1. Introduction
Poly (aniline), (PANI) is one of the promising conducting polymers because of its conductivity, reversible redox characteristics, electrochromic behavior and environmental stability in air. The properties inbuilt in PANI provide potential applications in rechargeable batteries [
1,
2], biosensor [
3], electrochromic device [
4,
5], photoelectrochemical cell [
6,
7] and corrosion protection [
8]. However, PANI does not show electroactivity at less acidic conditions and this limits its applications in biosensor. It becomes necessary to prepare derivatives of PANI having the required properties to use in biochemical applications.
In recent years, a great deal of attention has been paid on the synthesis of aniline-based copolymers. This becomes necessary due to the fact that it is difficult to synthesize new PANI derivatives with electrical properties and stability better than PANI. Simultaneous polymerization of aniline and other aniline derivatives offers the possibility to prepare copolymers of aniline. These copolymers retain the properties of PANI with additional newer properties. Copolymerization of aniline with another aniline derivative can be performed using chemical and electrochemical methods [
9-
11]. Aniline-based copolymers exhibit pH dependent electrical properties and could be used as sensors.
Horseradish peroxidase (HRP) has been commonly used as an enzyme for the construction of a hydrogen peroxide biosensor with or without a mediator. HRP contains heme as the prosthetic group, which is also the protein active site along with the heme-iron: Fe(III), that can catalyze the H
2O
2-dependent one-electron oxidation [
12].The enzyme electrodes were fabricated for sensing hydrogen peroxide by immobilization HRP into the electrode surface. Especially, the enzyme electrodes exhibited high sensor efficiency when Au nanoparticles are present as linking unit [
13].
There are few reports available on the preparation of modified electrodes dealing with the immobilization of HRP into conducting polymer matrix [
14-
16]. However, studies on the use of a mediator to augment the signal response are scarce. The present investigation has focused on fabricating the HRP based electrodes in different new environments such as the use of a mediator like gold nanoparticles (physical dispersion), or use of cross-linkable mediator (chemical reaction with HRP). It is to be noted that gold nanoparticles were used in combination with enzymes in the fabrication of electrode[
17,
18]. In these cases, gold nanoparticles were formed
insitu and the dispersion and sizes of the gold nanoparticles could not be effectively controlled. We have demonstrated from our pervious studies that metal nanoparticles could be effectively prepared with smaller sizes and good dispersion by gamma ray induced reduction processes[
19,
20]. Hence, we have designed the experiment in such a way to prepare the gold nanoparticles by gamma radiation induced reduction for obtaining well dispersed gold nanoparticles and to load them into HRP-conducting polymer matrix. The preparation of gold nanoparticles by gamma radiation was purposely done to know the influence of finely dispersed gold nanoparticles in the matrix on the amplification of response to hydrogen peroxide.
In this work, we explore the utility of film of poly (aniline-co-m-aminophenol) immobilized with HRP as sensor electrode. HRP was immobilized into the copolymer film by physical trapping and covalent modification approaches. In the physical trapping method, Au nanoparticles prepared by γ-irradiation induced reduction of HAuCl4 were dispersed into the copolymer film and further HRP was immobilized in the presence of glutaric dialdehyde (GDA). In the covalent functionalization method, HRP was covalently bonded to poly (aniline-co-m-aminophenol) film. The enzyme electrodes were tested for sensing characteristics of H2O2.
2. Experimental
2.1. Materials
Hydrogen tetrachloroaurate (III) (HAuCl4, 99.99%), aniline, m-aminophenol, horseradish peroxide (HRP, E.C. 1.11.1.7, Type VI, RZ > 3.0, >250U/mg), GDA (25wt% solution in water), sodium phosphate monobasic monohydrate, sodium phosphate dibasic heptahydrate were analytical reagent grade and supplied by Aldrich-Sigma Co. The 30% hydrogen peroxide solution was purchased from OCI (Seoul, Korea), and a fresh solution of H2O2 was prepared daily. Polyvinylpyrrolidone (PVP, MW. Av. 40,000) was obtained from Tokyo Kasi (Japan). Ultrapure water was prepared with quartz distillatory below boiling point and then purified with Milli-Q puls water purification system (Millipore Co. Ltd.), (the final resistance of water was 18.0 MΩ · cm-1). Other chemicals (reagent grade) were used without further purification.
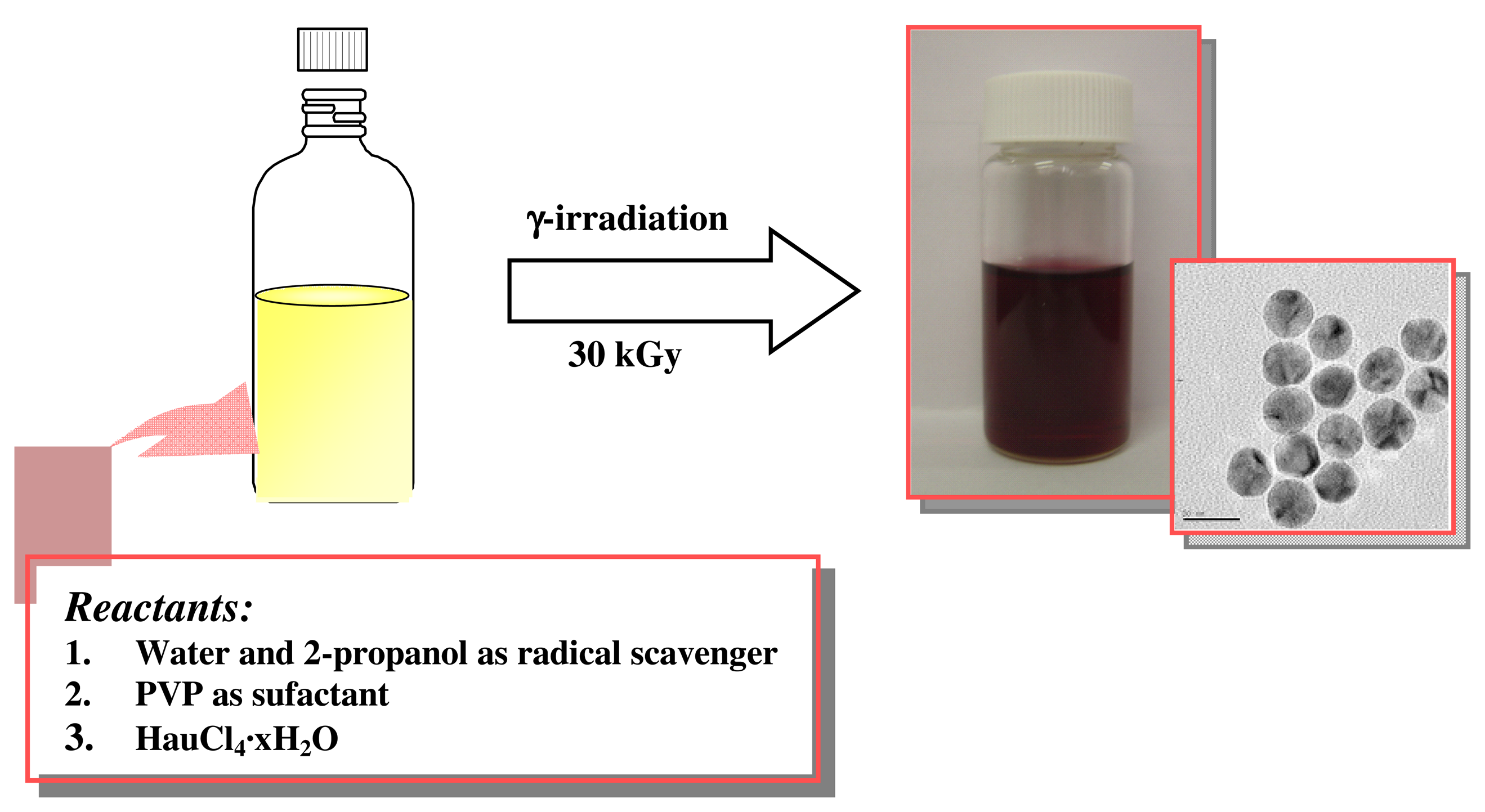
2.2. Preparation of Au nanoparticles by γ-irradiation
Scheme 1 shows the preparation procedure of the Au nanoparticles in aqueous solution by γ-irradiation. Colloids of Au were prepared as follows: Salts of HAuCl
4 (ca. 100ppm) were dissolved in water in the presence of PVP as colloidal stabilizer. Nitrogen gas was bubbled through the solution (20 min) to remove oxygen and then was irradiated by γ-ray (Co-60 source) under atmospheric pressure and ambient temperature. A total irradiation dose of 30 kGy (a dose rate = 6.48 × 105/h) was applied. Red-colored colloidal solution was obtained for Au. TEM image give an idea that nanoparticles of the size range 20-30 nm were formed in aqueous solution.
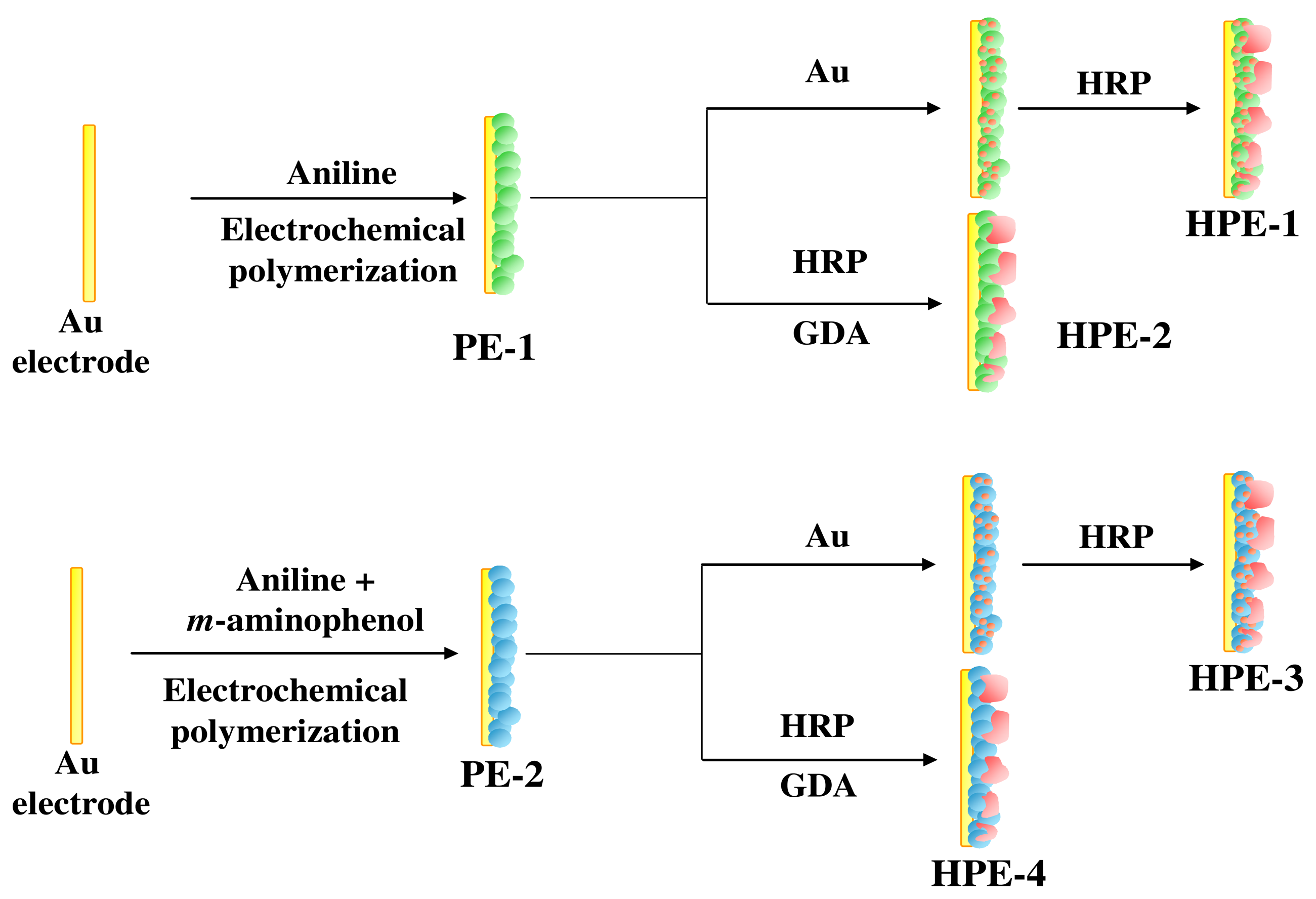
2.3. Preparation of the enzyme electrodes
Scheme 2 illustrates the procedure for fabrication of enzyme electrodes (HPE-1, HPE-2, HPE-3, and HPE-4). Poly (aniline), PANI film (PE-1) was electrodeposited by cycling the potential between 0.0 V and 1.2 V using a 0.1 M solution of aniline in 0.5 M H
2SO
4. Au nanoparticles prepared by γ-irradiation were loaded into PE-1 to obtain PE-1–Au electrode (physical trapping). HRP was entrapped into PANI-Au film to generate HPE-1. HPE-2 was fabricated by immobilization of HRP on the surface of PANI film (PE-1) electrode using GDA as the cross-linker (covalent bonding). It is to be noted that Au nanoparticles are not dispersed into PANI film before covalent modification while forming HPE-2.
Likewise, film of poly (aniline-co-m-aminophenol) electrode (PE-3) was electrodeposited on Au electrode through copolymerization of 0.1 M aniline with 2.5 mM m-aminophenol. HPE-3 and HPE-4 were fabricated using PE-2 in a similar manner to HPE-1 and HPE-2.
2.4. Measurements
Transmission Electron Microscopy photographs of the samples were recorded using Energy Filtered Transmission Electron Microscope (EF-TEM, EM 912 Omega, Carl Zeiss, Germany) and FE-TEM (Hitachi, S-4700, Japan) installed at Korea Basic Science Institute, Korea.
Amperometric and cyclic voltammetric experiments were performed using a Potentiostat/Galvanostat model 283 (Ametek PAR, U.S.A). All experiments were carried out using a conventional three-electrode system with the enzyme electrode as the working electrode, a platinum wire as the auxiliary electrode, and an Ag/AgCl (saturated KCl) electrode as the reference electrode. Electrolyte solutions were purged with high purity nitrogen prior to and blanked with nitrogen during electrochemical experiments. Electrochemical experiments were carried out in 0.5 M H2SO4 and 0.1 M phosphate buffer (pH 7.0). HPE-1, HPE-2, HPE-3 and HPE-4 are tested for sensor behavior towards H2O2.
3. Results and discussion
Fig. 1 presents the cyclic voltammograms (CVs) recorded during the electrochemical polymerization of aniline (0.1 M) and copolymerization of aniline (0.1 M) with
m-aminophenol (2.5 mM) in 0.5 M H
2SO
4. CVs for the electropolymerization of aniline exhibit three oxidation peaks around 0.32 V, 0.56 V and 0.92 V with their reduction counter waves around -0.04, 0.39, and 0.64 V respectively. An increasing trend in current with potential cycles in both anodic and cathodic sides signifies the layer by layer deposition of PANI on the electrode (PE-1). The redox pairs, A/A'and C/C' correspond to leucoemeraldine/emeraldine and emeraldine/pernigraniline transformations of PANI, respectively [
21]. The redox pair B/B' is due to formation dimmer and/or hydrolysis products. These redox characteristics are online with published literature on electrochemical polymerization of aniline. However, we could notice additional features in the CVs for the copolymerization of aniline with
m-aminophenol. Besides the peaks corresponding to PANI, there is a new oxidation peak around 0.41 V in the CV curves. This may correspond to polaronic conversion of
m-aminophenol units in the co polymer with a reduction peak at 0.31 V. In the case of copolymerization, new redox characteristics could also be seen around 0.70 V representing the bipolaronic conversion of
m-aminophenol units. These CV features in copolymer growth strongly favor the inclusion of
m-aminophenol units in the film (PE-2). We have used an upper anodic limit of 1.2 V for maintaining the same condition for the preparation of conducting polymer matrixes, Poly (aniline) and poly (aniline-co-m-aminophenol). It must be noted that for obtaining copolymer of m-aminophenol with aniline, a potential of 1.2 V is needed since the oxidation potential of the monomer is nearer to 1.2 V. Hence, in the case of polyaniline deposition also we have used an upper potential of 1.2 V.
The film-coated electrodes, (PANI and copolymer) were loaded with Au nanoparticles prepared by γ-irradiation.
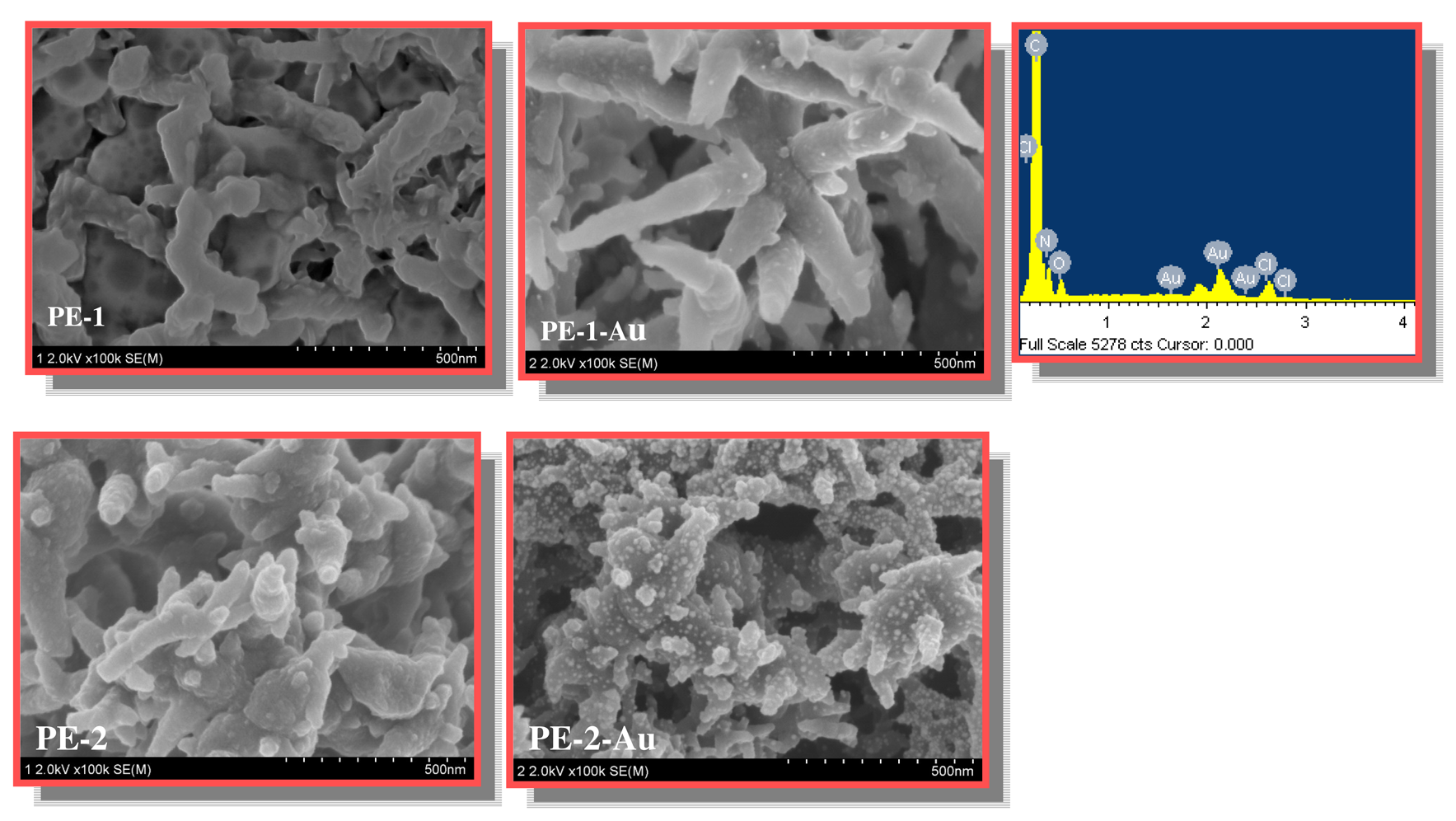
Fig. 2 presents the SEM images and EDX results of PE-1 and PE-2. The film morphology of PANI (PE-1) is fibrous having an average diameter of 50 nm. However, the fibrous morphology with a non-uniform diameter can be seen for the copolymer. The PANI fibers are comparatively longer than the fibers of copolymer. On loading Au nanoparticles into PANI (PE-1-Au) and copolymer films (PE-2-Au), the diameter of the polymer fibers decreased. It is probable that upon loading gold nanoparticles, a part of the fiberous surface of the PANI might undergo dissolution due to the use of organic solvent in the preparation of gold colloids.
EDX results confirm the loading Au nanoparticles into the surface of PANI or copolymer (
Fig. 2). There are differences in the sizes and shape of Au nanoparticles that are incorporated into PANI or copolymer film. The Au nanoparticles loaded in PANI has a spherical morphology. However, Au nanoparticles are present within the swollen fibers in the case of copolymer. Comparatively, the loading of Au nanoparticles in PANI is higher than in copolymer. HRP was then immobilized to obtain HPE-1 and HPE-3 in the case of PANI and copolymer, respectively. Also, HRP was covalently immobilized to PANI and copolymer film to obtain HPE-2 and HPE-4, respectively, in the absence of loading of Au nanoparticles.
Fig. 3 shows the SEM images of HPE-1, HPE-2, HPE-3 and HPE-4 (see,
Scheme 2). The dispersed fibrous morphology could be witnessed in these cases. Au nanoparticles act as connecting linker between the enzyme and groups in PANI. GDA is bi-functional and generally used for chemical modifications of proteins and polymers. The carbonyl group in GDA links covalently the amine groups in the protein molecules. PANI has –NH groups in the backbone chain that could be linked with HRP through carbonyl group in GDA. The binding /immobilization of HRP to PANI and copolymer thus may be considered different due to the fact that the immobilization occurs through physical trapping of Au nanoparticles and HRP in the case of PANI, whilst the –OH groups in copolymer may bind Au nanoparticles and HRP in the copolymer.
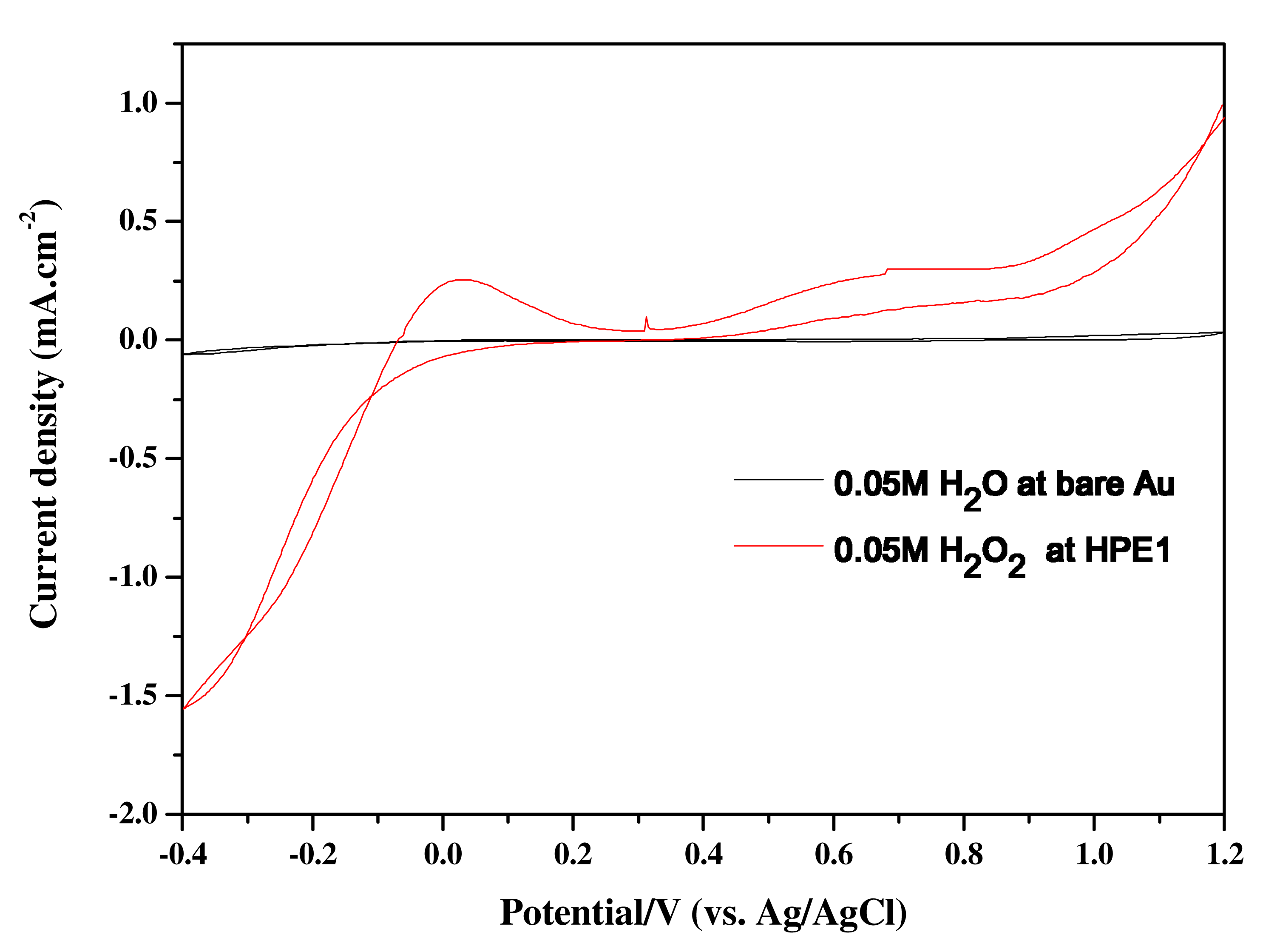
Amperometric response of an enzyme electrodes (HPE-1, HPE-2, HPE-3, and HPE-4) were recorded towards oxidation of hydrogen peroxide and the current response for the electrochemical oxidation of H
2O
2 were recorded for 0.05M of H
2O
2. For the purpose of comparison, electro oxidation of H
2O
2 was performed at the bare Au electrode. An oxidation wave was noticed around 0.05 V with a current density of 0.25 mA/cm
2 for 0.05M of H
2O
2 at HPE-1 electrode in comparison to the negligible current (in the order of microampere /cm
2 at the bare Au electrode . Thus, it is clear that enzyme modified electrodes have pronounced current response for the oxidation of 0.05 M of H
2O
2 than at the bare Au electrode (
Fig. 4).
Fig. 5 shows the amperometric response of HRP electrodes recorded at 0.05 V as a function of H
2O
2 concentration in 0.1 M phosphate buffer (pH 7.0). The efficiency of HRP-1, HRP-2, HRP-3 and HRP-4 towards electrochemical sensing of H
2O
2 was tested. The current-time transients for the oxidation of increasing concentration of H
2O
2 (
Fig. 5) at HPE-1 indicate that current density increased with increasing in H
2O
2 concentration. A similar observation was noticed for other HPE electrodes also.
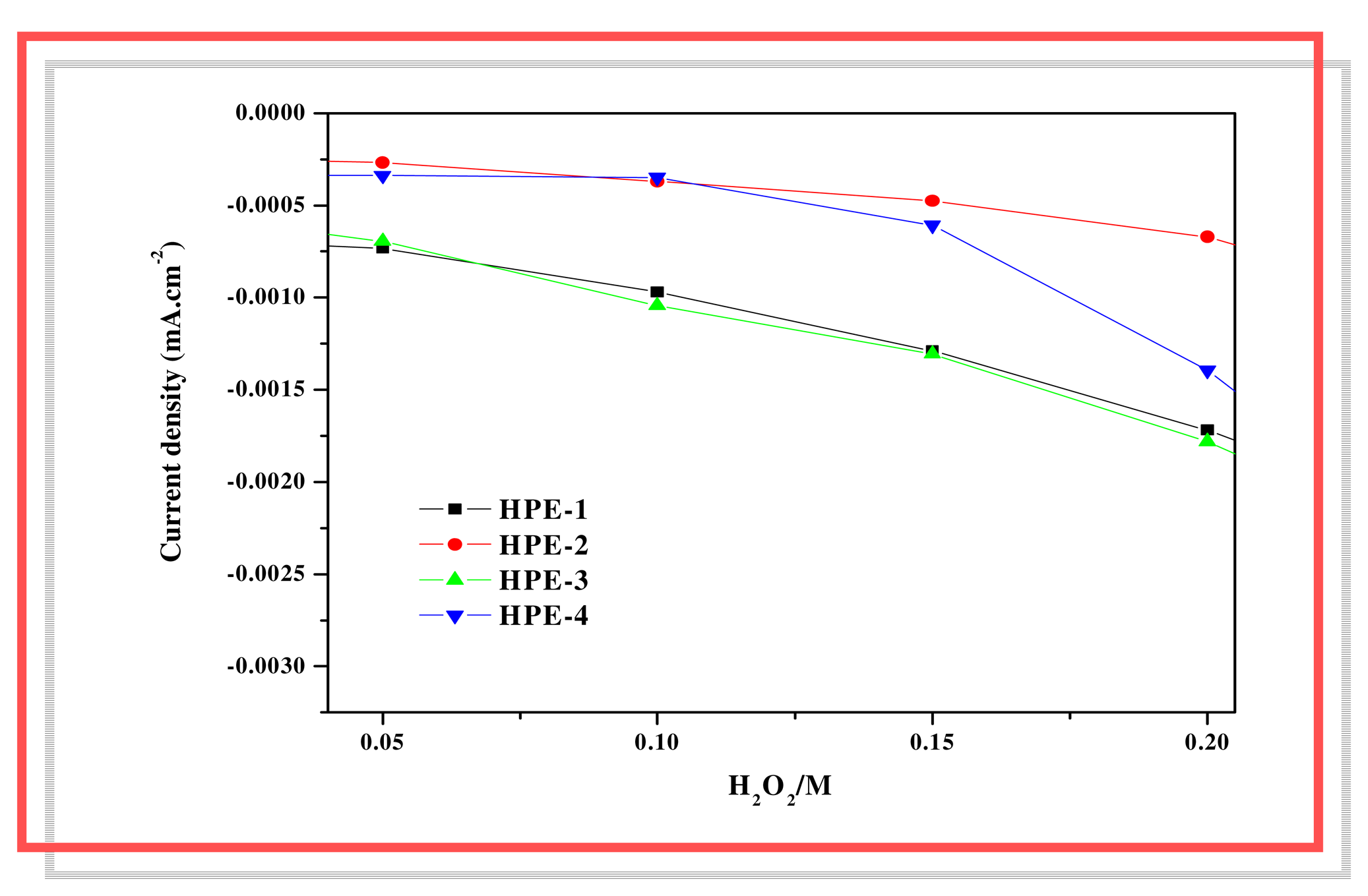
The current densities for the oxidation of H
2O
2 were recorded for various concentration of H
2O
2 at HPE-1, HPE-2, HPE-3 and HPE-4 presented in
Fig. 6. It is to be noticed that covalently functionalized enzyme electrodes (HPE-2 and HPE-4) show lower electrocatalytic current in comparison to physically trapped enzyme electrodes (HPE-1 and HPE-3).