SU-8 Cantilevers for Bio/chemical Sensing; Fabrication, Characterisation and Development of Novel Read-out Methods
Abstract
:1. Introduction
2. SU-8 as device material
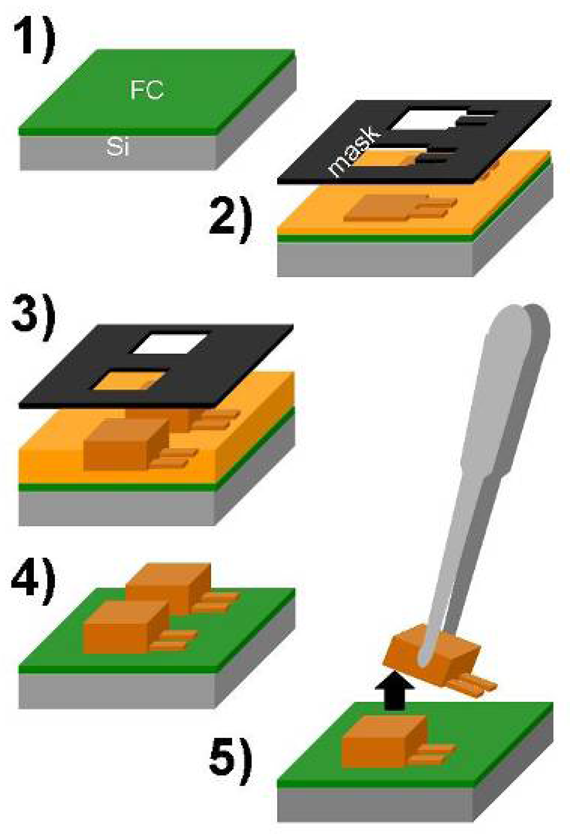
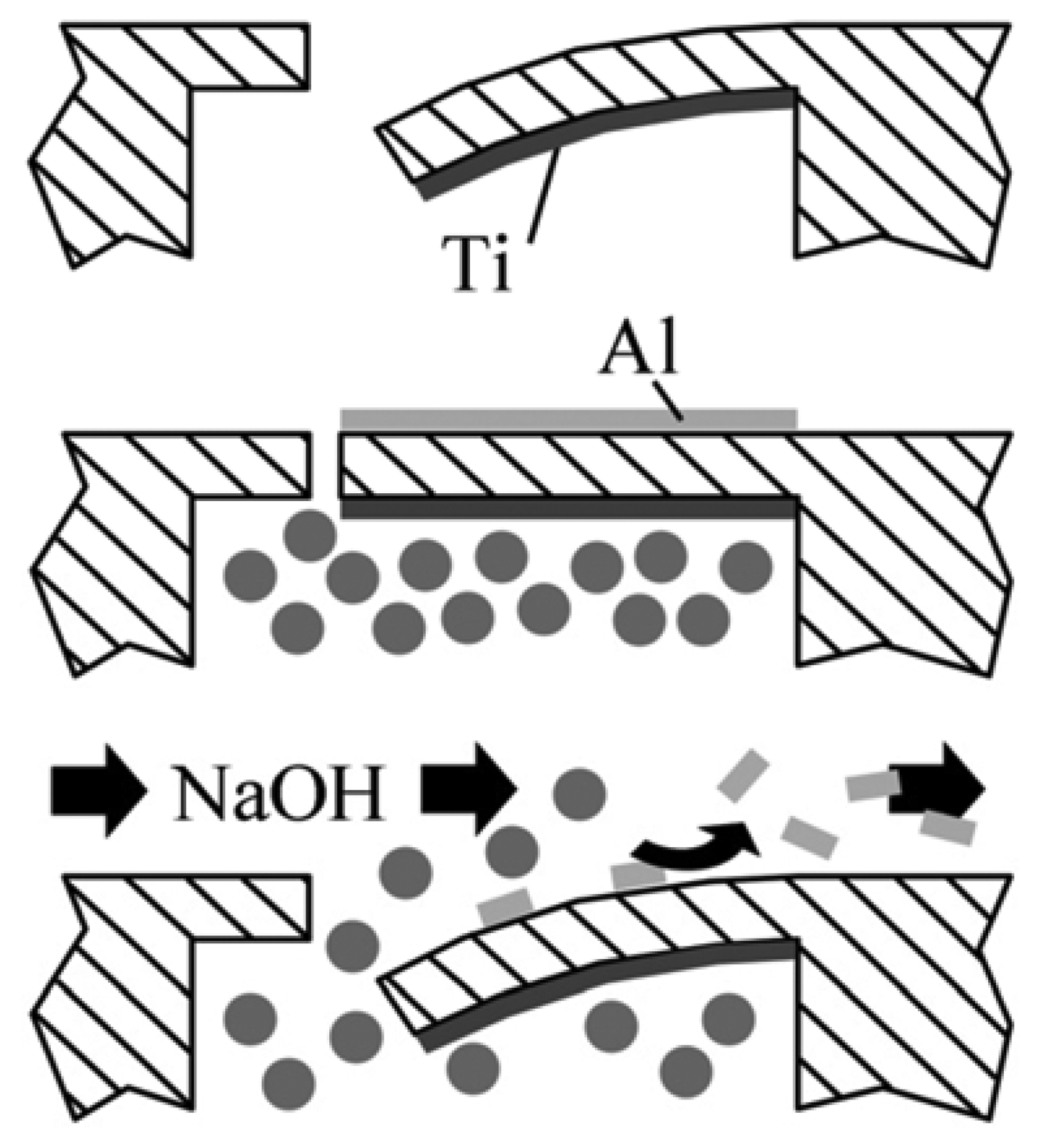
2.1. Fabrication
- An advanced silicon etch device is used to deposit a fluorocarbon coating which serves as release layer at the end.
- A first step of photo-lithography with SU-8 2002 or SU-8 2005 (MicroChem, USA) allows for the definition of the cantilever layer. The cantilever thickness is determined by the speed and acceleration of the spin-coater as well as the viscosity of the SU-8.
- A 200-μm-thick film of SU-8 2075 is spin-coated and patterned to form the support body of the cantilever chip.
- The non-exposed SU-8 is developed in propyleneglycolmethyletheracetate (PGMEA)
- The chips are mechanically released from the substrate by the use of tweezers.
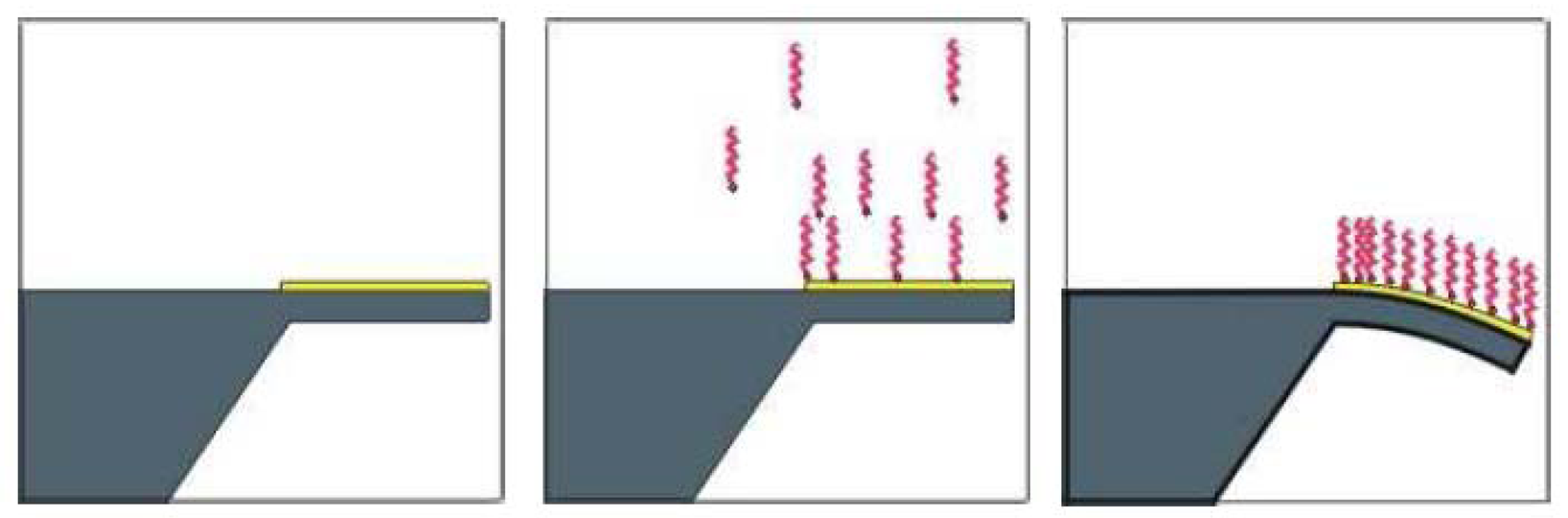
2.2. SU-8 surface functionalisation
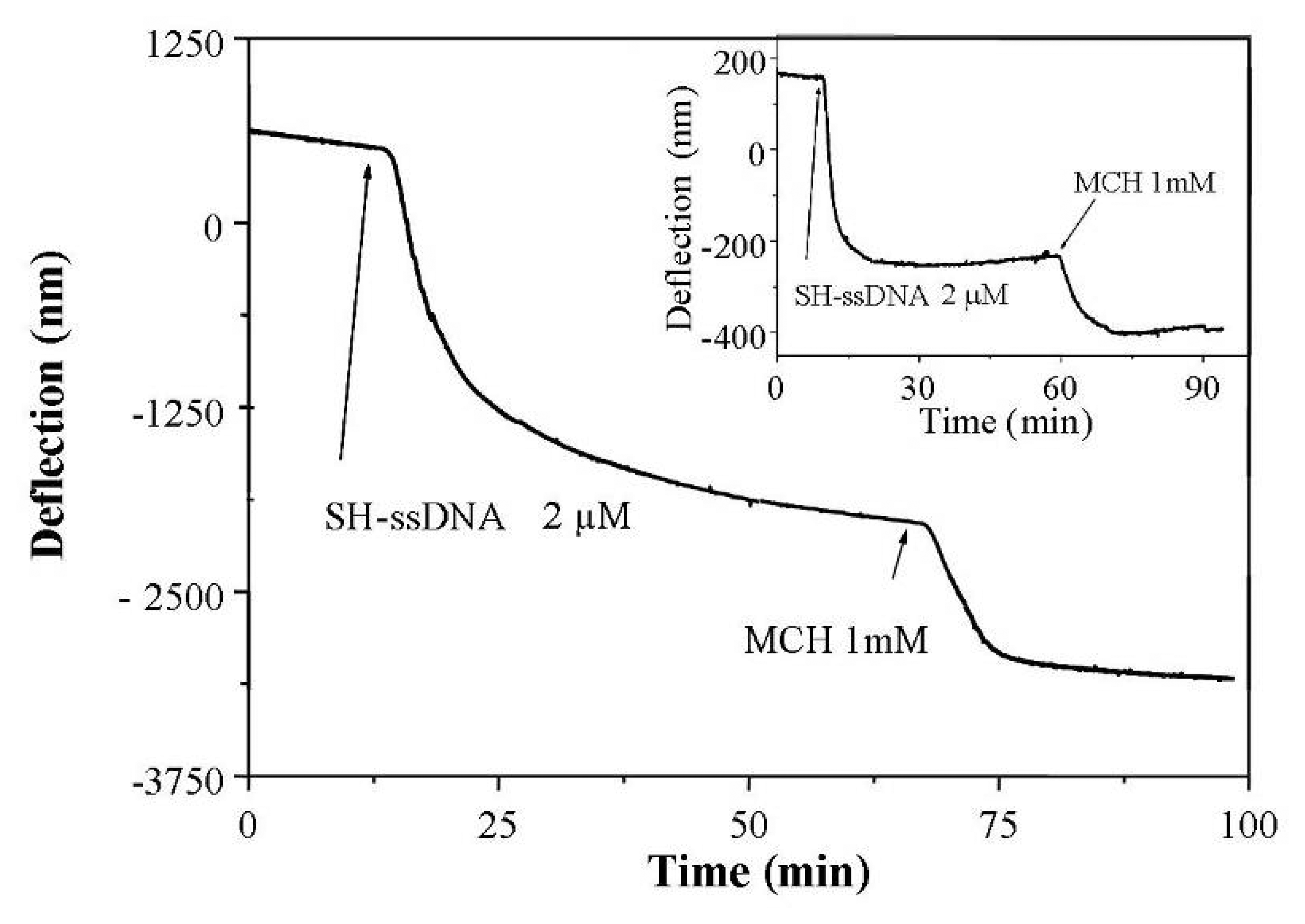
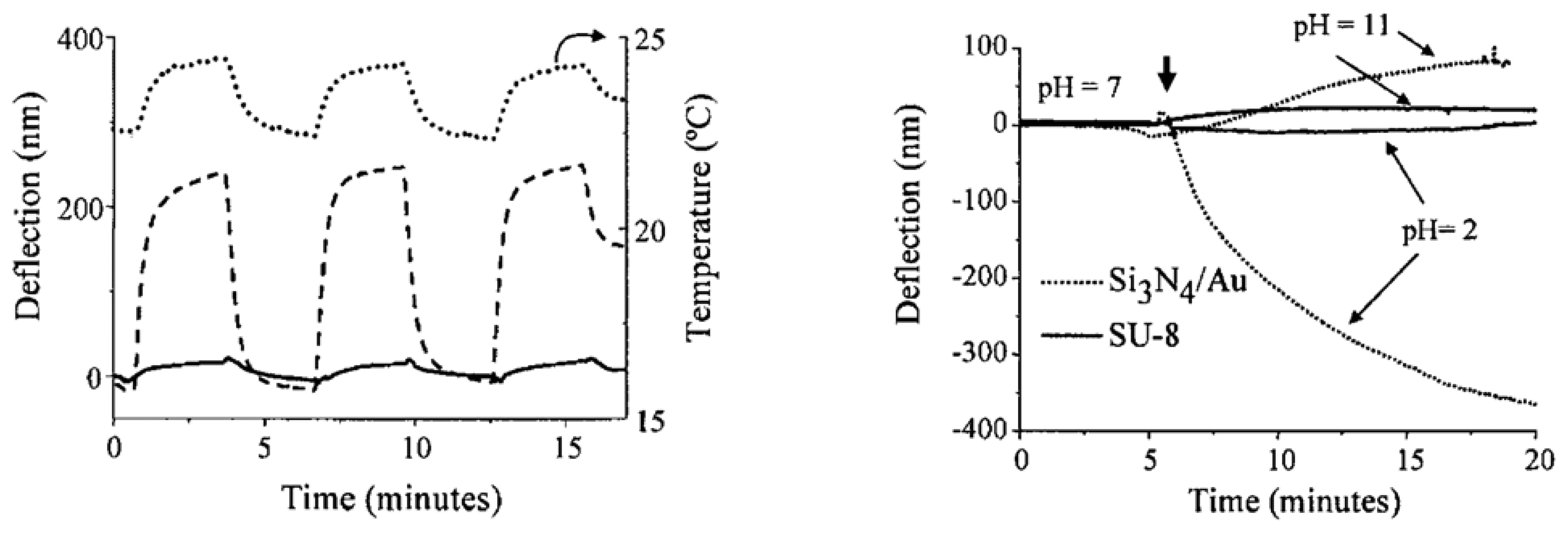
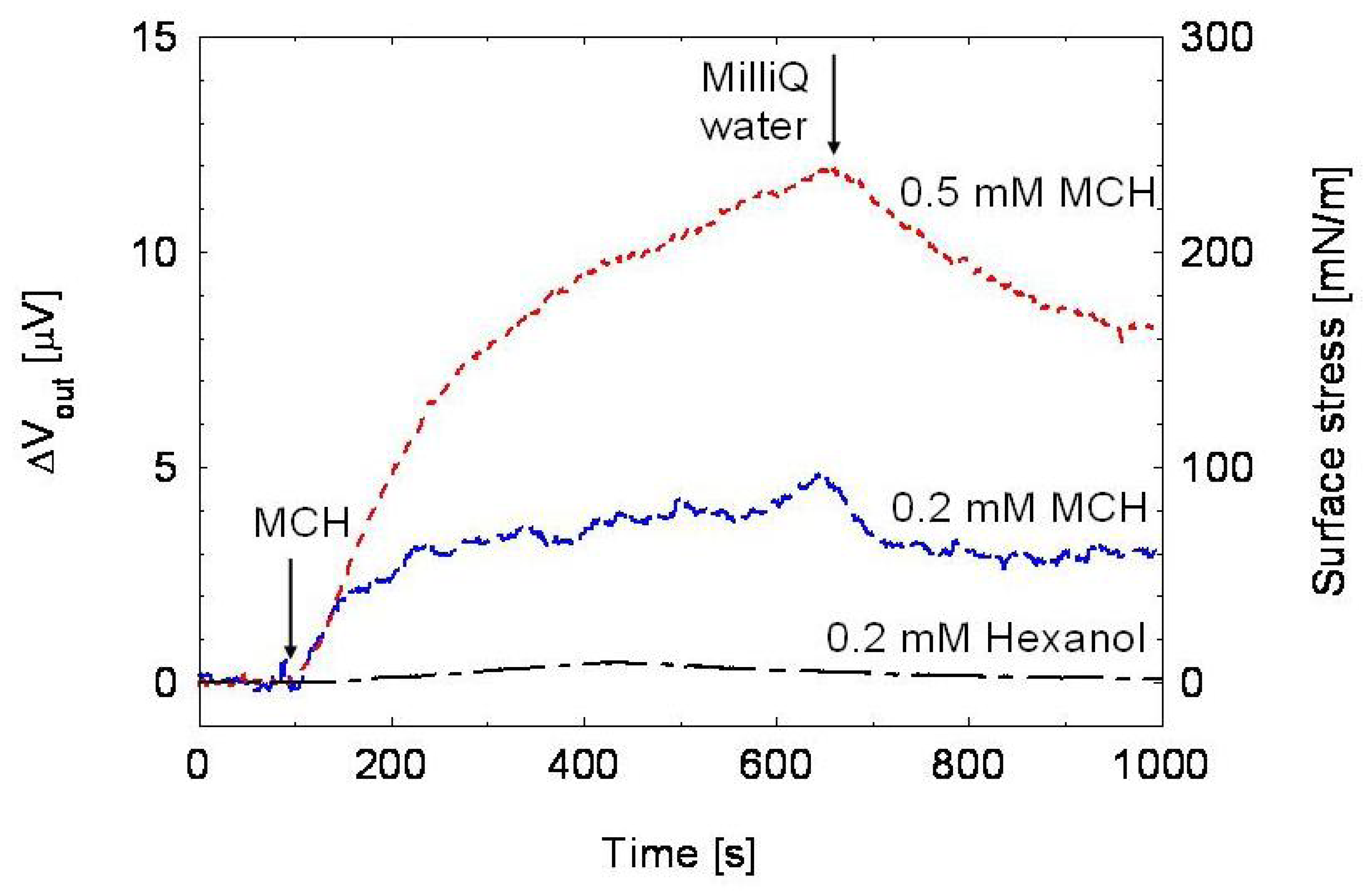
2.3 Experimental results
3. Read-out principles
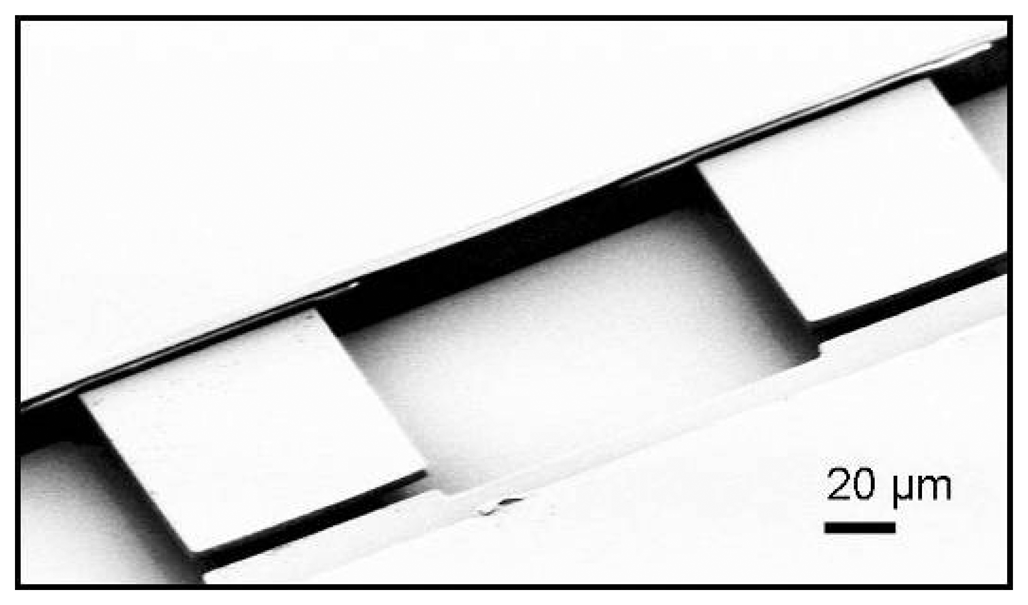
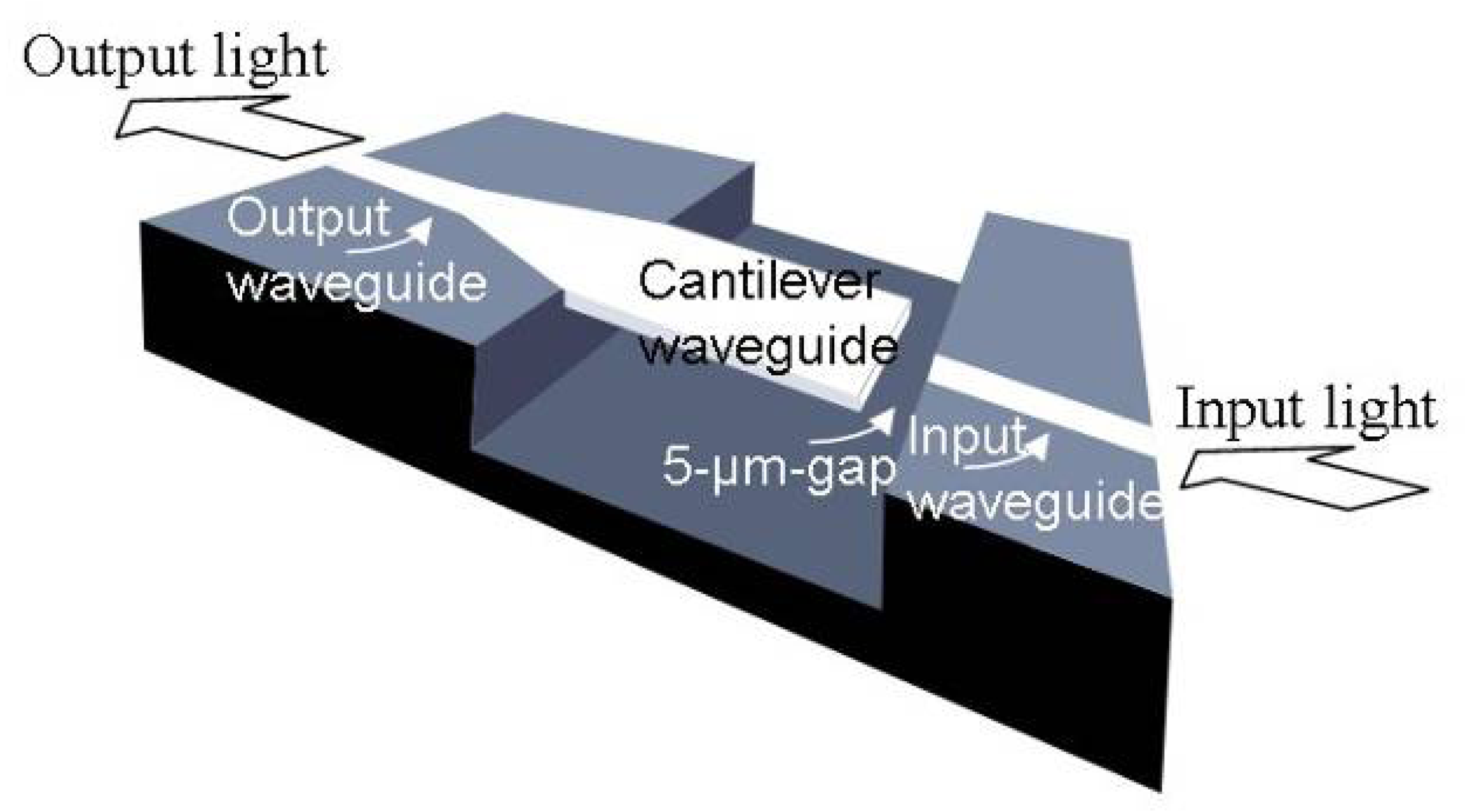
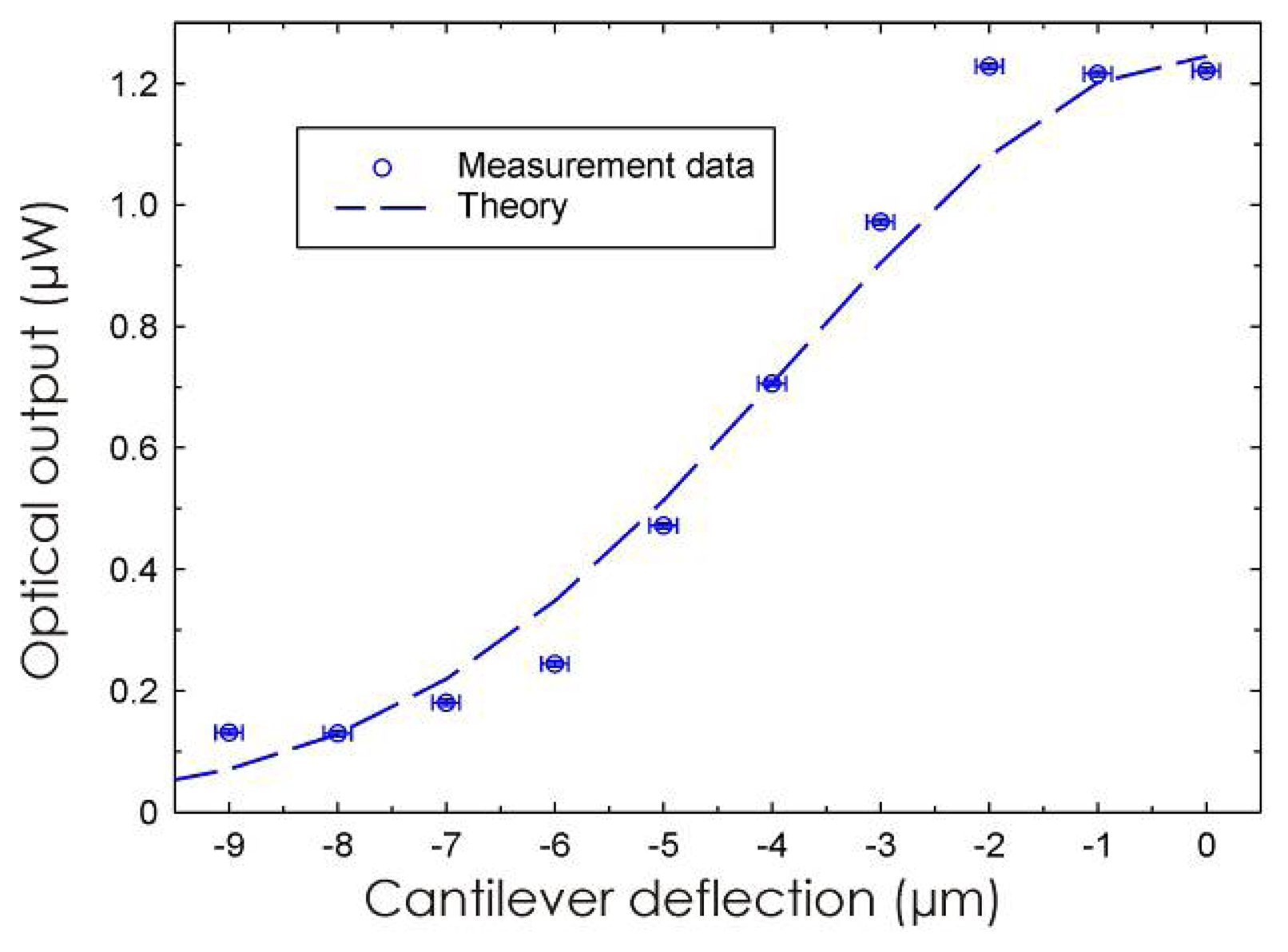
3.1 Integrated optical read-out
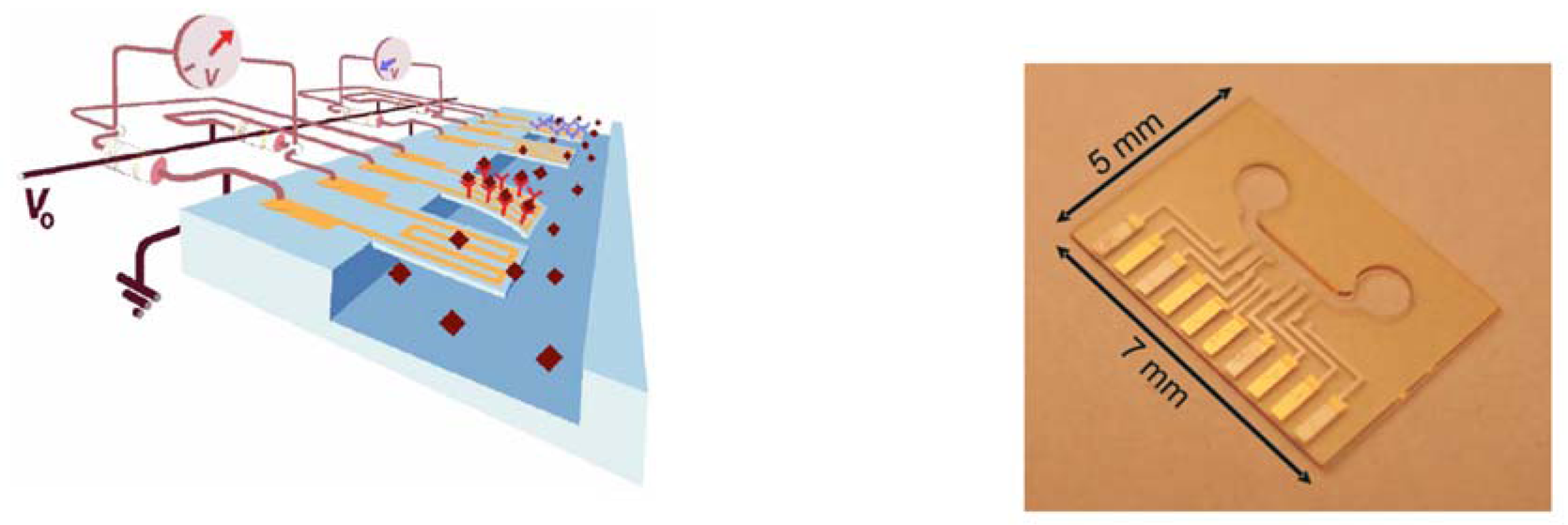
3.2 Piezo-resistive read-out
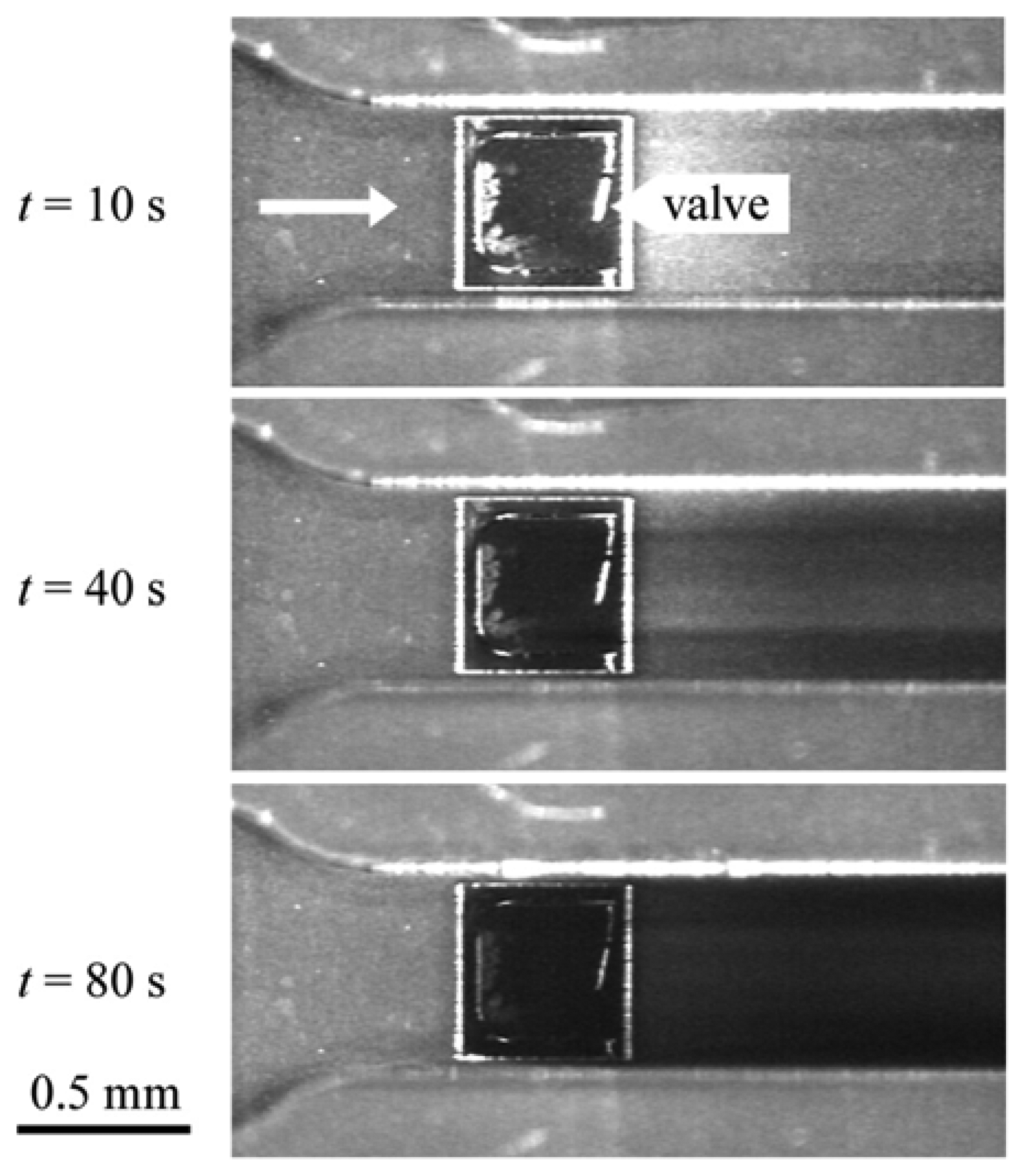
3.3 Autonomous read-out
4. Conclusion and Outlook
Acknowledgments
References and Notes
- Álvarez, M.; Carrascosa, L.G.; Moreno, M.; Calle, A.; Zaballos, Á.; Lechuga, L.M.; Martínez-A, C.; Tamayo, J. Nanomechanics of the formation of DNA self-assembled monolayers and hybridization on microcantilevers. Langmuir 2004, 20, 9663–9668. [Google Scholar]
- Backmann, N.; Zahnd, C.; Huber, F.; Bietsch, A.; Plückthun, A.; Lang, H.-P.; Güntherodt, H.-J.; Hegner, M.; Gerber, Ch. A label-free immunosensor array using single-chain antibody fragments. Proceedings of National Academy of Science US 2005, 102, 14587–14592. [Google Scholar]
- McKendry, R.; Zhang, J.; Arntz, Y.; Strunz, T.; Hegner, M.; Lang, H.-P.; Baller, M.K.; Certa, U.; Meyer, E.; Güntherodt, H.-J.; Gerber, Ch. Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proceedings of National Academy of Science US 2002, 99, 9783–9788. [Google Scholar]
- Tabard-Cossa, V.; Godin, M.; Beaulieu, L.Y.; Grütter, P. A differential microcantilever-based system for measuring surface stress changes induced by electrochemical reactions. Sensors and Actuators B 2005, 107, 233–241. [Google Scholar]
- Desikan, R.; Armel, S.; Meyer, H.M.; Thundat, T. Effect of chain length on nanomechanics of alkanethiol self-assembly. Nanotechnology 2007, 18, 424028. [Google Scholar]
- Raiteri, R.; Butt, H.-J. Measuring electrochemically induced surface stress with an Atomic Force Microscope. Journal of Physical Chemistry 1995, 99, 15728–15732. [Google Scholar]
- Biswal, S.L.; Raorane, D.; Chaiken, A.; Birecki, H.; Majumdar, A. Nanomechanical detection of DNA melting on microcantilever surfaces. Analytical Chemistry 2006, 78, 7104–7109. [Google Scholar]
- Hyun, S.-J.; Kim, H.-S.; Kim, Y.-J.; Jung, H.-I. Mechanical detection of liposomes using piezoresistive cantilever. Sensors and Actuators B 2006, 117, 415–419. [Google Scholar]
- Lee, J.H.; Hwang, K.S.; Park, J.; Yoon, K.H.; Yoon, D.S.; Kim, T.S. Immunoassay of prostate-specific antigen (PSA) using resonant frequency shift of piexoelectric Nanomechanical microcantilever. Biosensors & Bioelectronics 2005, 20, 2157–2162. [Google Scholar]
- Joshi, M.; Kale, N.; Lal, R.; Rao, V.R.; Mukherji, S. A novel dry method for surface modification of SU-8 for immobilization of biomolecules in Bio-MEMS. Biosensors & Bioelectronics 2007, 22, 2429–2435. [Google Scholar]
- Fritz, J.; Baller, M.K.; Lang, H.P.; Rothuizen, H.; Vettiger, P.; Meyer, E.; Güntherodt, H.-J.; Gerber, Ch.; Gimzewski, J.K. Translating biomolecular recognition into nanomechanics. Science 2000, 288, 316–318. [Google Scholar]
- Meyer, G.; Amer, N.M. Novel optical approach to atomic force microscopy. Applied Physics Letters 1988, 53, 1045–1047. [Google Scholar]
- Boisen, A.; Thaysen, J.; Jensenius, H.; Hansen, O. Environmental sensors base don micromachined cantilever with integrated read-out. Ultramicroscopy 2000, 82, 11–16. [Google Scholar]
- Mukhopadhyay, R.; Lorentzen, M.; Kjems, J.; Besenbacher, F. Nanomechanical sensing of DNA sequences using piezoresistive cantilevers. Langmuir 2005, 21, 8400–8408. [Google Scholar]
- Lorenz, H.; Despont, M.; Fahrni, N.; LaBianca, N.; Renaud, P.; Vettiger, P. SU-8: a low-cost negative resist for MEMS. Journal of Micromechanics and Microengineering 1997, 7, 121–124. [Google Scholar]
- Gersborg-Hansen, M.; Thamdrup, L.H.; Mironov, A.; Kristensen, A. Combined electron beam and UV lithography in SU-8. Microelectronic Engineering 2007, 84, 1058–1061. [Google Scholar]
- Haefliger, D.; Nordström, M.; Rasmussen, P.A.; Boisen, A. Dry release of all-polymeric structures. Microelectronic Engineering 2005, 78-79, 88–92. [Google Scholar]
- Nordström, M. Fabrication of cantilever chip with complementary micro channel system in SU-8 for biochemical detection. MSc Thesis, Lund University & Technical University of Denmark, 2004. [Google Scholar]
- Keller, S.; Haefliger, D.; Boisen, A. Optimized plasma-deposited fluorocarbon coating for dry release and passivation of thin SU-8 cantilevers. Journal of Vacuum Science and Technology B. Accepted for publication.
- Bertilsson, L.; Liedberg, B. Infrared study of thiol monolayer assemblies on gold: preparation, characterization, and functionalisation of mixed monolayers. Langmuir 1993, 9, 141–149. [Google Scholar]
- Begley, M.R. The impact of materials selection and geometry on multi-functional bilayer micro-sensors and actuators. Journal of Micromechanics and Microengineering 2005, 15, 2379–2388. [Google Scholar]
- www.microchem.com
- Nordström, M.; Marie, R.; Calleja, M.; Boisen, A. Rendering SU-8 hydrophilic to facilitate use in micro channel fabrication. Journal of Micromechanics and Microengineering 2004, 14, 1614–1617. [Google Scholar]
- Iranpoor, N.; Salehi, P. Ceric ammonium nitrate: a mild and efficient reagent for conversion of epoxides to Beta-nitrato alcohols. Tetrahedron 1995, 51, 909–912. [Google Scholar]
- Marie, R.; Schmid, S.; Johansson, A.; Ejsing, L.; Nordström, M.; Haefliger, D.; Christensen, C.B.V.; Boisen, A.; Dufva, M. Immobilisation of DNA to polymerised SU-8 photoresist. Biosensors & Bioelectronics 2006, 21, 1327–1332. [Google Scholar]
- Blagoi, G.; Johansson, A.; Keller, S.; Boisen, A. Functionalizing Polymeric Cantilevers with Proteins. Proceedings of the International Workshop on Nanomechanical Sensors, Copenhagen, Denmark; 2006. [Google Scholar]
- Wu, T.-L.; Tsao, K.-C.; Chang, P.-Y.; Li, C.-N.; Sun, C.-F.; Wu, J.T. Development of ELISA on microplate for serum C-reactive protein and establishment of age-dependent normal reference range. Clinica Chimica Acta 2002, 322, 163–168. [Google Scholar]
- Hopcroft, M.; Kramer, T.; Kim, G.; Takashima, K.; Higo, Y.; Moore, D.; Brugger, J. Micromechanical testing of SU-8 cantilevers. Fatigue Fracture Engineering Materials and Structures 2005, 28, 735–742. [Google Scholar]
- http://www.memsnet.org/material/siliconnitridesi3n4/
- Calleja, M.; Nordström, M.; Álvarez, M.; Tamayo, J.; Lechuga, L.M.; Boisen, A. Highly sensitive polymer-based cantilever-sensors for DNA detection. Ultramicroscopy 2005, 105, 215–222. [Google Scholar]
- Calleja, M.; Tamayo, J.; Nordström, M.; Boisen, A. Low-noise polymeric nanomechanical biosensors. Applied Physics Letters 2006, 88, 113901. [Google Scholar]
- Nordström, M.; Zauner, D.A.; Calleja, M.; Hübner, J.; Boisen, A. Integrated optical readout for miniaturisation of cantilever-based sensor system. Applied Physics Letters 2007, 91, 103512. [Google Scholar]
- Johansson, A.; Blagoi, G.; Boisen, A. Polymeric cantilever-based biosensor with integrated readout. Applied Physics Letters 2006, 89, 173505. [Google Scholar]
- Haefliger, D.; Marie, R.; Boisen, A. Self-actuated polymeric valve for autonomous sensing and mixing. Proceedings of Transducers ’05, Seoul, Korea; 2005. [Google Scholar]
- Ruano-López, J.M.; Aguirregabiria, M.; Tijero, M.; Arroyo, M.T.; Elizalde, J.; Berganzo, J.; Aramburu, I.; Blanco, F.J.; Mayora, K. A new SU-8 process to integrate buried waveguides and sealed microchannels for a Lab-on-a-chip. Sensors and Actuators B 2006, 114, 542–551. [Google Scholar]
- Bêche, B.; Pelletier, N.; Gaviot, E.; Zyss, J. Single-mode TE00-TM00 optical waveguides on SU-8 polymer. Optics Communications 2004, 230, 91–94. [Google Scholar]
- Nordström, M.; Zauner, D.A.; Boisen, A.; Hübner, J. Single-mode waveguides with SU-8 polymer core and cladding for MOEMS applications. Journal of Lightwave Technology 2007, 25, 1284–1289. [Google Scholar]
- Nordström, M.; Calleja, M.; Hübner, J.; Boisen, A. Novel fabrication technique for free-hanging homogeneous polymeric cantilever waveguides. Journal of Micromechanics and Microengineering. Accepted for publication.
- Wu, G.; Ji, H.; Hansen, K.; Thundat, T.; Datar, R.; Cote, R.; Hagan, M.F.; Chakraborty, A.K.; Majumdar, A. Origin of nanomechanical cantilever motion generated from biomolecular interactions. Proceedings of National Academy of Science US 2001, 98, 1560–1564. [Google Scholar]
- Johansson, A.; Janting, J.; Schultz, P.; Hoppe, K.; Hansen, I.N.; Boisen, A. SU-8 cantilever chip interconnection. Journal of Micromechanics and Microengineering 2006, 16, 314–319. [Google Scholar]
- Johansson, A.; Hansen, O.; Hales, J.; Boisen, A. Temperature effects in Au piezoresistors integrated in SU-8 cantilever chips. Journal of Micromechanics and Microengineering 2006, 16, 2564–2569. [Google Scholar]
- Berger, R.; Delamarche, E.; Lang, H.-P.; Gerber, Ch.; Gimzewski, J.K.; Meyer, E.; Güntherodt, H.-J. Surface stress in the self-assembly of alkanethiols on gold. Science 1997, 276, 2021–2024. [Google Scholar]
- Johansson, A. SU-8 cantilever sensor with integrated readout. PhD Thesis, Technical University of Denmark, 2006. [Google Scholar]
- Rasmussen, P.A.; Thaysen, J.; Hansen, O.; Eriksen, S.C.; Boisen, A. Optimised cantilever biosensor with piezoresistive read-out. Ultramicroscopy 2003, 97, 371–376. [Google Scholar]
- Gammelgaard, L.; Rasmussen, P.A.; Calleja, M.; Vettiger, P.; Boisen, A. Microfabricated photoplastic cantielver with integrated photoplastic/carbon based piezoresistive strain sensor. Applied Physics Letters 2006, 88, 113508. [Google Scholar]
- Mateiu, R.; Lillemose, M.; Hansen, T.S.; Boisen, A.; Geschke, O. Reliability of poly 3,4-ethylenedioxythiophene strain gauge. Microelectronic Engineering 2007, 84, 1270–1273. [Google Scholar]
- Richter, J.; Hansen, O.; Nylandsted, A.; Lundsgaard Hansen, J.; Eriksen, G.F.; Thomsen, E.V. Piezoresistance of Silicon and Strained Si0.9Ge0.1. Sensors and Actuators A 2005, 123-124, 388–396. [Google Scholar]
- Lillemose, M.; Spieser, M.; Christiansen, N.O.; Christensen, A.; Boisen, A. Inherently conductive polymer thin film piezoresistors. Proceedings of MNE ’07, Copenhagen, Denmark, 2007.
- Patent WO 2006/128472 A1.
- Haefliger, D.; Hansen, O.; Boisen, A. Self-positioning of polymer membranes driven by thermodynamically induced plastic deformation. Advanced Materials 2006, 18, 238–241. [Google Scholar]



© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Nordström, M.; Keller, S.; Lillemose, M.; Johansson, A.; Dohn, S.; Haefliger, D.; Blagoi, G.; Havsteen-Jakobsen, M.; Boisen, A. SU-8 Cantilevers for Bio/chemical Sensing; Fabrication, Characterisation and Development of Novel Read-out Methods. Sensors 2008, 8, 1595-1612. https://doi.org/10.3390/s8031595
Nordström M, Keller S, Lillemose M, Johansson A, Dohn S, Haefliger D, Blagoi G, Havsteen-Jakobsen M, Boisen A. SU-8 Cantilevers for Bio/chemical Sensing; Fabrication, Characterisation and Development of Novel Read-out Methods. Sensors. 2008; 8(3):1595-1612. https://doi.org/10.3390/s8031595
Chicago/Turabian StyleNordström, Maria, Stephan Keller, Michael Lillemose, Alicia Johansson, Søren Dohn, Daniel Haefliger, Gabriela Blagoi, Mogens Havsteen-Jakobsen, and Anja Boisen. 2008. "SU-8 Cantilevers for Bio/chemical Sensing; Fabrication, Characterisation and Development of Novel Read-out Methods" Sensors 8, no. 3: 1595-1612. https://doi.org/10.3390/s8031595



