Development and Electrochemical Investigations of an EIS- (Electrolyte-Insulator-Semiconductor) based Biosensor for Cyanide Detection
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
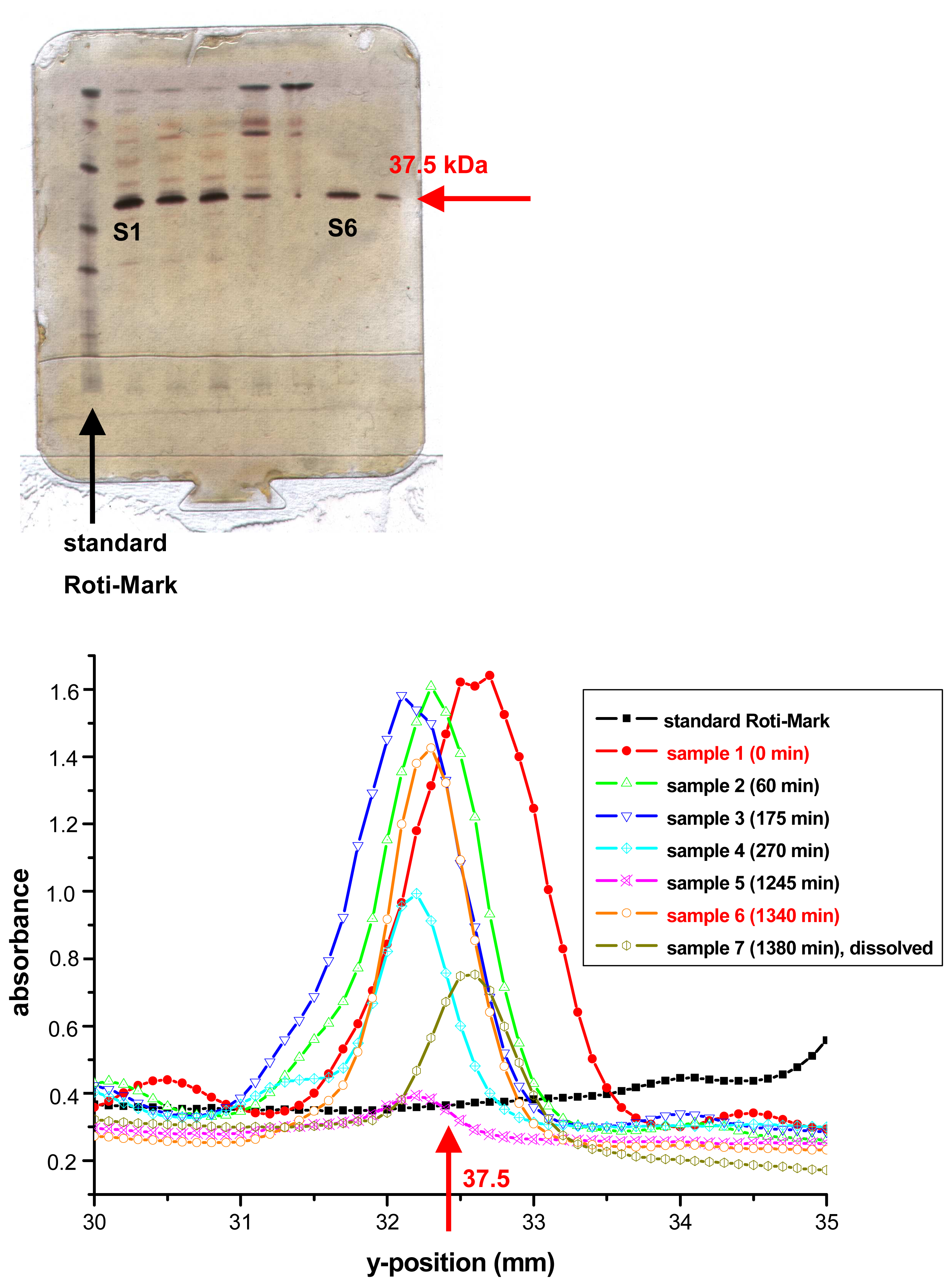
3.1. Immobilisation of cyanidase to NHS-activated Sepharose™
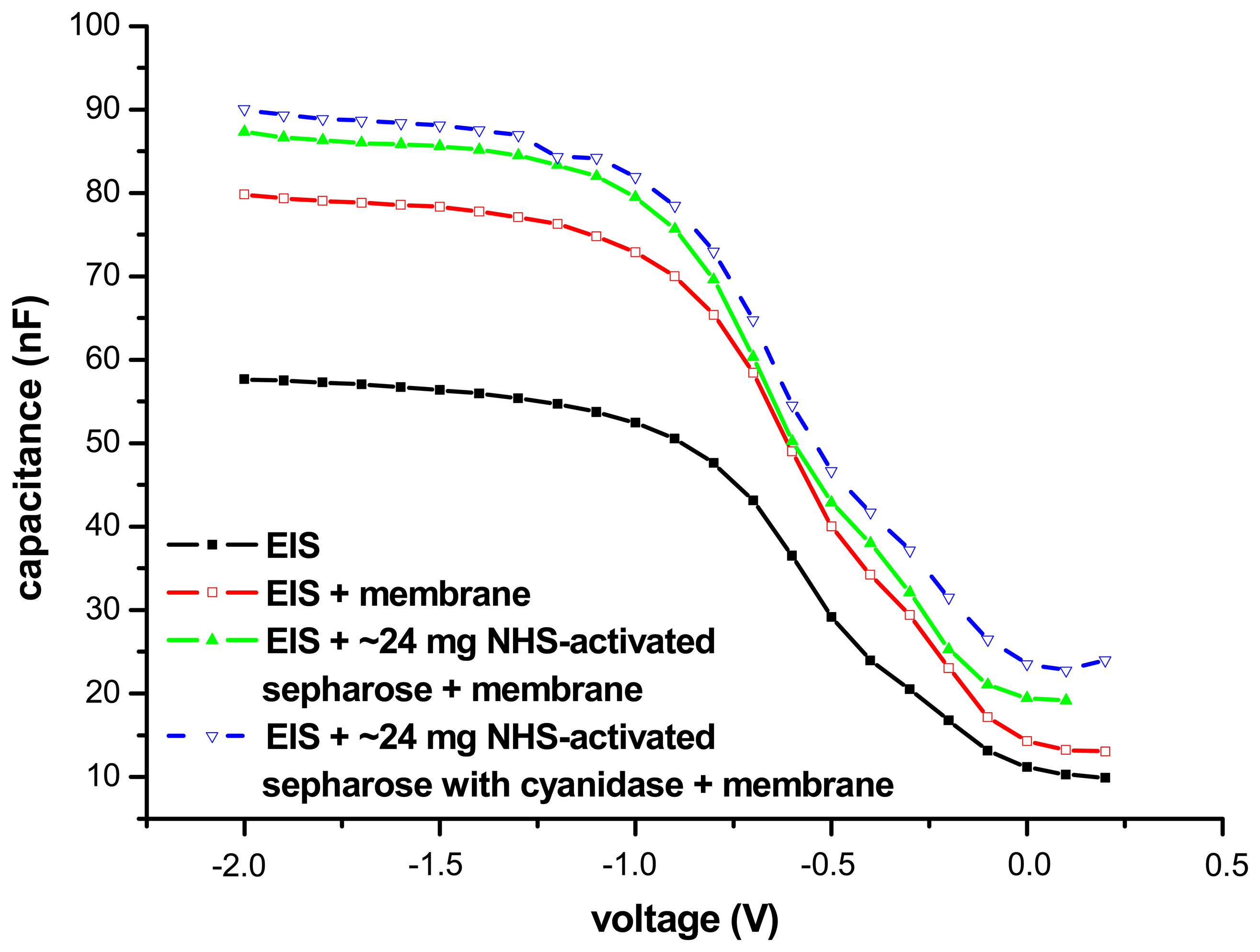
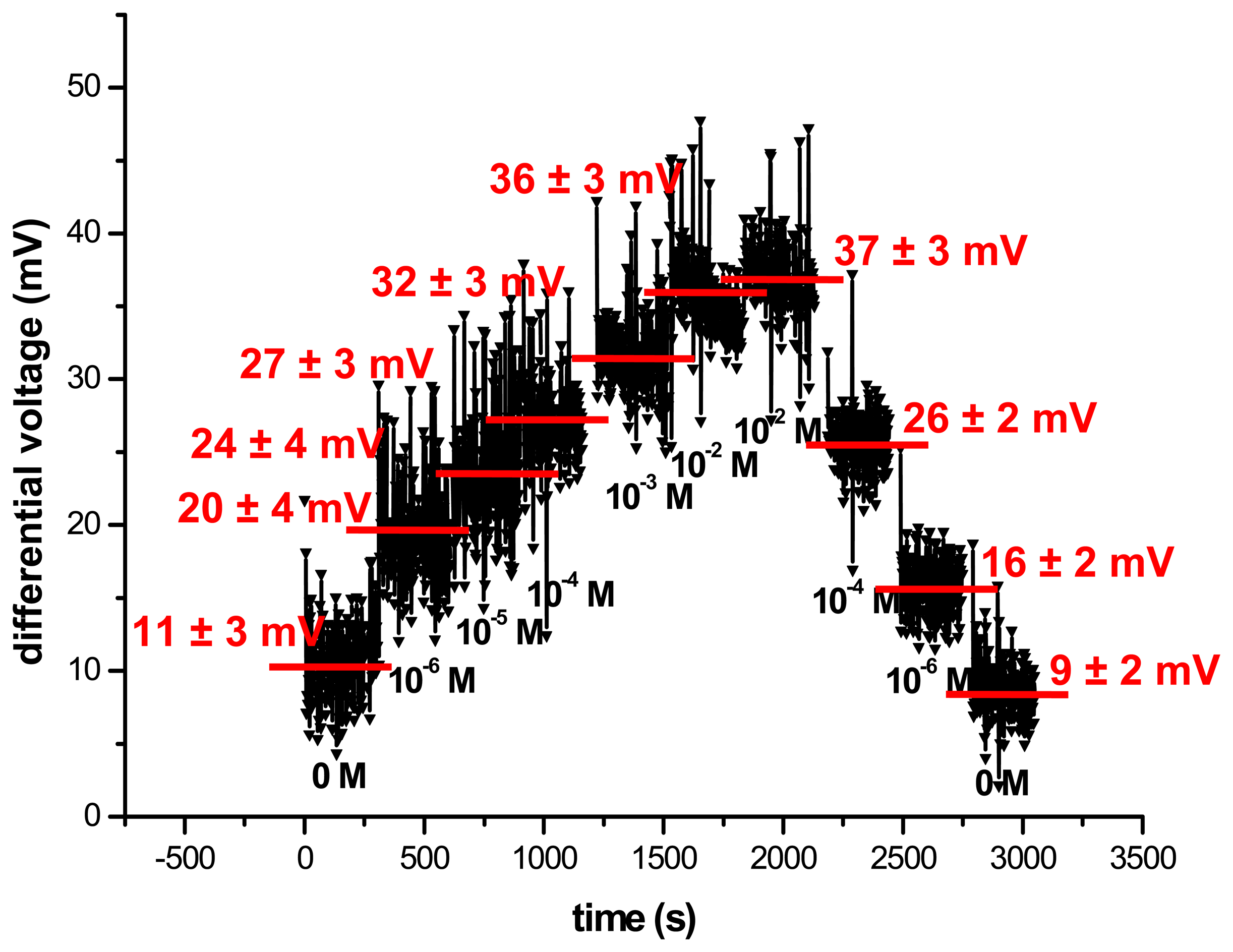
3.2. Electrochemical characterisation of the cyanide biosensor
4. Conclusions
Acknowledgments
References
- Marquardt, H.; Schäfer, S. G. Lehrbuch der Toxikologie; Spektrum Akademischer Verlag: Heidelberg Berlin, 1997; pp. 555–565. [Google Scholar]
- U.S. Department of health and human services, public health service, agency for toxic substances and disease registry. Toxicological profile for cyanide. 2006.
- Mak, K. K. W.; Law, A. W. C.; Tokuda, S.; Yanase, H.; Renneberg, R. Application of cyanide hydrolase from klebsiella sp. in a biosensor system for detection of low-lewel cyanide. Appl. Microbiol. Biotechnol. 2005, 67, 631–636. [Google Scholar]
- Badugu, R.; Lakowicz, J. R.; Geddes, C. D. Enhanced fluorescence cyanide detection at physiologically lethal levels: reduced ICT-based signal transduction. J. Am. Chem. Soc. 2005, 127, 3635–3641. [Google Scholar]
- Jones, D. A. Why are so many food plants cyanogenic? Phytochemistry 1997, 47(2), 155–162. [Google Scholar]
- Keusgen, M. Biosensors: new approaches in drug discovery. Naturwissenschaften 2002, 89, 433–444. [Google Scholar]
- Yeoh, H.-H.; Tatsuma, T.; Oyama, N. Monitoring the cyanogenic potential of cassava: the trend towards biosensor development. Trends Anal. Chem. 1998, 17(4), 234–240. [Google Scholar]
- Mak, K. K. W.; Yanase, H.; Renneberg, R. Cyanide fishing and cyanide detection in coral reef fish using chemical tests and biosensors. Biosens. Bioelectron. 2005, 20, 2581–2593. [Google Scholar]
- Watanabe, A.; Yano, K.; Ikebukuro, K.; Karube, I. Cyanide hydrolysis in a cyanide-degrading bacterium, pseudomonas stutzeri AK61, by cyanidase. Microbiology 1998, 144, 1677–1682. [Google Scholar]
- Von Tümpling, W. Schäden durch Cyanide im Flusswasser. Chemie 2006, 4, 67–67. [Google Scholar]
- Aldridge, W. N. A new method for the estimation of micro quantities of cyanide and thiocyanide. Analyst 1944, 69, 262–265. [Google Scholar]
- Epstein, J. Estimation of microquantities of cyanide. Anal. Chem. 1947, 19(4), 272–274. [Google Scholar]
- Lundquist, P.; Sörbo, B. Rapid determination of toxic cyanide concentration in blood. Clin. Chem. 1989, 35(4), 617–619. [Google Scholar]
- Afkhami, A.; Sarlak, N. A novel cyanide sensing phase based on immobilization of methyl violet on triacetylcellulose membrane. Sens. Actuators B 2007, 122, 437–441. [Google Scholar]
- Shan, D.; Mousty, C.; Cosnier, S. Subnanomolar cyanide detection at polyphenol oxidase/clay biosensors. Anal. Chem. 2004, 76, 178–183. [Google Scholar]
- Weinberg, H. S.; Cook, S. J. Segmented flow injection, UV digestion, and amperometric detection for the determination of total cyanide in wastewater treatment plant effluents. Anal. Chem. 2002, 74, 6055–6063. [Google Scholar]
- Keusgen, M.; Kloock, J. P.; Knobbe, D.-T.; Jünger, M.; Krest, I.; Goldbach, M.; Klein, W.; Schöning, M. J. Direct determination of cyanides by potentiometric biosensors. Sens. Actuators B 2004, 103, 380–385. [Google Scholar]
- Porter, T. L.; Vail, T. L.; Eastman, M. P.; Stewart, R.; Reed, J.; Venedam, R.; Delinger, W. A solid-state sensor platform for the detection of hydrogen cyanide gas. Sens. Actuators B 2007, 123, 313–317. [Google Scholar]
- Hachiya, H.; Ito, S.; Fushinuki, Y.; Masadome, T.; Asano, Y.; Imato, T. Continuous monitoring for cyanide in waste water with a galvanic hydrogen cyanide sensor using a purge system. Talanta 1999, 48, 997–1004. [Google Scholar]
- Hofton, M. Continuous determination of free cyanide in effluents using Ag2S-ISE. Environ. Sci. Technol. 1976, 10(3), 277–280. [Google Scholar]
- Ipatov, A.; Ivanov, M.; Makarychev-Mikhailov, S.; Kolodnikov, V.; Legin, A.; Vlasov, Y. Determination of cyanide using flow-injection multisensor system. Talanta 2002, 58, 1071–1076. [Google Scholar]
- Neshkova, M. T.; Pancheva, E. M.; Pashova, V. A new generation of CN sensing silver chalcogenide-selective membranes for FIA application I. Flow-injection detector for CN based on thin Ag2+dSe1-xTex electropolated membrane. Sens. Actuators B 2006, 119, 625–631. [Google Scholar]
- Volotovsky, V.; Kim, N. Cyanide determination by an ISFET-based peroxidase biosensor. Biosens. Bioelectron. 1998, 13, 1029–1033. [Google Scholar]
- Watanabe, A.; Yano, K.; Ikebukuro, K.; Karube, I. Cloning and expression of a gene encoding cyanidase from pseudomonas stutzeri AK61. Appl. Microbiol. Biotechnol. 1998, 50, 93–97. [Google Scholar]
- Schöning, M. J.; Brinkmann, D.; Rolka, D.; Demuth, C.; Poghossian, A. CIP (cleaning-in-place) suitable “non-glass” pH sensor based on Ta2O5-gate EIS structure. Sens. Actuators B 2005, 111-112, 423–429. [Google Scholar]
- Poghossian, A.; Thust, M.; Schroth, P.; Steffen, A.; Lüth, H.; Schöning, M. J. Penicillin detection by means of silicon-based field-effect structures. Sens. Mater. 2001, 13(4), 207–223. [Google Scholar]
- Poghossian, A.; Schöning, M. J. Silicon-based chemical and biological field-effect sensors. In Encyclopedia of Sensors; Grimes, C. A., Dickey, E. C., Pishko, M. V., Eds.; American Scientific Publishers, Stevenson Ranch: USA, 2006; Vol. 9, pp. 463–534. [Google Scholar]
- Ingvorsen, K.; Hojer-Pedersen, B.; Godtfredsen, S. E. Novel cyanide-hydrolyzing enzyme from alcaligenes xylosoxidans subsp. denitrificans. Appl. Environ. Microbiol. 1991, 57, 1783–1789. [Google Scholar]


© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Turek, M.; Ketterer, L.; Claßen, M.; Berndt, H.K.; Elbers, G.; Krüger, P.; Keusgen, M.; Schöning, M.J. Development and Electrochemical Investigations of an EIS- (Electrolyte-Insulator-Semiconductor) based Biosensor for Cyanide Detection. Sensors 2007, 7, 1415-1426. https://doi.org/10.3390/s7081415
Turek M, Ketterer L, Claßen M, Berndt HK, Elbers G, Krüger P, Keusgen M, Schöning MJ. Development and Electrochemical Investigations of an EIS- (Electrolyte-Insulator-Semiconductor) based Biosensor for Cyanide Detection. Sensors. 2007; 7(8):1415-1426. https://doi.org/10.3390/s7081415
Chicago/Turabian StyleTurek, Monika, Lothar Ketterer, Melanie Claßen, Heinz K. Berndt, Gereon Elbers, Peter Krüger, Michael Keusgen, and Michael J. Schöning. 2007. "Development and Electrochemical Investigations of an EIS- (Electrolyte-Insulator-Semiconductor) based Biosensor for Cyanide Detection" Sensors 7, no. 8: 1415-1426. https://doi.org/10.3390/s7081415




