6.15 |
| 17.08 | 85.41 | ||
| 20.44 | 102.19 | ||
| 19.48 | 97.42 | ||
| 19.29 | 96.46 |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Loh, K.-S.; Lee, Y.H.; Musa, A.; Salmah, A.A.; Zamri, I. Use of Fe3O4 Nanoparticles for Enhancement of Biosensor Response to the Herbicide 2,4-Dichlorophenoxyacetic Acid. Sensors 2008, 8, 5775-5791. https://doi.org/10.3390/s8095775
Loh K-S, Lee YH, Musa A, Salmah AA, Zamri I. Use of Fe3O4 Nanoparticles for Enhancement of Biosensor Response to the Herbicide 2,4-Dichlorophenoxyacetic Acid. Sensors. 2008; 8(9):5775-5791. https://doi.org/10.3390/s8095775
Chicago/Turabian StyleLoh, Kee-Shyuan, Yook Heng Lee, Ahmad Musa, Abdul Aziz Salmah, and Ishak Zamri. 2008. "Use of Fe3O4 Nanoparticles for Enhancement of Biosensor Response to the Herbicide 2,4-Dichlorophenoxyacetic Acid" Sensors 8, no. 9: 5775-5791. https://doi.org/10.3390/s8095775




