Electrochemical Detection of a Dengue-related Oligonucleotide Sequence Using Ferrocenium as a Hybridization Indicator
Abstract
:1. Introduction
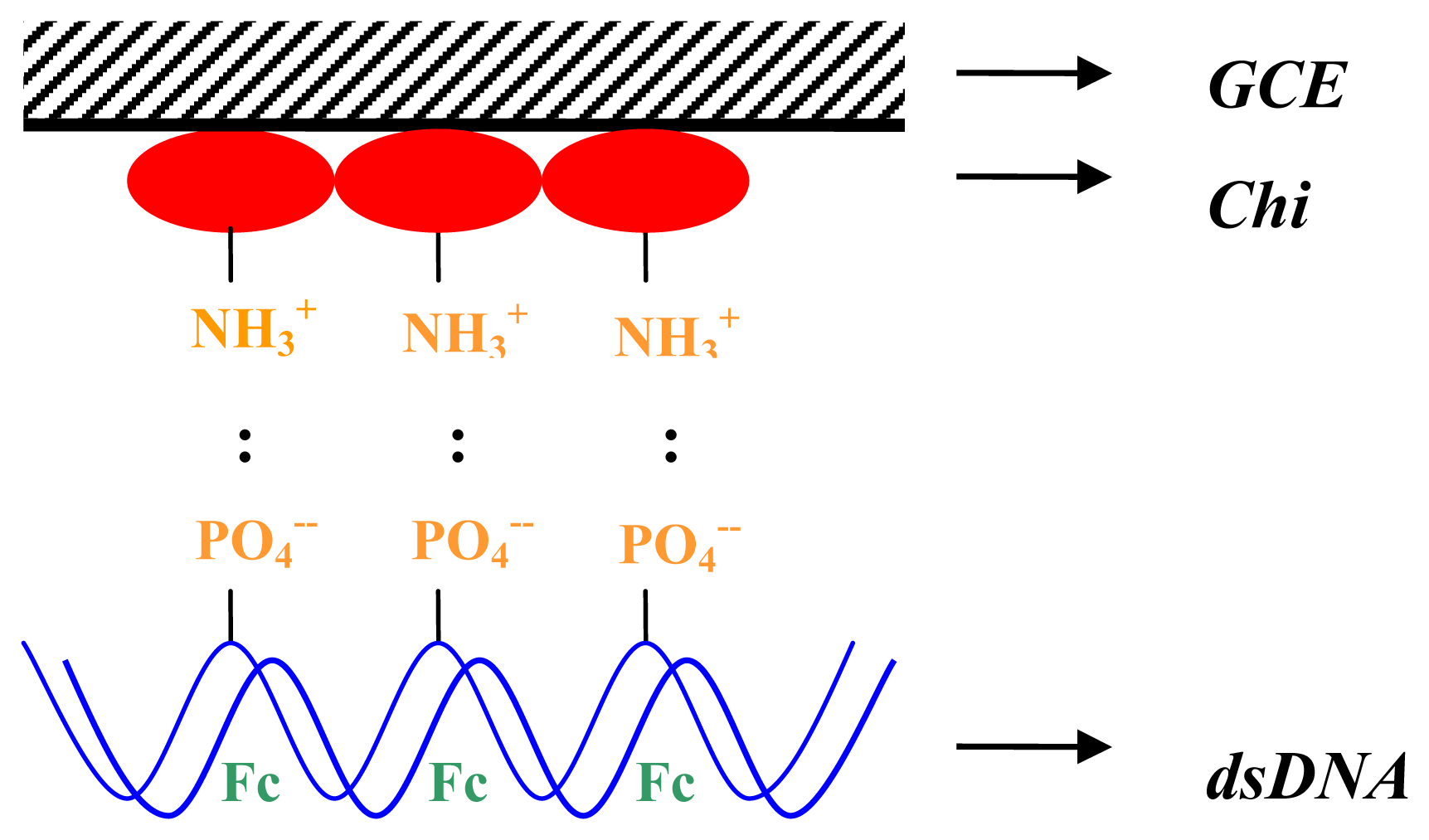
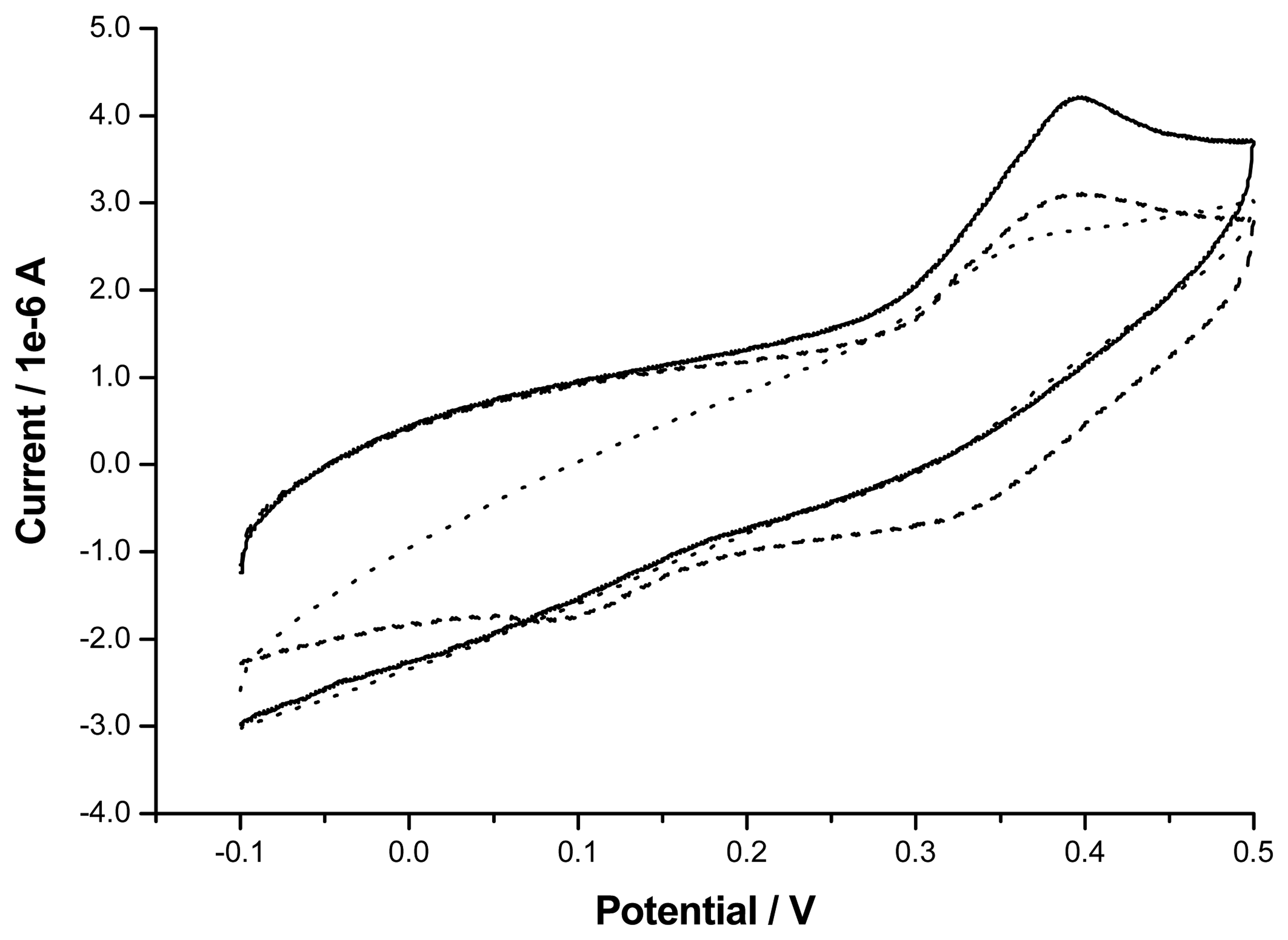
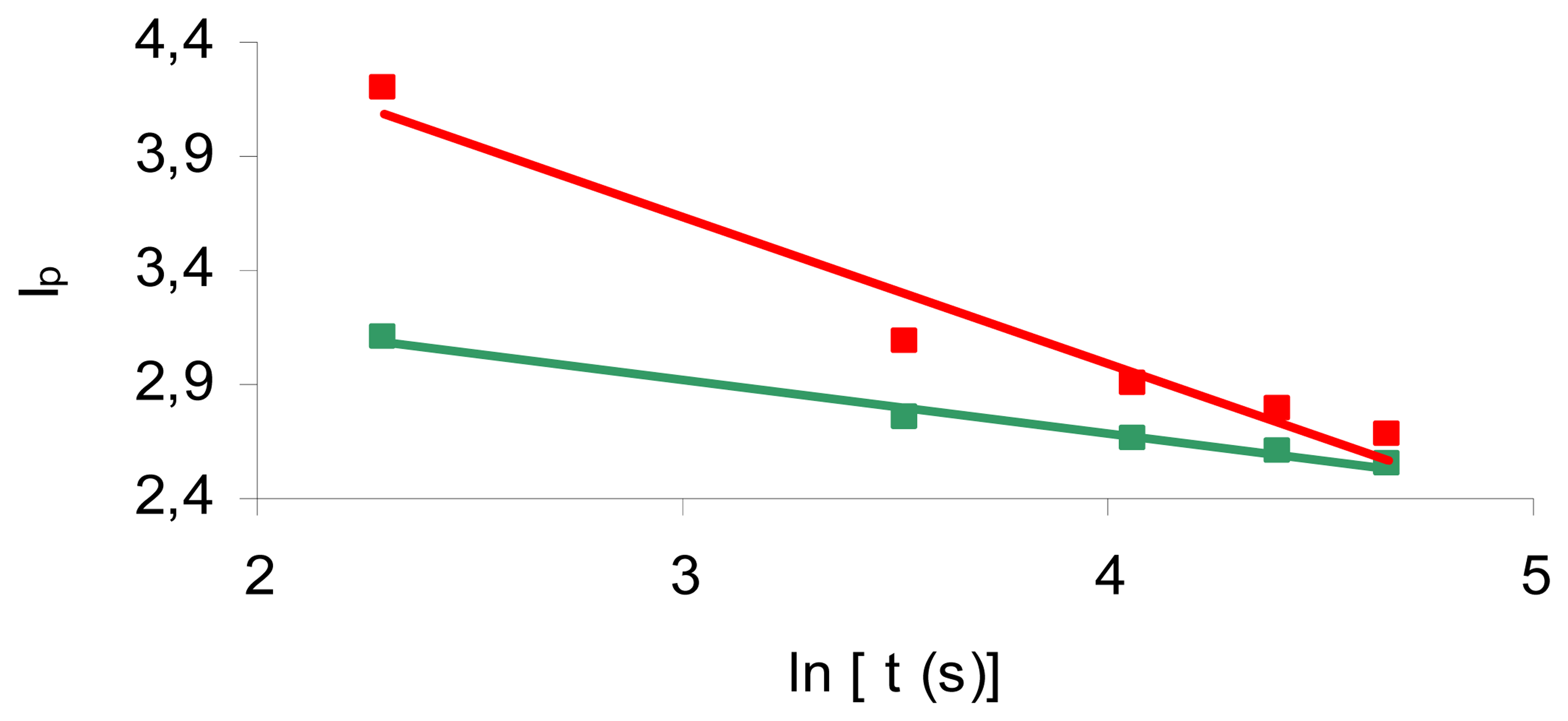
2. Results and Discussion
3. Experimental Section
3.1 Apparatus
3.2 Reagents
3.3. Methods
3.3.1 Electrode preparation
3.3.2 Probe immobilization
3.3.3 Hybridization
3.3.4 Indicator binding and electrochemical detection
4. Conclusions
Acknowledgments
References
- Olejnik, J.; Olejnik, E.K.; Rotschild, K.J. Photocleavable aminotag phosphoramidites for 5′-termini DNA/RNA labeling. Nucleic Acids Res. 1998, 26, 3572–3576. [Google Scholar]
- Girotti, S.; Ferri, E.; Ghini, S.; Musiani, M.; Zerbini, M.L.; Gibellini, D.; Gentilomi, G. Direct quantitative chemiluminescent assays for the detection of viral DNA. Anal. Chim. Acta 1991, 255, 387–394. [Google Scholar]
- Livache, T.; Fouque, B.; Roget, A.; Marchand, J.; Bidan, G.; Teoule, R.; Mathis, G. Polypyrrole DNA chip on a silicon device: example of hepatitis C virus genotyping. Anal. Biochem. 1998, 255, 188–194. [Google Scholar]
- Xu, D.K.; Ma, L.R.; Liu, Y.Q.; Jing, Z.H. Development of chemiluminscent biosensing of nucleic acids based on oligonucleotide-immobilized gold surfaces. Analyst 1999, 124, 533–536. [Google Scholar]
- Mandenius, C.F.; Chollet, A.; Mecklenburg, M.; Lundström, I.; Mosbach, K. Optical surface methods for detection of nucleic acid binding. Anal. Letters 1989, 22, 2961–2973. [Google Scholar]
- Vo-Dinh, T.; Houck, K.; Stokes, D.L. Surface-enhanced Raman gene probes. Anal. Chem. 1994, 66, 3379–3383. [Google Scholar]
- Su, X.; Li, S.F.Y.; Liu, W.; Kwang, J. Piezoelectric quartz cristal based screening test for porcine reproductive and respiratory syndrome virus infection in pigs. Analyst 2000, 125, 725–730. [Google Scholar]
- Su, X.; Li, S.F.Y. Serological determination of Helicobacter pylori infection using sandwiched and enzymatically amplified piezoelectric biosensor. Anal. Chim. Acta 2001, 429, 27–36. [Google Scholar]
- Aizawa, H.; Kurosawa, S.; Tanaka, M.; Yoshimoto, M.; Miyake, J.; Tanaka, H. Rapid diagnosis of Treponema pallidum in serum using latex piezoelectric immunoassay. Anal. Chim. Acta 2001, 437, 167–169. [Google Scholar]
- Zhang, H.; Tan, H.; Wang, R.; Wei, W.; Yao, S. Immobilization of DNA on silver surface of bulk acoustic wave sensor and its application to the study of UV-C damage. Anal. Chim. Acta 1998, 374, 31–38. [Google Scholar]
- Su, H.; Kallury, K.M.R.; Thompson, M.; Roach, A. Interfacial nucleic acid hybridization studied by random primer 32P labeling and liquid-phase acoustic network analysis. Anal. Chem. 1994, 66, 769–777. [Google Scholar]
- Xu, C.; Cai, H.; Xu, Q.; He, P.; Fang, Y. Characterization of single-stranded DNA on chitosan-modified electrode and its application to the sequence-specific DNA detection. Fresenius J. Anal. Chem. 2001, 369, 428–432. [Google Scholar]
- Zhao, Y.-D.; Pang, D.-W.; Wang, Z.-L.; Cheng, J.-K.; Qi, Y.-P. DNA-modified electrodes. Part 2. Electrochemical characterization of gold electrodes modified with DNA. J. Electroanal. Chem. 1997, 431, 203–209. [Google Scholar]
- Millan, K.M.; Mikkelsen, S.R. Sequence-selective biosensor for DNA based on electroactive hybridization indicators. Anal. Chem. 1993, 65, 2317–2323. [Google Scholar]
- Millan, K.M.; Saraullo, A.; Mikkelsen, S.R. Voltammetric DNA biosensor for cystic fibrosis based on a modified carbon paste electrode. Anal. Chem. 1994, 66, 2943–2948. [Google Scholar]
- Liu, S.; Ye, J.; He, P.; Fang, Y. Voltammetric determination of sequence-specific DNA by electroactive intercalator on graphite electrode. Anal. Chim. Acta 1996, 335, 239–243. [Google Scholar]
- Erdem, A.; Kerman, K.; Meric, B.; Akarca, U.S.; Ozsoz, M. DNA electrochemical biosensor for the detection of short DNA sequences related to the hepatitis B virus. Electroanalysis 1999, 11, 586–588. [Google Scholar]
- Wang, J.; Cai, X.; Rivas, G.; Shiraishi, H.; Farias, P.A.M.; Dontha, N. DNA electrochemical biosensor for the detection of short DNA sequences related to the human immunodeficiency virus. Anal. Chem. 1996, 68, 2629–2634. [Google Scholar]
- Hashimoto, K.; Ito, K.; Ishimori, Y. Sequence-specific gene detection with a gold electrode modified with DNA probes and an electrochemically active dye. Anal. Chem. 1994, 66, 3830–3833. [Google Scholar]
- Sun, X.; He, P.; Liu, S.; Ye, J.; Fang, Y. Immobilization of single-stranded deoxyribonucleic acid on gold electrode with self-assembled aminoethanethiol monolayer for DNA electrochemical sensor applications. Talanta 1998, 47, 487–495. [Google Scholar]
- Kerman, K.; Oznan, D.; Kara, P.; Meric, B.; Gooding, J.J.; Ozsoz, M. Voltammetric determination of DNA hybridization using methylene blue and self-assembled alkanethiol monolayer on gold electrodes. Anal. Chim. Acta 2002, 462, 39–47. [Google Scholar]
- Xu, C.; He, P.; Fang, Y. Electrochemical labeled DNA probe for the detection of sequence-specific DNA. Anal. Chim. Acta 2000, 411, 31–36. [Google Scholar]
- Xu, C.; Cai, H.; He, P.; Fang, Y. Electrochemical detection of sequence-specific DNA using a DNA probe labeled with aminoferrocene and chitosan modified electrode immobilized with ssDNA. Analyst 2001, 126, 62–65. [Google Scholar]
- Hayatsu, H.; Tanaka, Y.; Negishi, K. Preparation of DNA-chitosan columns and their applications: binding of carcinogens to the column. Nucleic Acids Symp. Ser. 1997, 37, 139–140. [Google Scholar]
- Ju, H.; Ye, B.; Gu, J. Supermolecular interaction of ferrocenium with yeast DNA and application in electrochemical sensing for hybridization recognition of yeast DNA. Sensors 2004, 4, 71–83. [Google Scholar]
- Houng, H.H.-S.; Chen, R.C.-M.; Vaughn, D.W.; Kanesa-thasan, N. Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1-4 using conserved and serotype-specific 3′ noncoding sequences. J. Virol. Methods 2001, 95, 19–32. [Google Scholar]
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ribeiro Teles, F.R.; França dos Prazeres, D.M.; De Lima-Filho, J.L. Electrochemical Detection of a Dengue-related Oligonucleotide Sequence Using Ferrocenium as a Hybridization Indicator. Sensors 2007, 7, 2510-2518. https://doi.org/10.3390/s7112510
Ribeiro Teles FR, França dos Prazeres DM, De Lima-Filho JL. Electrochemical Detection of a Dengue-related Oligonucleotide Sequence Using Ferrocenium as a Hybridization Indicator. Sensors. 2007; 7(11):2510-2518. https://doi.org/10.3390/s7112510
Chicago/Turabian StyleRibeiro Teles, Fernando Rodrigues, Duarte Miguel França dos Prazeres, and José Luiz De Lima-Filho. 2007. "Electrochemical Detection of a Dengue-related Oligonucleotide Sequence Using Ferrocenium as a Hybridization Indicator" Sensors 7, no. 11: 2510-2518. https://doi.org/10.3390/s7112510



