Supramolecular Based Membrane Sensors
Abstract
:1. Introduction
2. Classification of Supramolecular Families
2.1. Linear Components for Supramolecular Networks
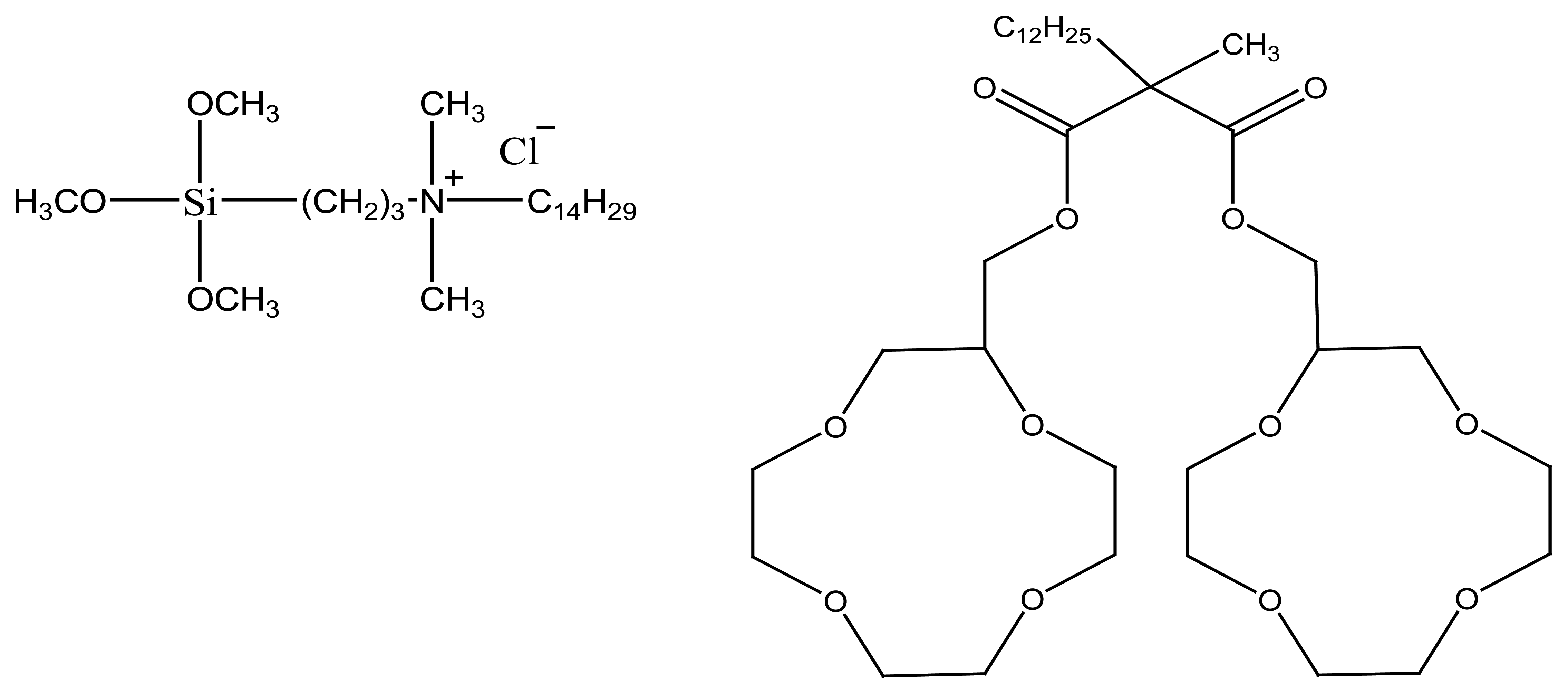
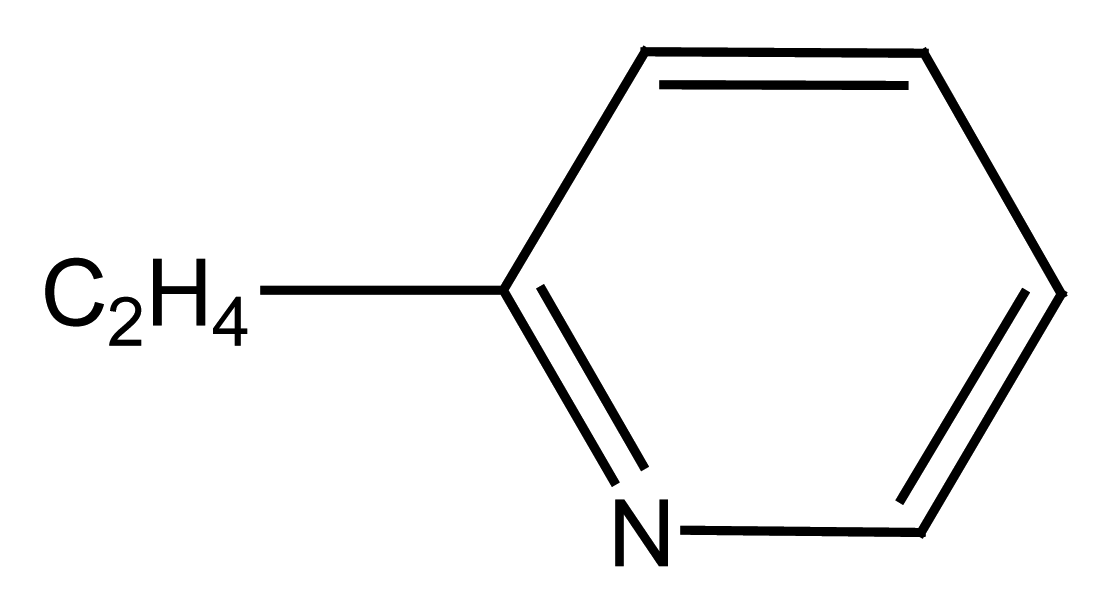
2.1.1. Podands
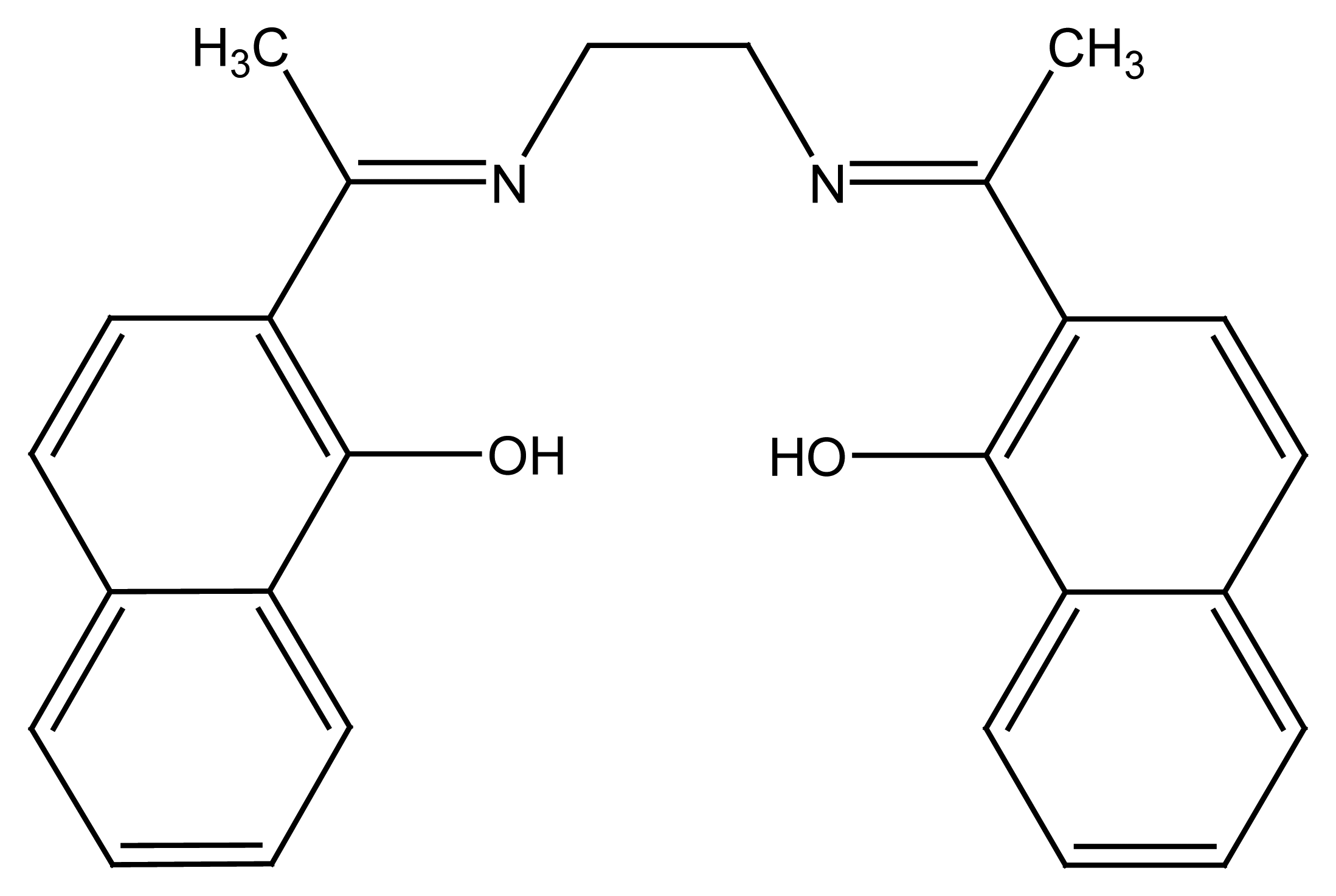
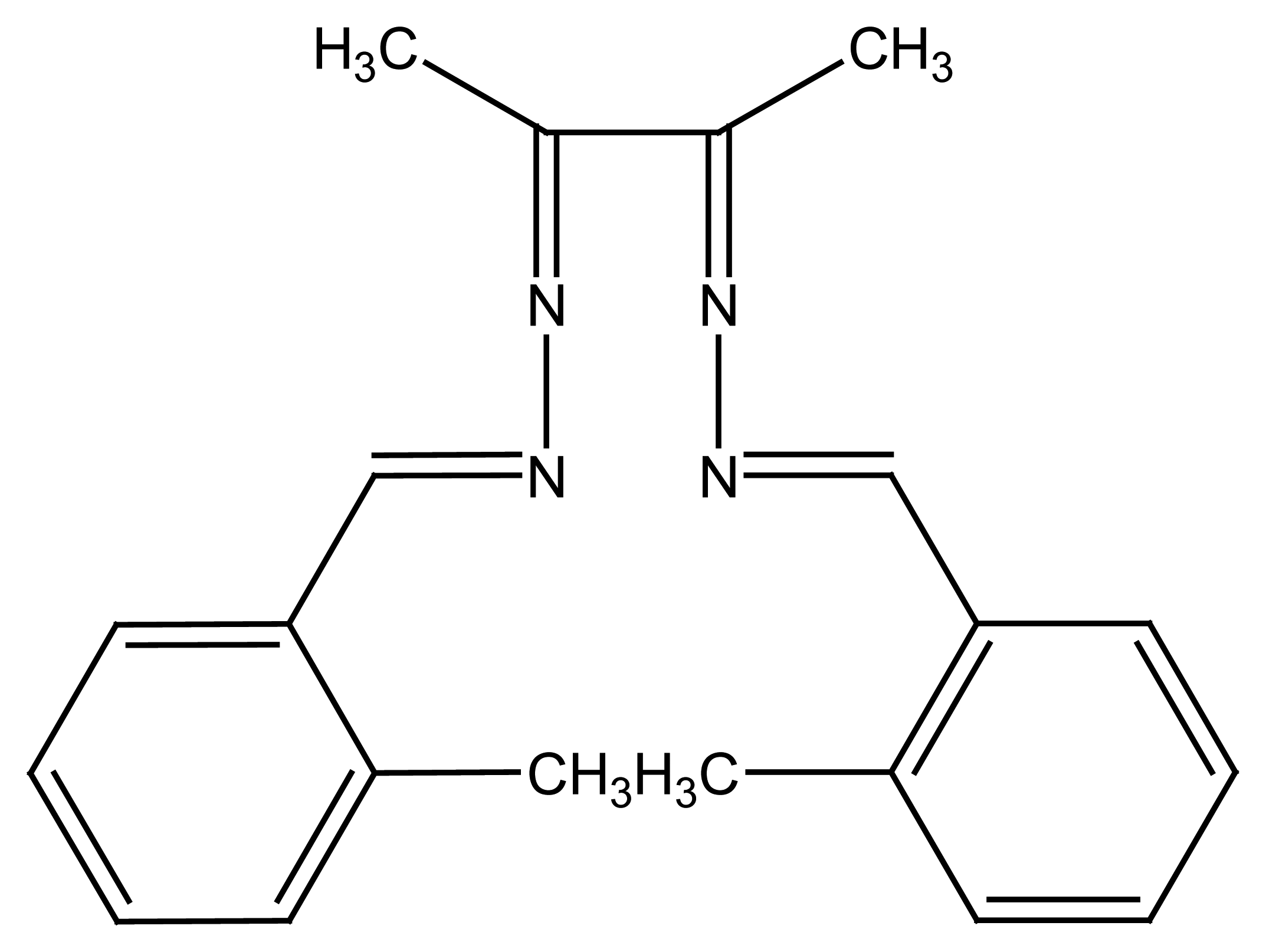
2.1.2. Rigid Components from Schiff's Bases
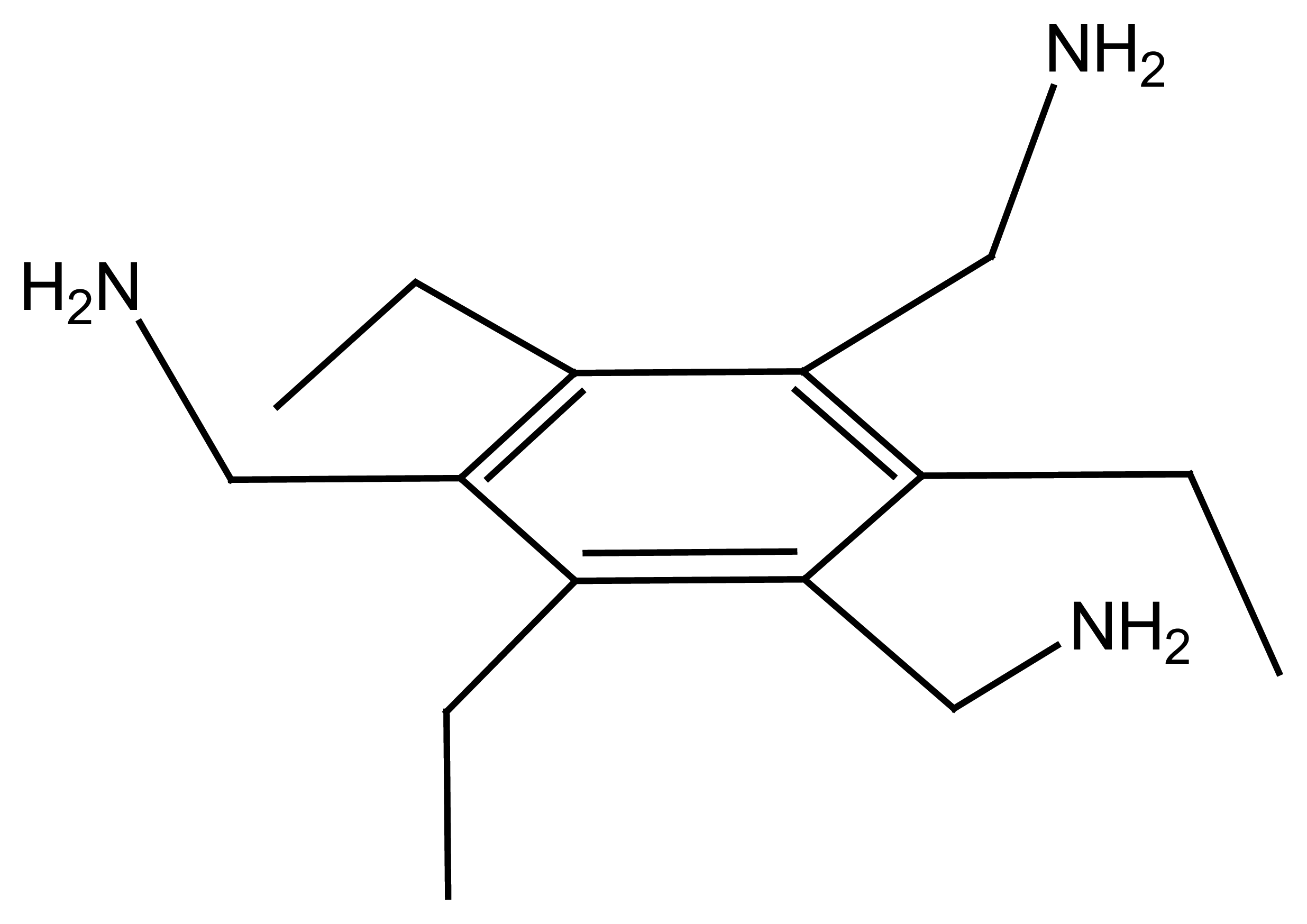
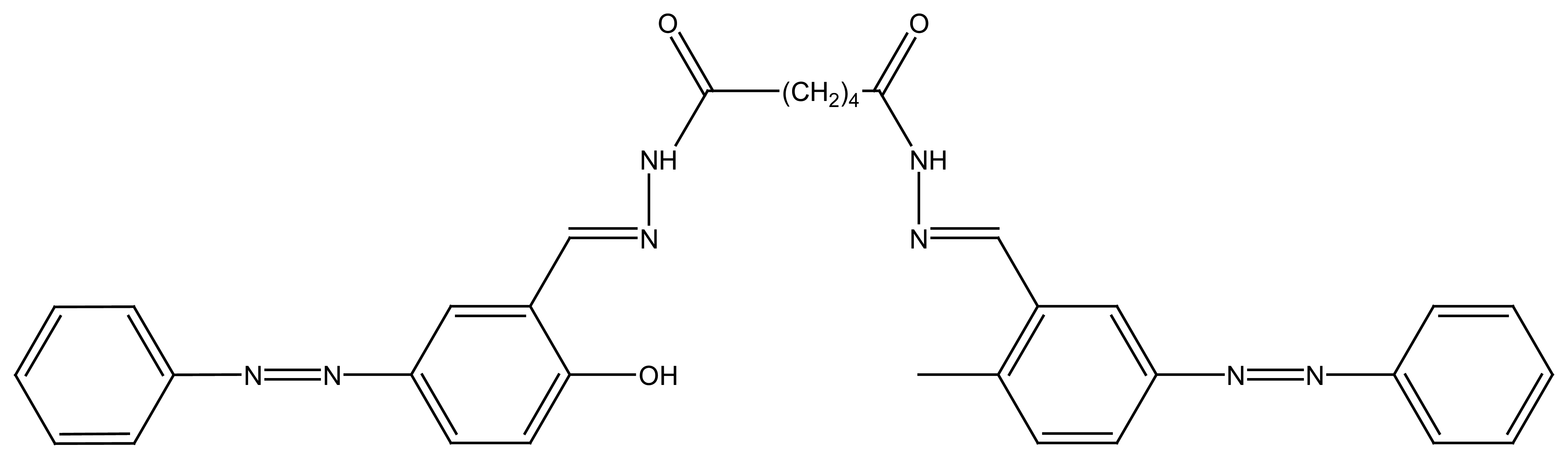
2.1.3. Flexible Tripod Ligands
2.1.4. Rigid Tripod Ligands
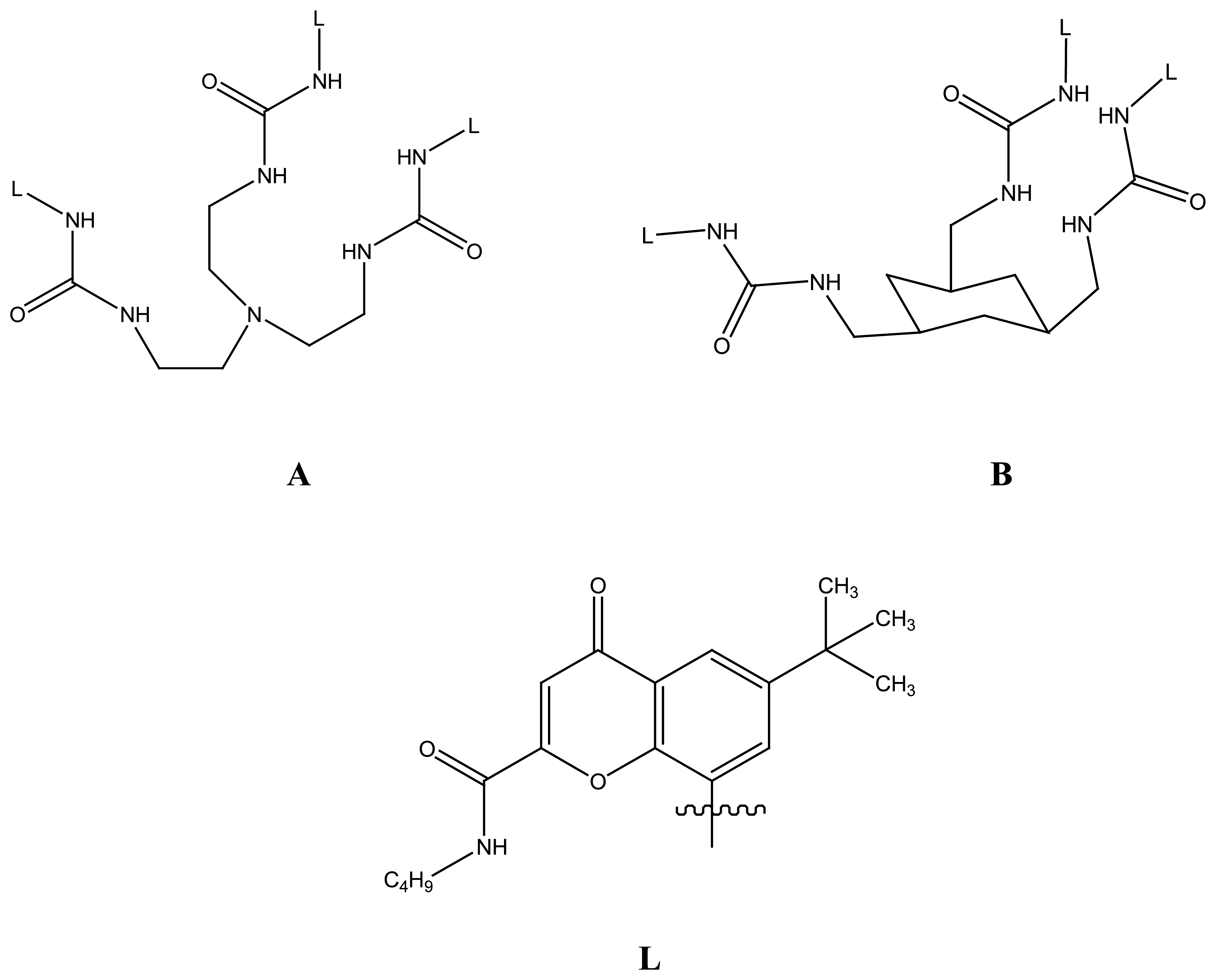
2.1.5. Guandinium Based Linear Anionic Hosts
2.2. Cyclic Compounds
2.2.1. Naturally Occurring Planar Microcycles
2.2.2. Synthetic Planar Microcycles
2.2.3. Catananes and Rotaxanes
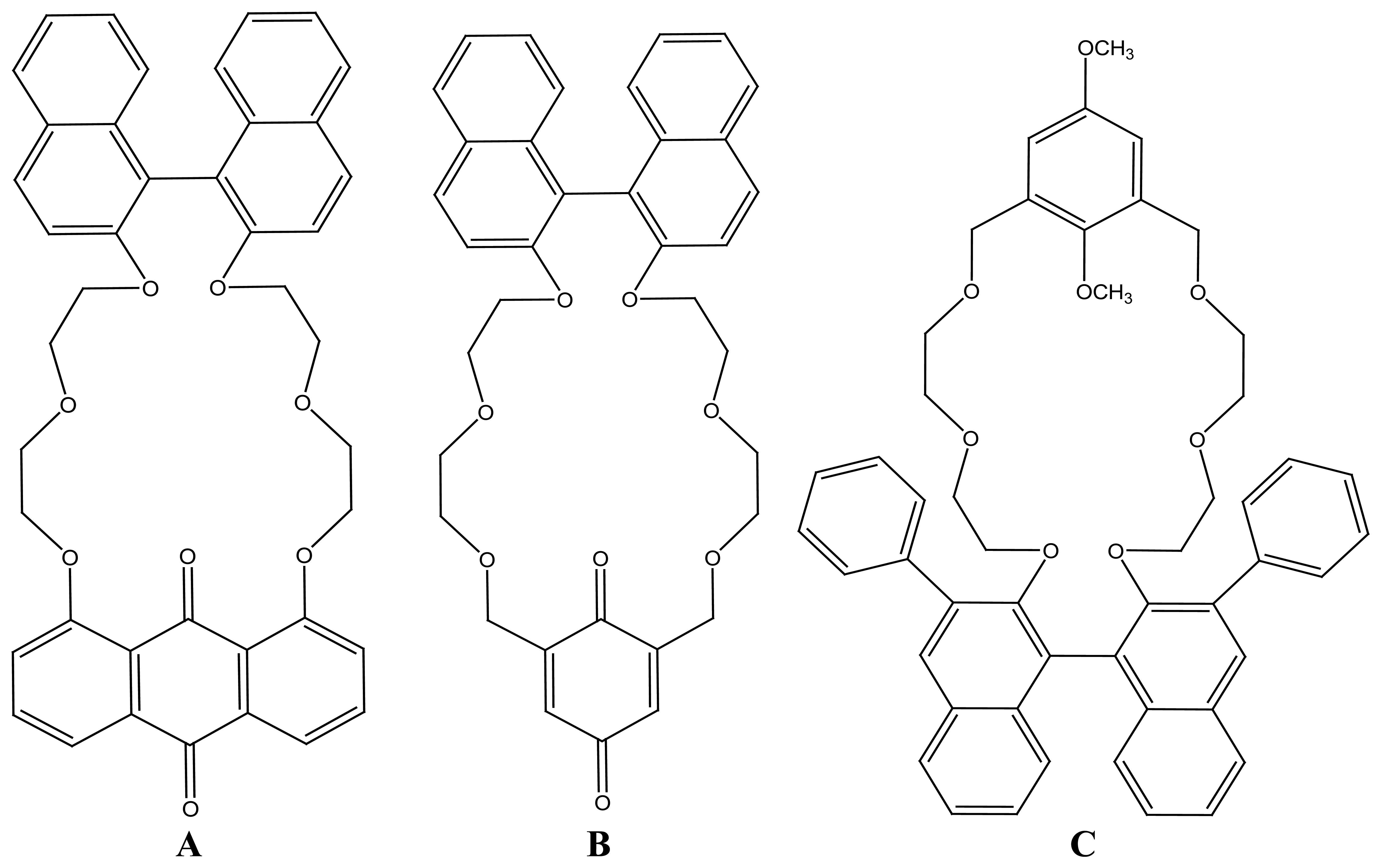
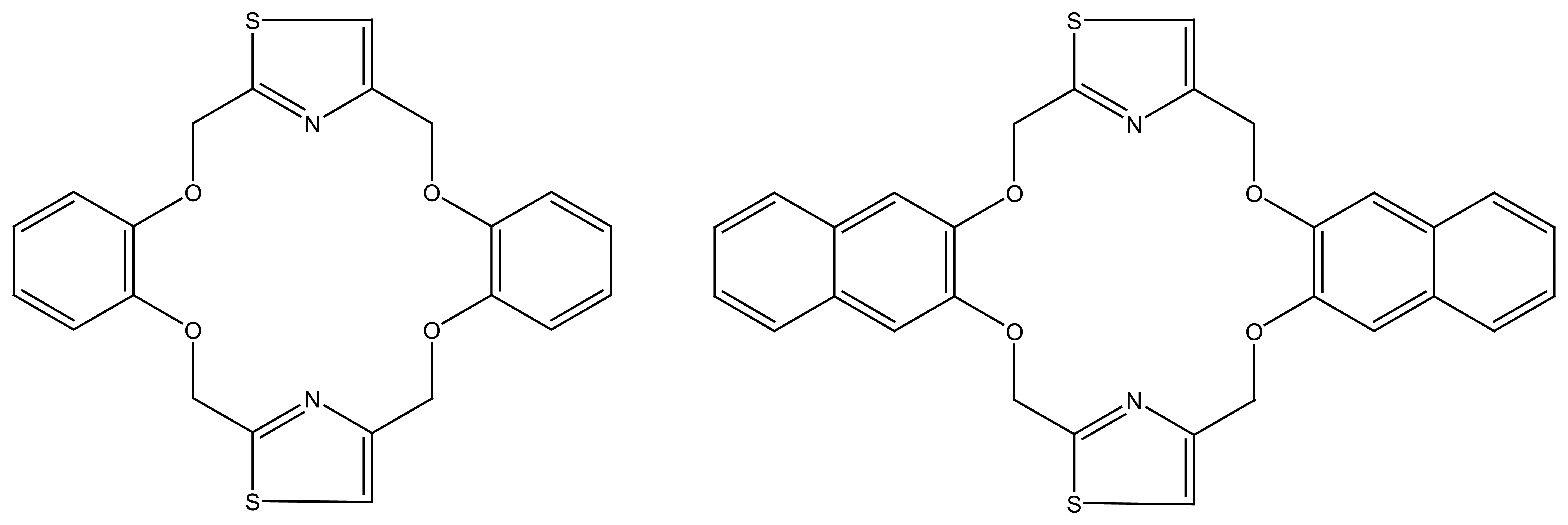
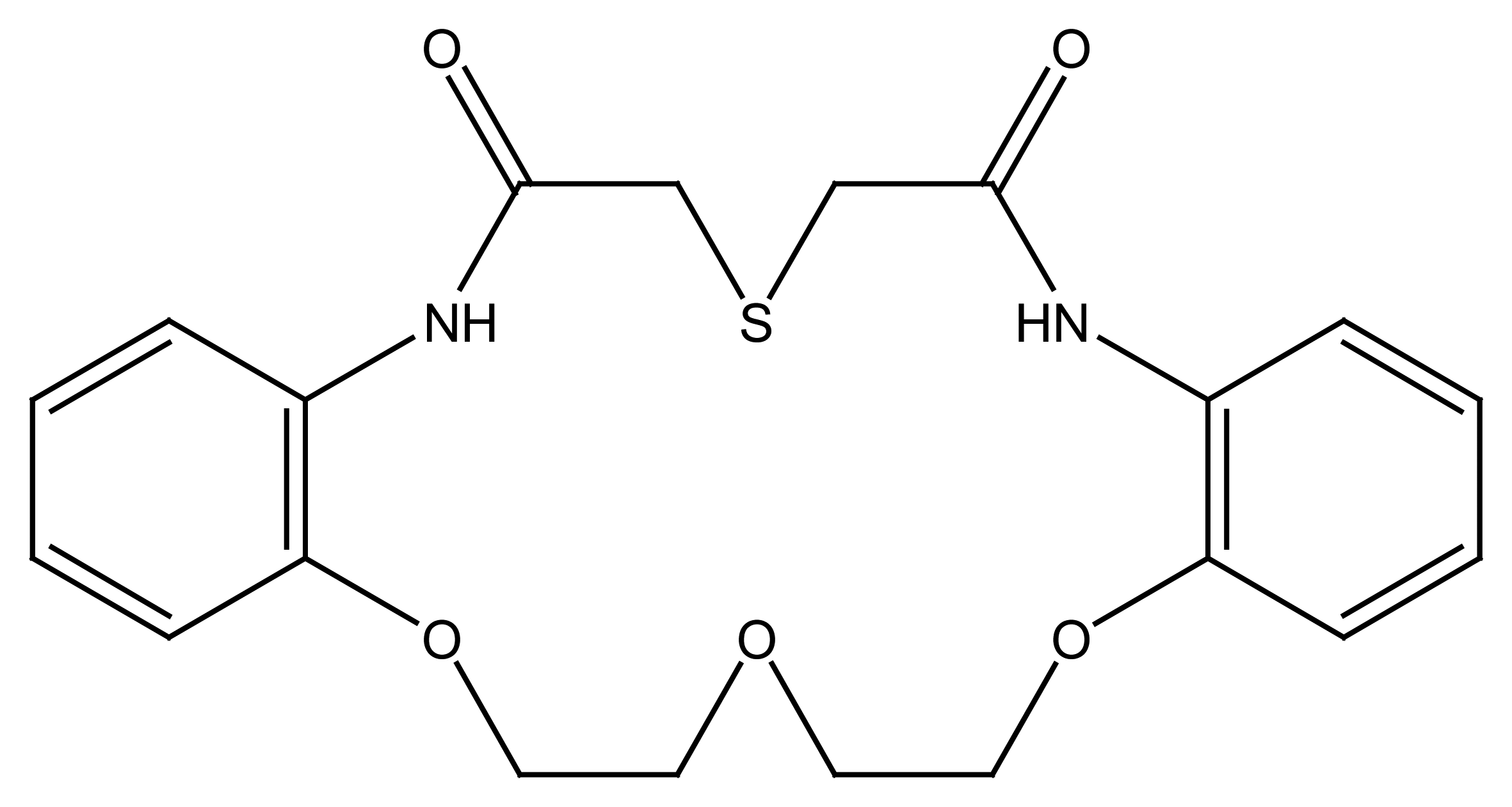
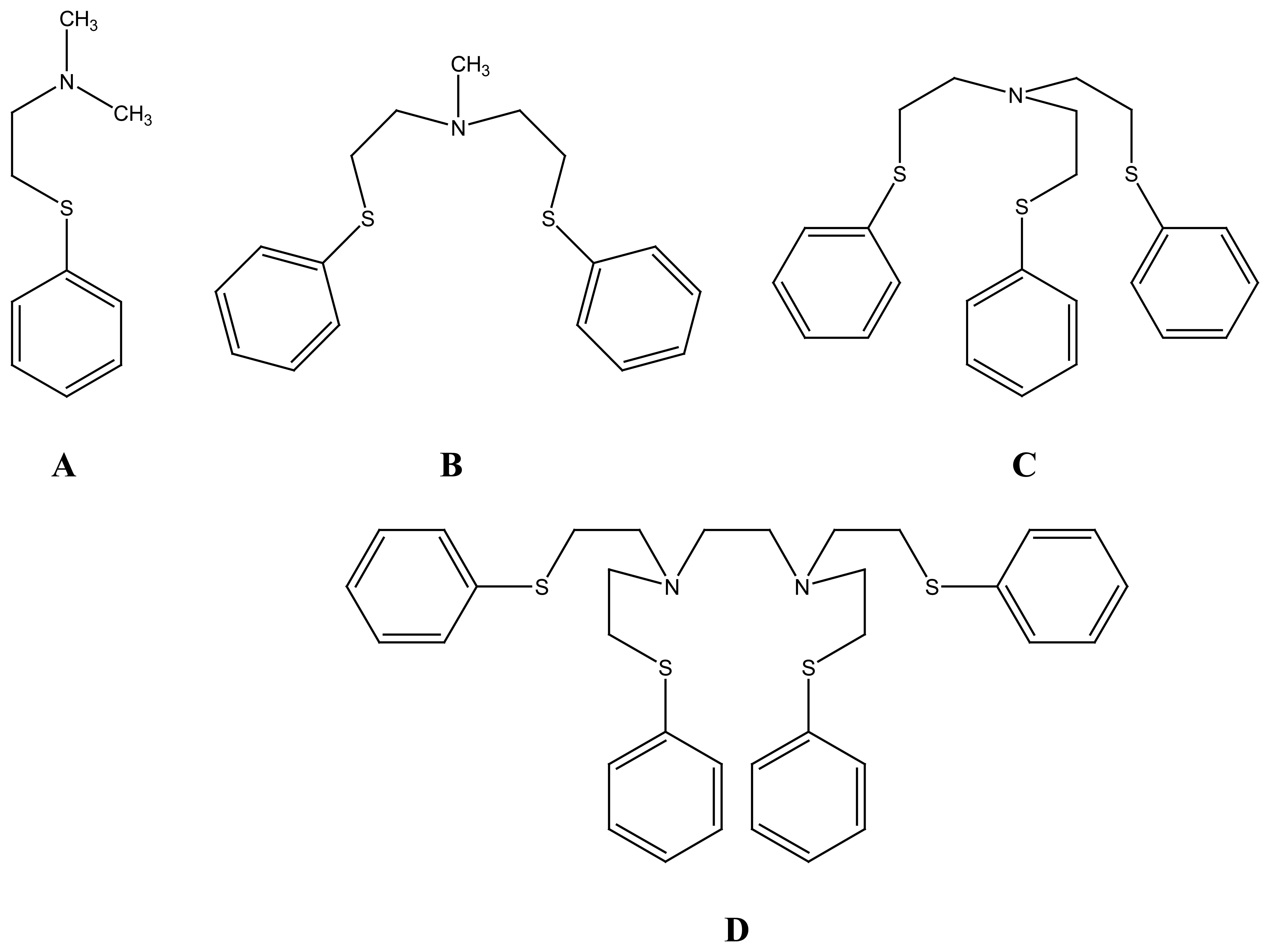
2.2.4. Other Macrocycles
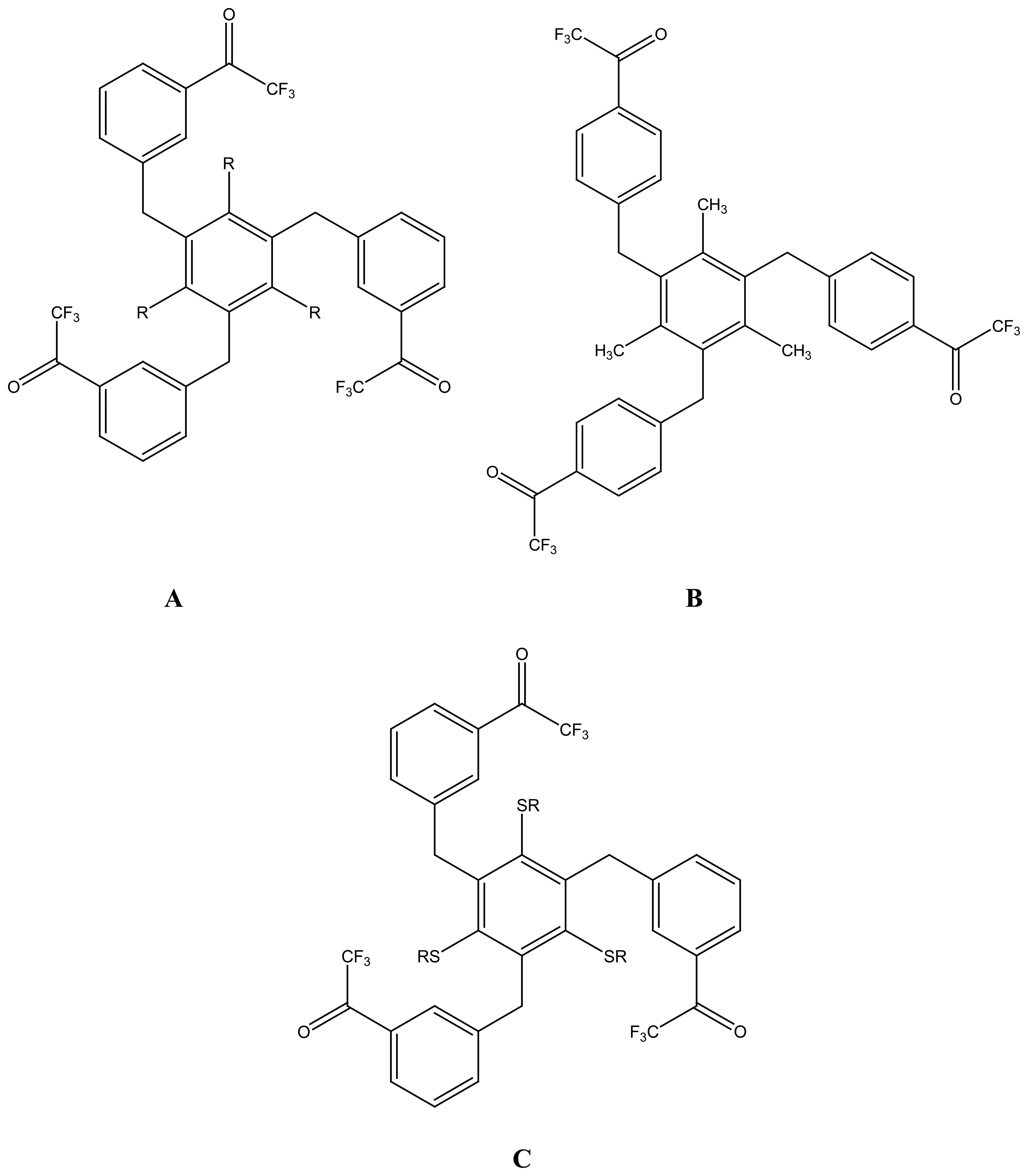
2.3. Molecular Baskets and Cages
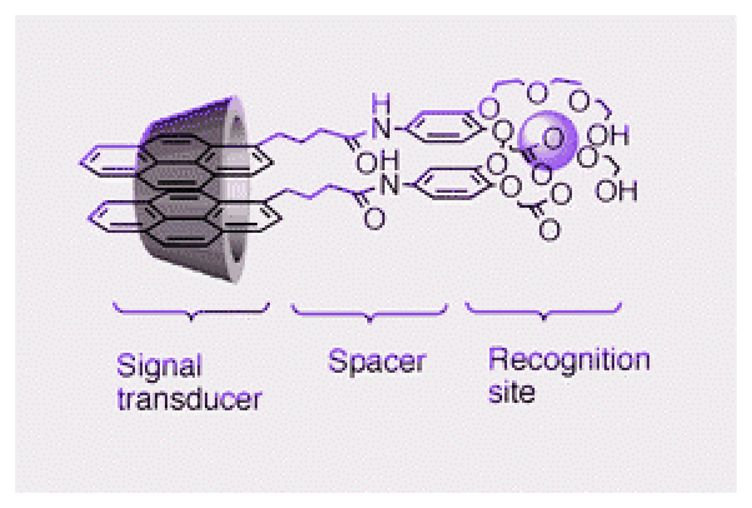
3. Construction of Supramolecule Based Ion Sensors
- 1)
- The size of the cavity of the host should be large enough to accommodate the guest species. As the complexation happens, the hydration shell of the target species is removed and substituted by the donor atoms of the host (ligand).
- 2)
- The number of donor atoms in the ligand should be sufficient, to match the coordination number of the target species.
- 3)
- The flexibility of holding of donor atoms by the host backbone must be limited, so that their positions are suitable to match the shape of the coordination sphere of the target species.
- 4)
- Using the macrocyclic backbone, an excess of certain ligating groups can be avoided, thus improving the selectivity. For example in the case of a divalent cationic target two ionizable groups can be introduced into the host, which could hold more of them. The remaining two binding groups fill up the coordination sphere.
- 5)
- There is a possibility to attach chelating moieties onto host molecules, thus combining the chelate and the macrocyclic effect. However, the chelating groups increase the flexibility of the ligand and may reduce the overall selectivity.
- 6)
- Branched groups can be attached to proper cites of the host for high hydrophobicity and avoiding crystallized membrane phases.
4. Application of the Concept of Supramolecular Chemistry in the Design of Liquid Membrane Sensors for the Potentiometric Detection of Ionic Species
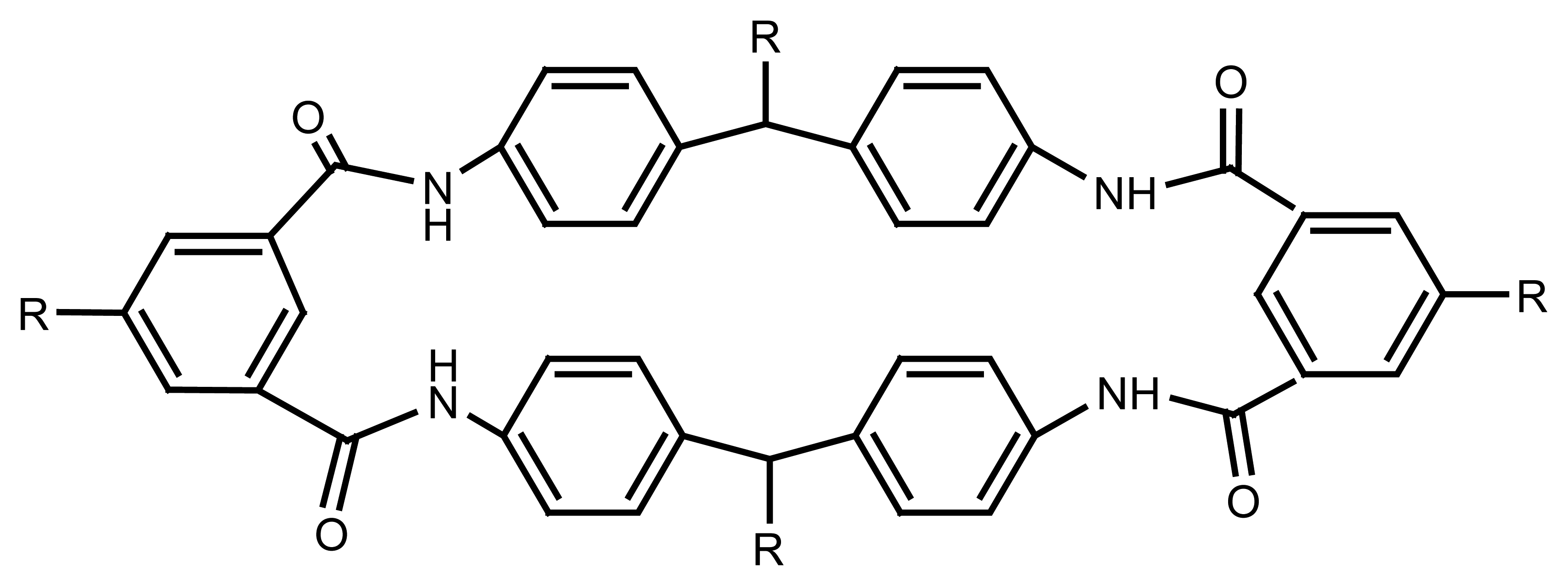
4.1. Calixarene Based Ion Selective Sensors
4.1.1. Calixarene-Based Main Group ion Selective Electrodes
4.1.2. Calixarene-Based Transition Metal Ion Selective Electrodes
4.1.3. Calixarene-Based Lanthanide and Actinide Ion Selective Electrodes
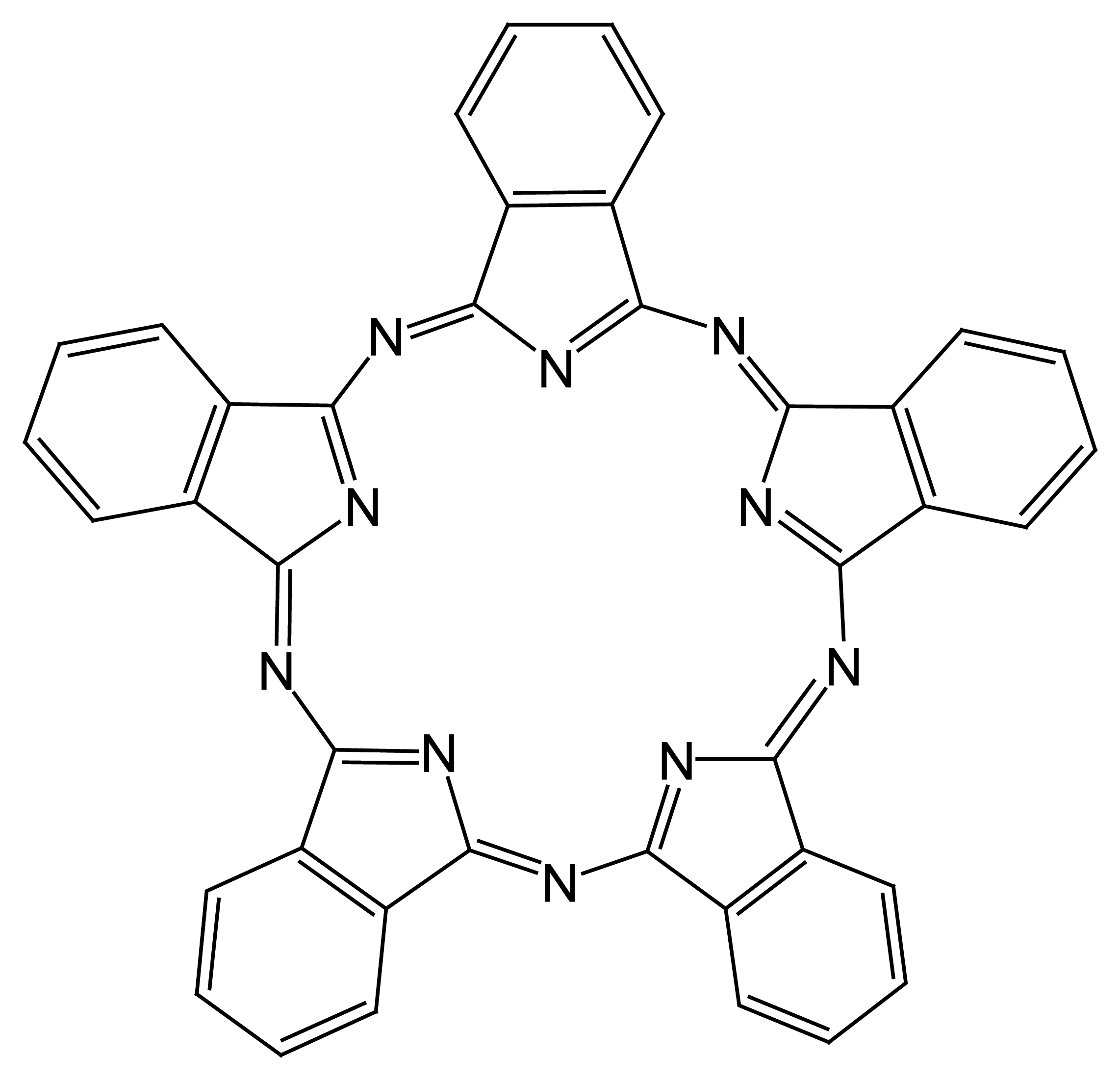
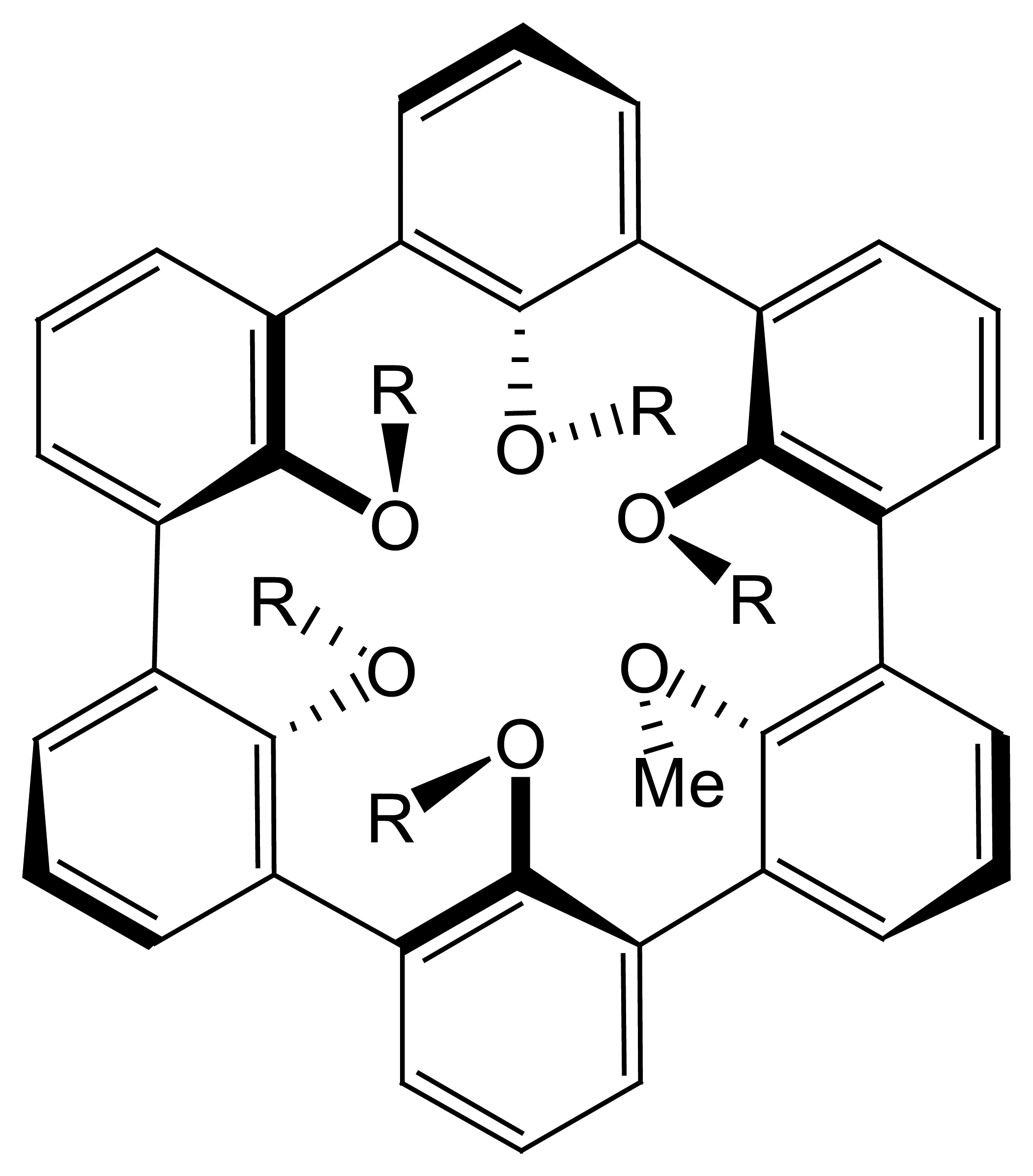
4.2. Porphyrin Based Ion Selective Sensors
4.2.1. Porphyrin Based Main Group Anion Selective Electrodes
4.2.2. Porphyrin-Based Transition Metal, and Lanthanide Selective Electrodes
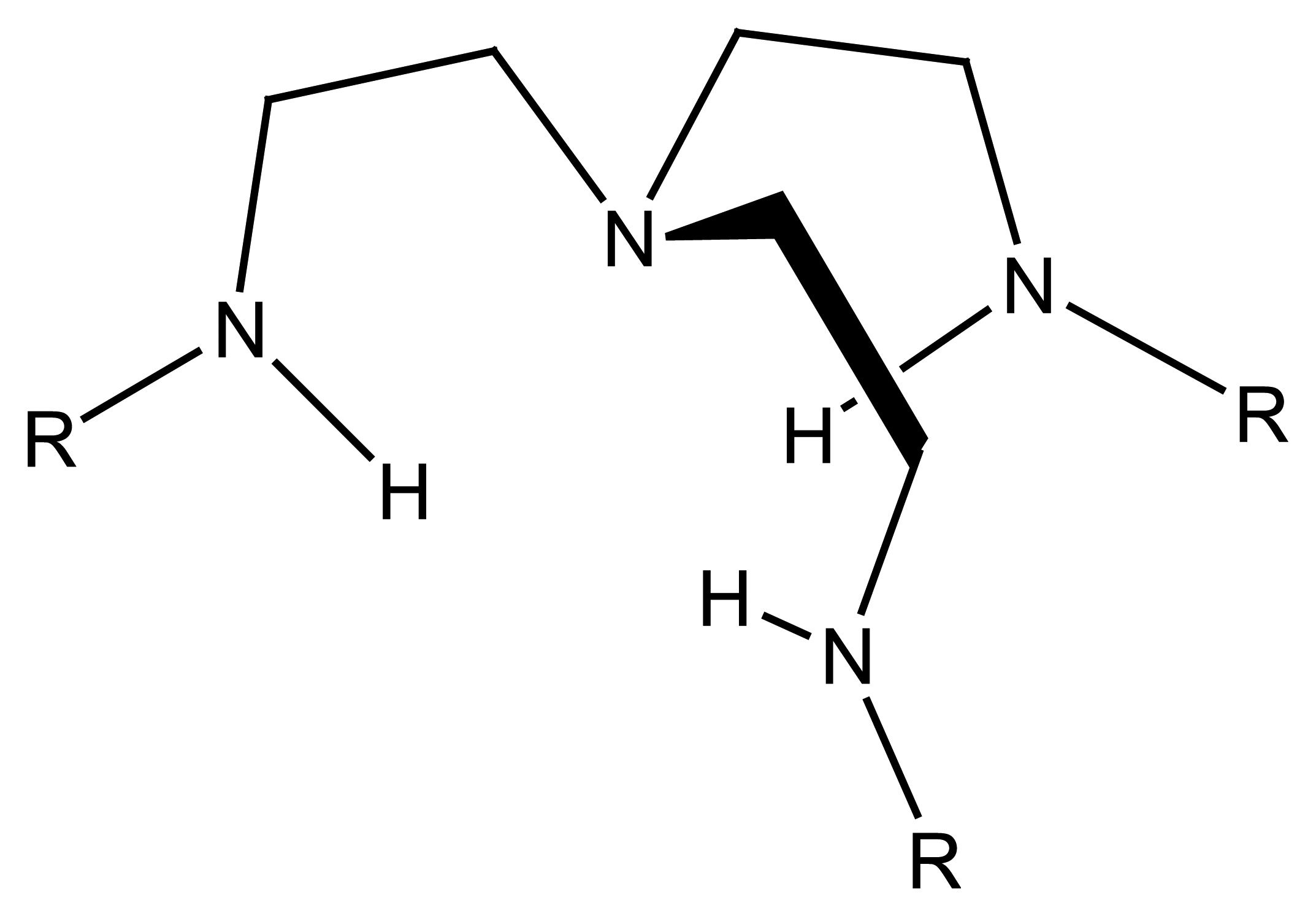
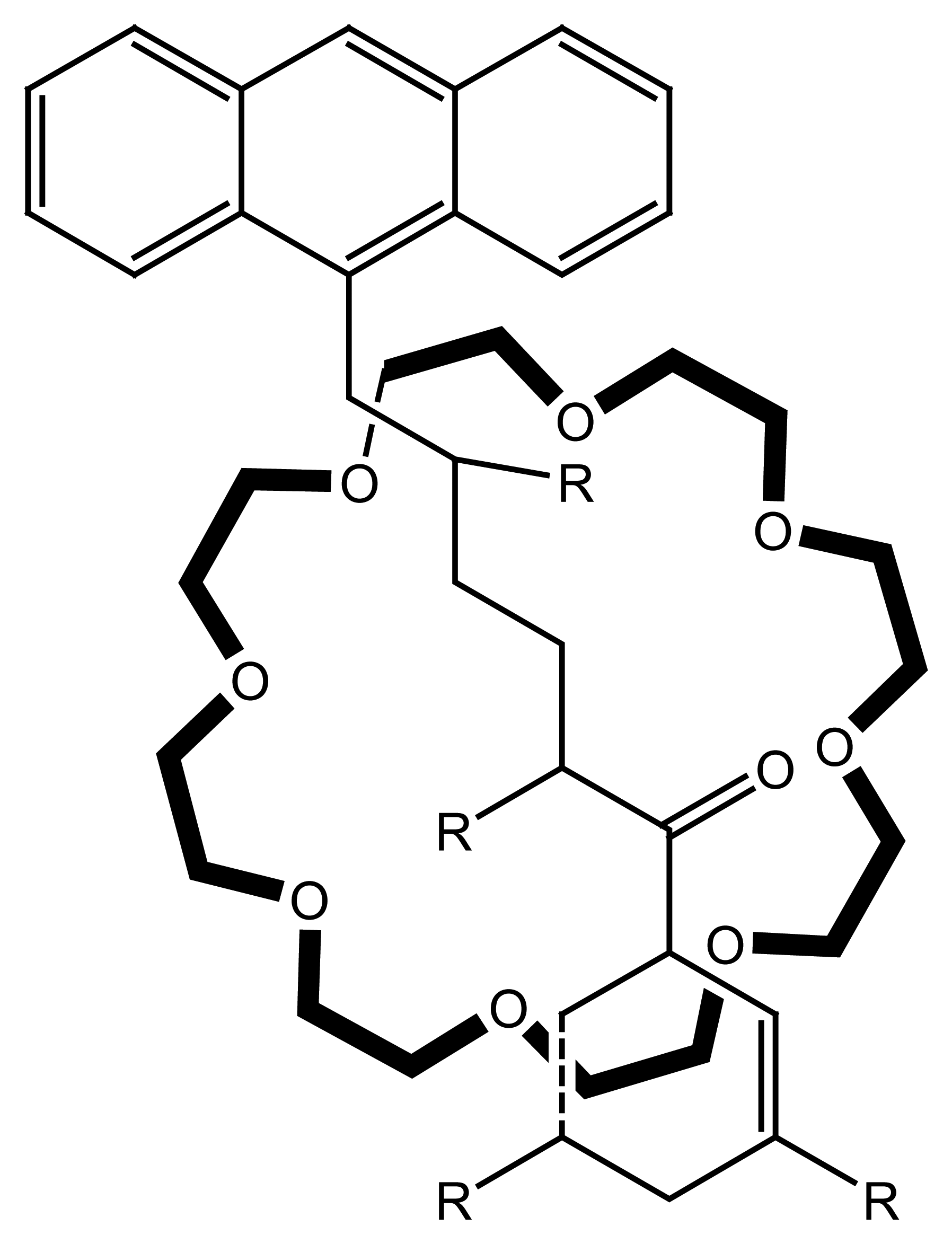
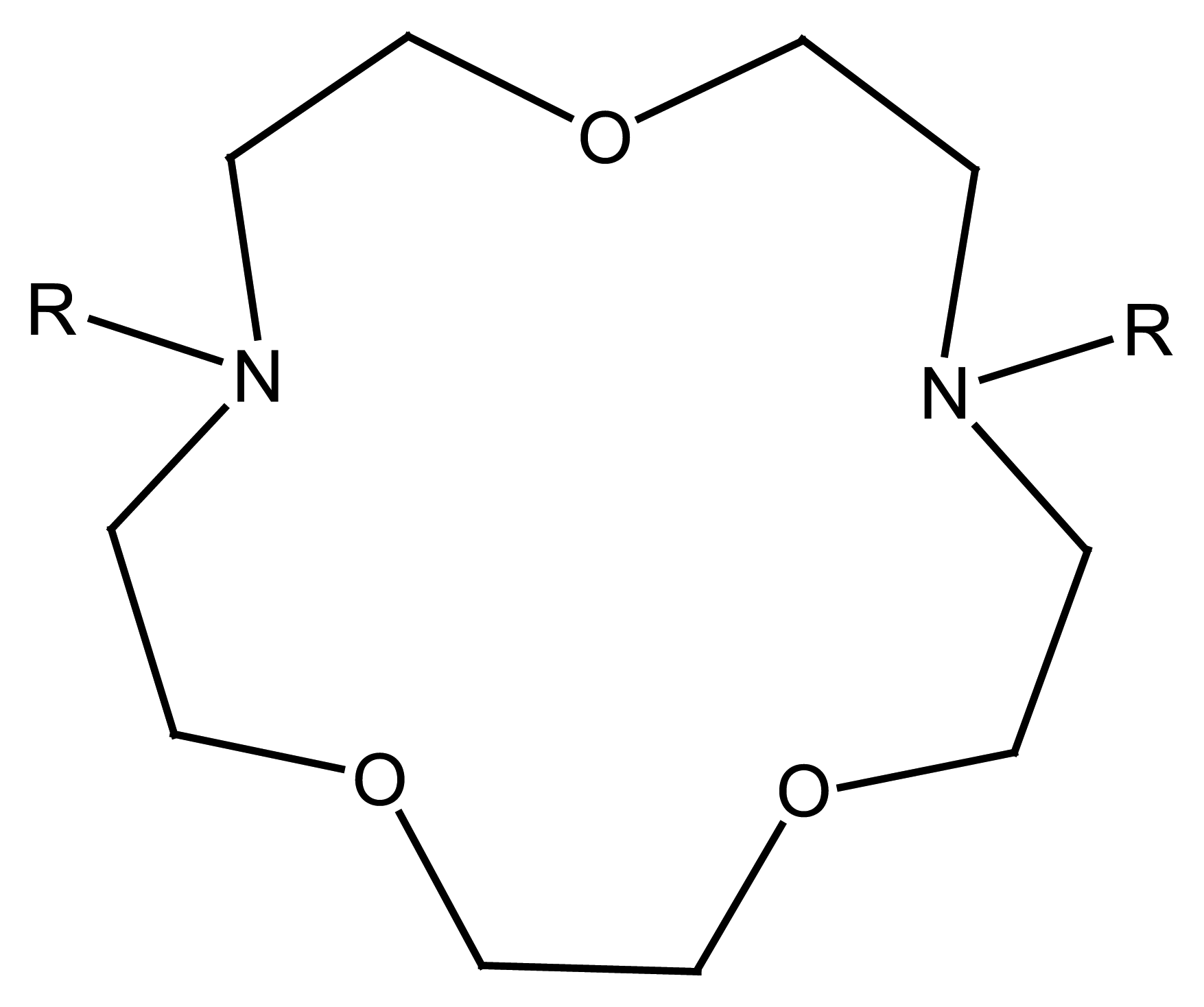
4.3. Cryptand Based Ion Selective Sensors
4.3.1. Cryptand Based Main Group Ion Selective Electrodes
4.3.2. Cryptand Based Transition Metal Selective Electrodes
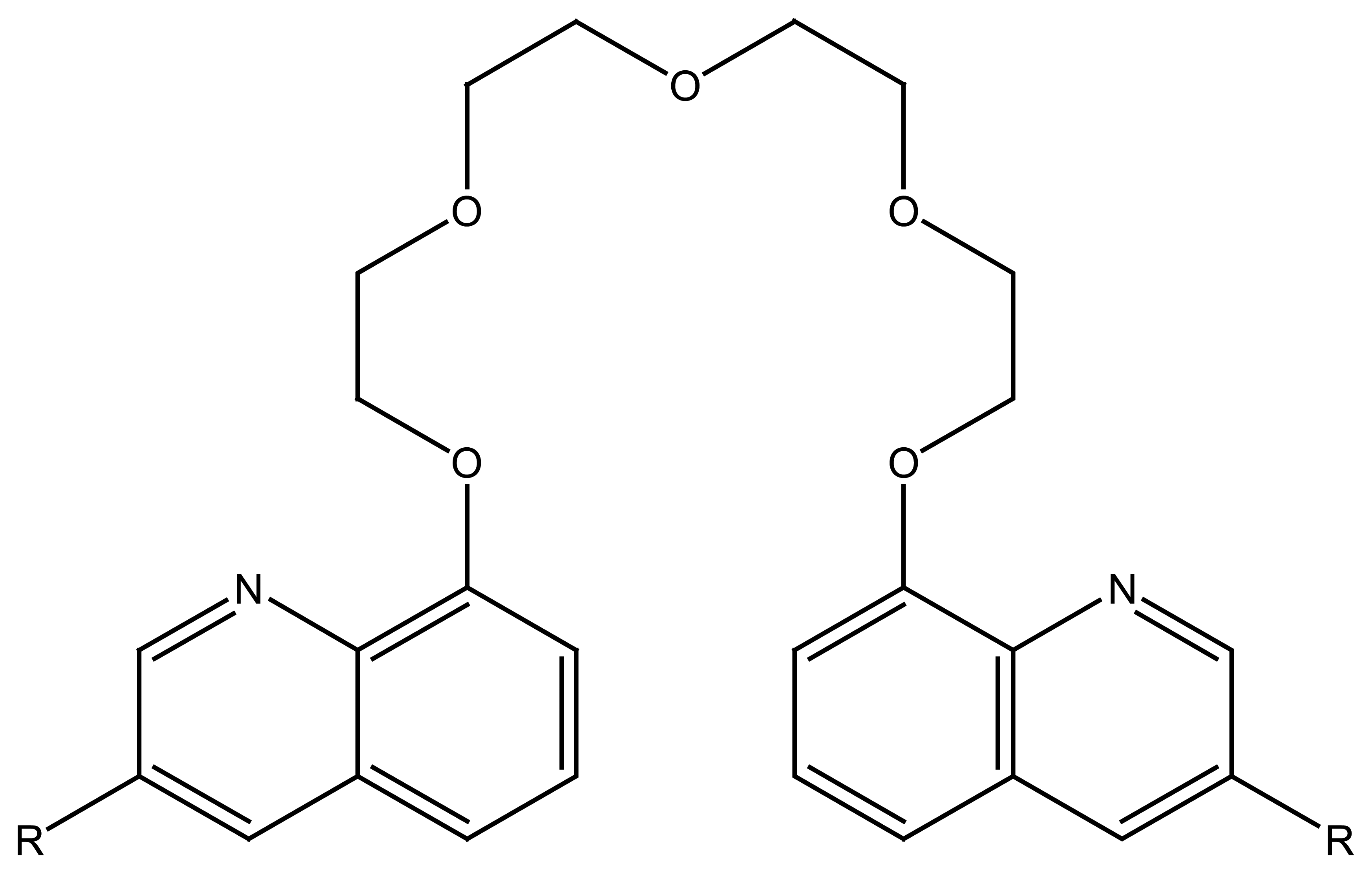
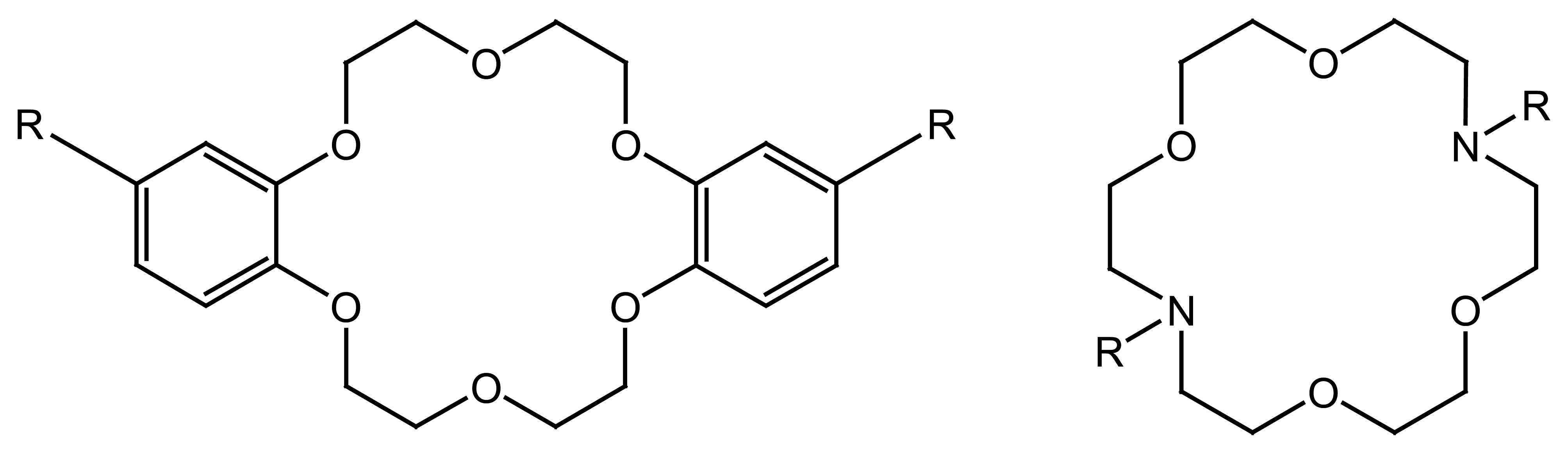
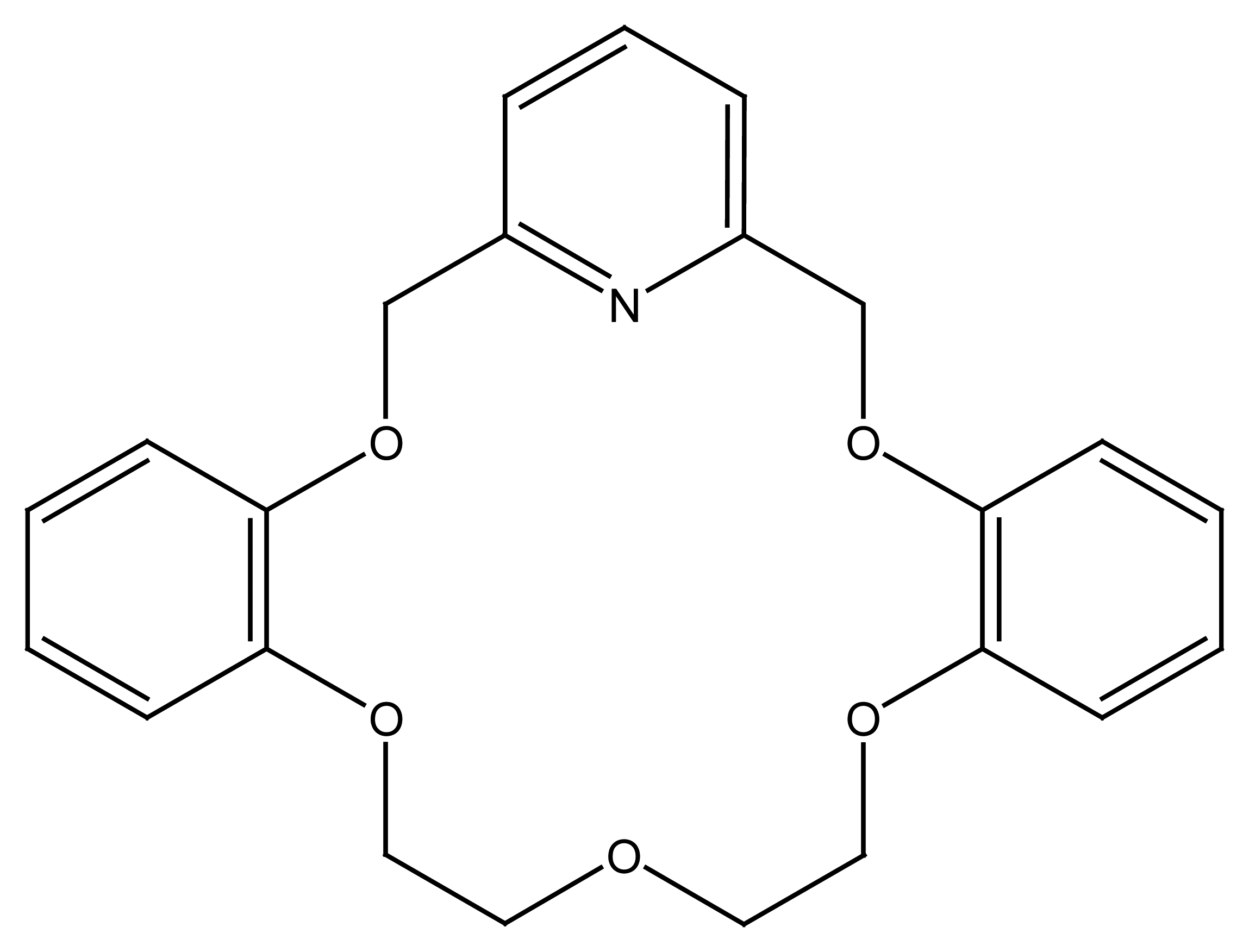
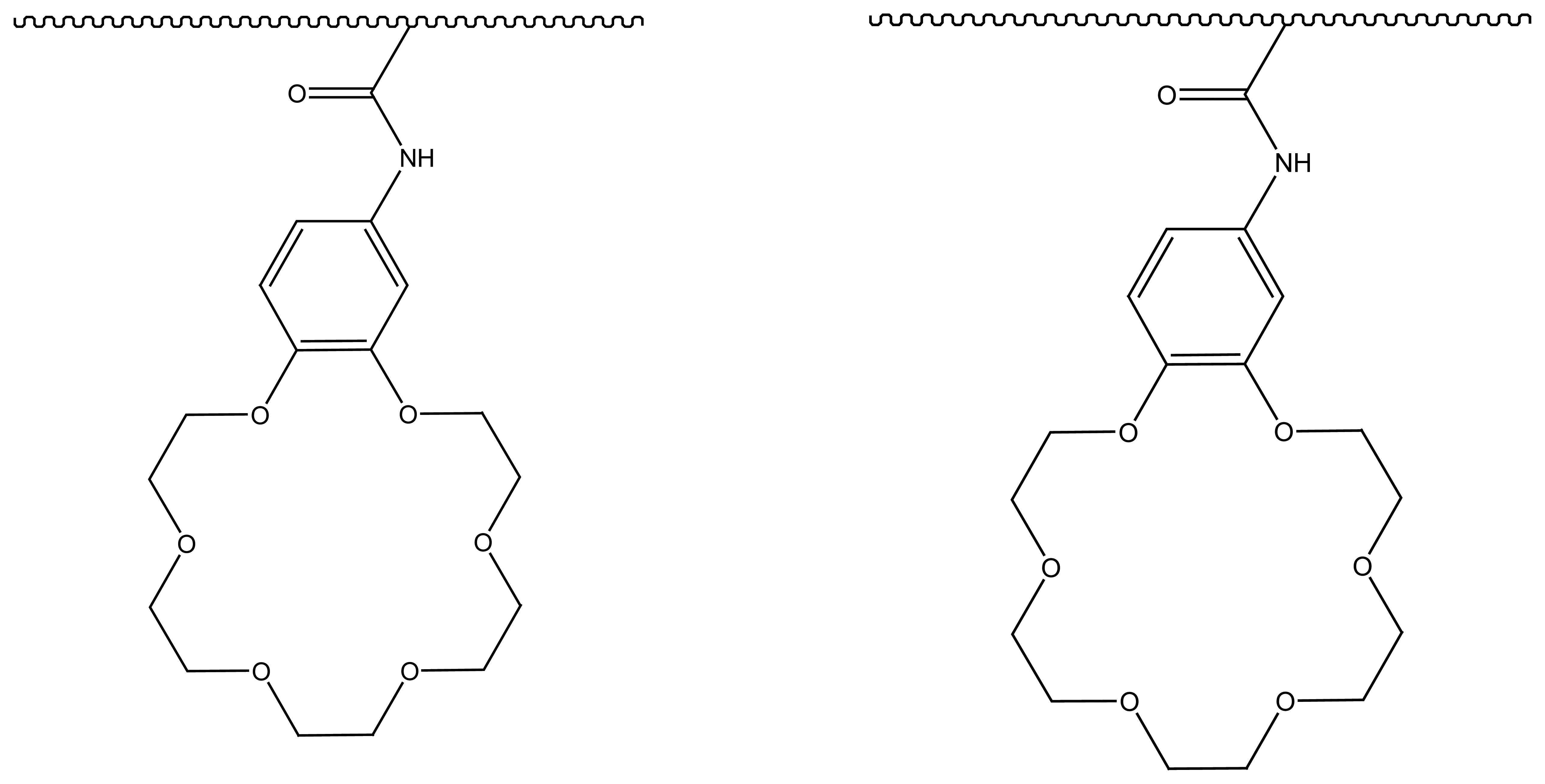
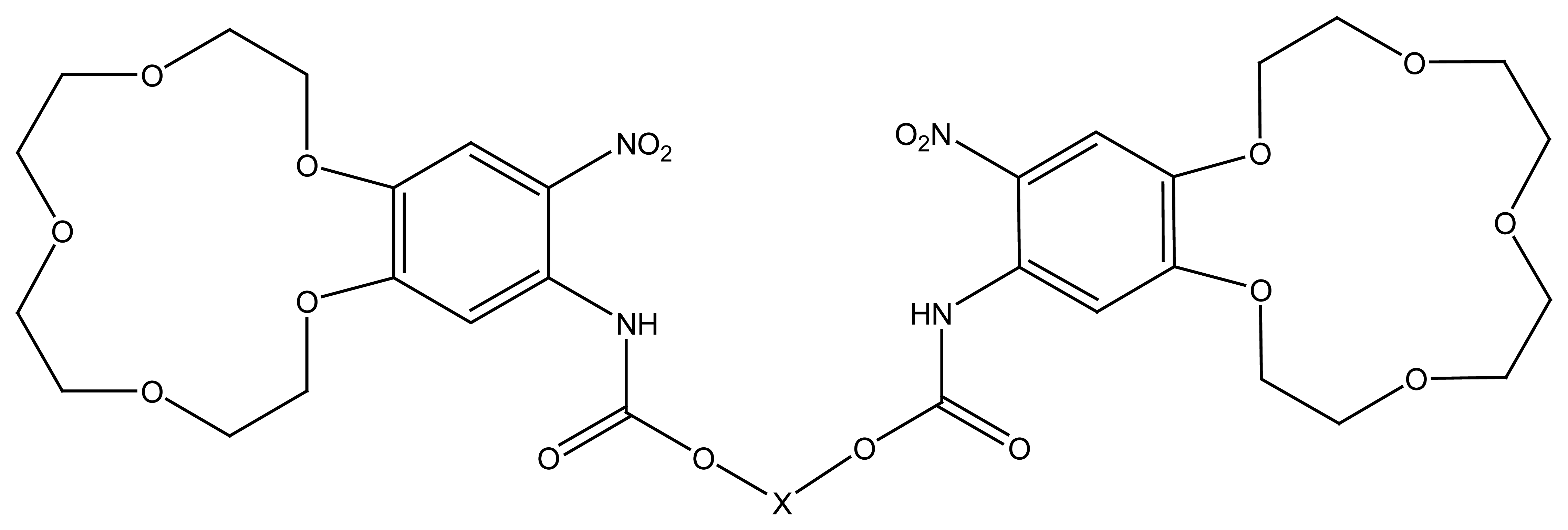
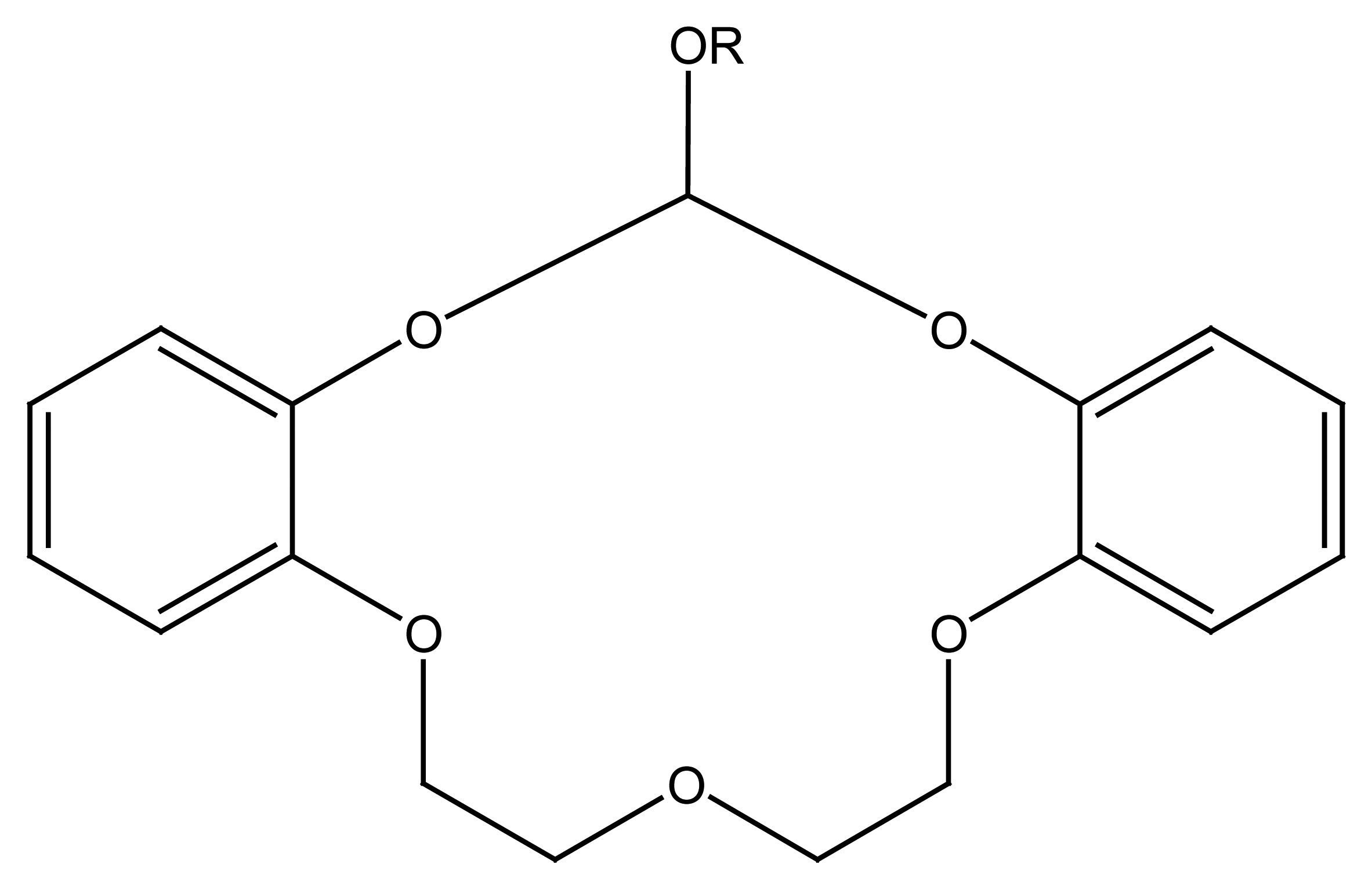
4.4. Crown Ethers Based Ion Selective Sensors
4.4.1. Crown Ethers Based Main Group Ion Selective Electrodes
4.4.2. Crown Ether Based Transition Group Ion Selective Electrodes
4.5. Podand Based Ion Selective Sensors
4.6 Tripod Based Ion Selective Sensors
4.7 Rigid Schiff's Bases Based Ion Selective Sensors
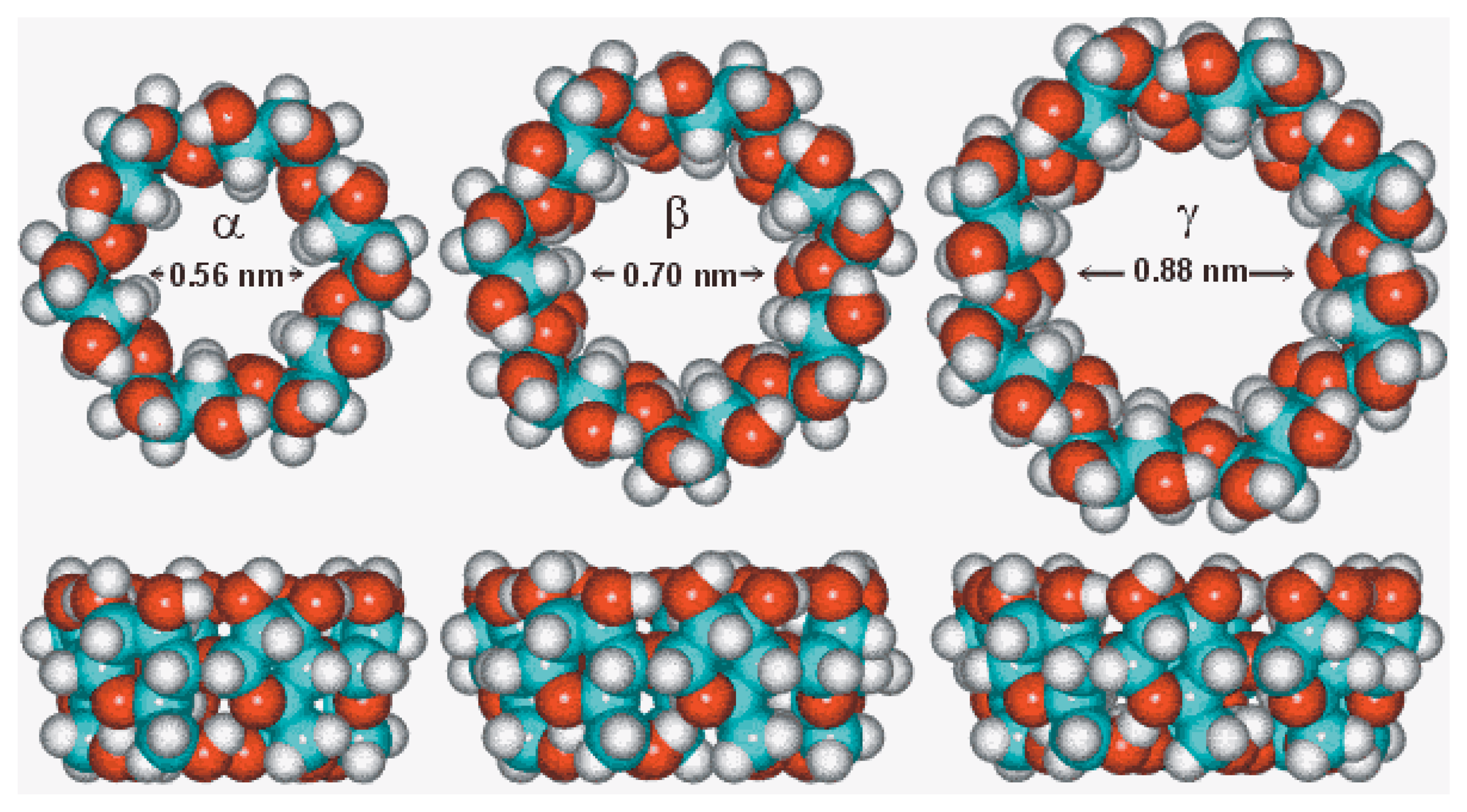
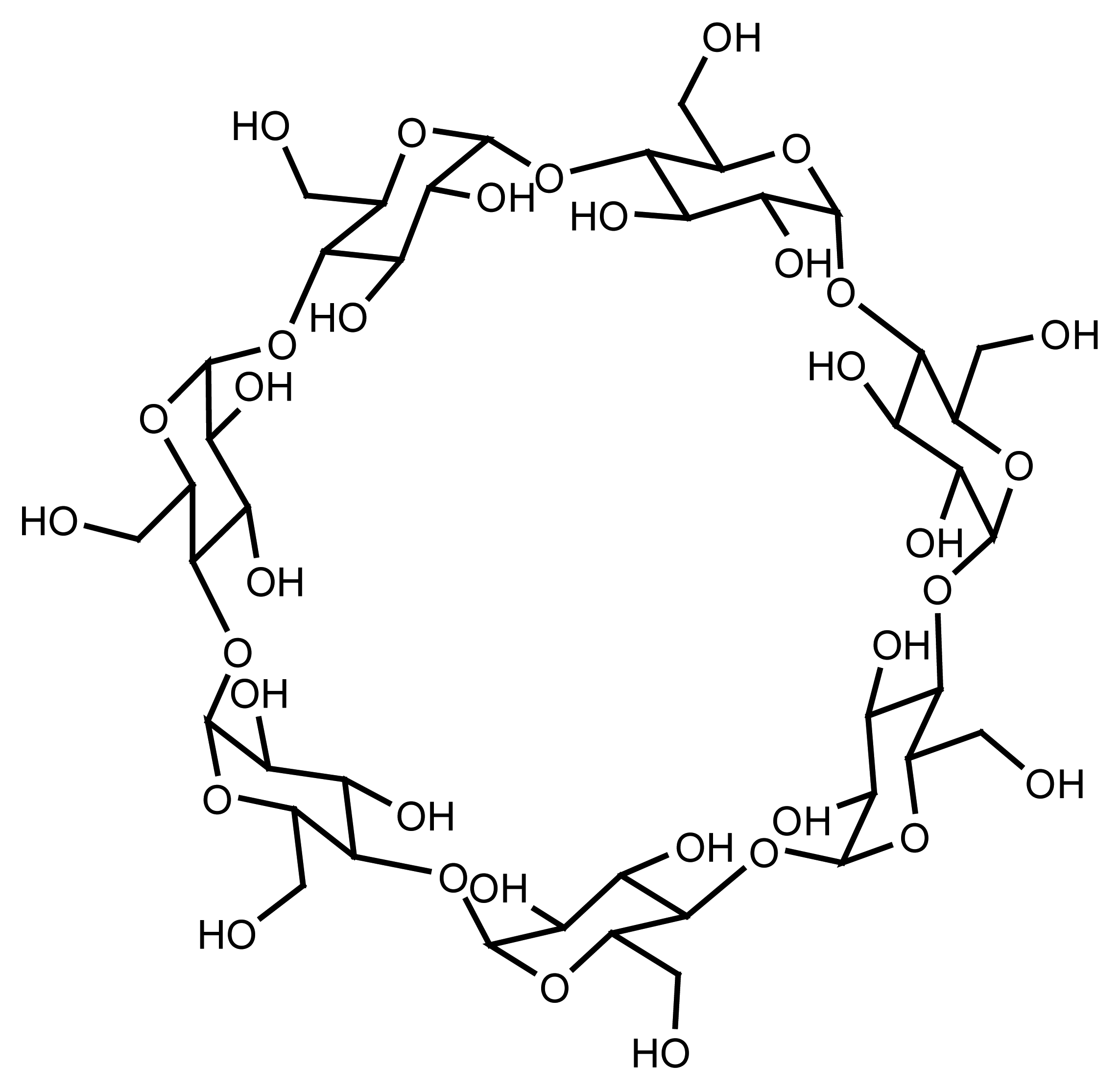
4.8. Cyclodextrin Based Ion Selective Sensors
5. Supra Molecule based Amperometric Sensors
Acknowledgments
References
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1976, 89, 7017–7036. [Google Scholar]
- Weber, E.; Vögel, F. Cristaline 1:1-alkalimetalkomplexe nichtcycclischer neutralligandens. Tetrahedron Lett. 1975, 16, 2415–2418. [Google Scholar]
- Lehn, J.M. Supramolecular chemistry-scope and perspectives. Molecules, supermolecules, and molecular devices (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar]
- Fischer, E. Einflus der Configuration auf die Wirkung der Enzyme. Ber: Dtsch., Chem. Ges 1894, 27, 2985. [Google Scholar]
- Cragg, P.J. A Practical Guide to Supramolecular Chemistry. In John Wiley & Sons; England, 2005. [Google Scholar]
- Ciampolini, M.; Nardi, N. Five-coordinated high-spin complexes of bivalent cobalt, nickel and copper with tris(2-dimethylaminoethyl)amine. Inorg. Chem. 1966, 5, 41–44. [Google Scholar]
- Perisic, O.; Fong, S.; Lynch, D.E.; Bycroft, M.; Williams, R.L. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase. A2. J. Biol. Chem. 1998, 273, 1596–1604. [Google Scholar]
- Kaim, W.; Schwederski, B. Bioorganic Chemistry: Inorganic Chemistry of Life-An Introduction and Guide. In John Wiley & Sons; Chichester, 1994. [Google Scholar]
- Greene, R.N. 18-Crown-6: a strong complexing agent for alkali metal cations. Tetrahedron Lett. 1972, 13, 1793–1796. [Google Scholar]
- Encyclopedia of Sensors. Potentiometric Ion Sensors. American Scientific Publishers 2006, Vol. 8, 197–288. [Google Scholar]
- Ludwig, R.; Dzung, N.T.K. Calixarene-Based Molecules for Cation Recognition. Sensors 2002, 2, 397–416. [Google Scholar]
- Cajan, M.; Lhotak, P.; Lang, J.; Dvorakova, H.; Stibor, I.; Koca, J. Conformational Behavior of Calixarenes: The Pinched Cone Pinched Cone Interconversion. J. Chem. Soc., Perkin. Trans. 2 2002, 2, 1922–1929. [Google Scholar]
- Cajan, M.; Damborsky, J.; Stibor, I.; Koca, J. Stability of Complexes of Aromatic Amides with Bromide Anion: Quantitative Structure-Property Relationships. J. Chem. Inf. Comp. Sci. 2000, 40, 1151–1157. [Google Scholar]
- Cajan, M.; Stibor, I.; Koca, J. Computational Studies on the Stability of [Amide]Br- Complexes. J. Phys. Chem. A 1999, 103, 3778–3782. [Google Scholar]
- Stibor, I.; Hafeed, D.S.M.; Lhotak, P.; Hodacova, J.; Koca, J.; Cajan, M. From the Amide Bond Activation to Simultaneous Recognition of Anion-Cation Couple. Gazz. Chim. Ital 1997, 127, 673–685. [Google Scholar]
- O'Connor, K.M.; Cherry, M.; Svehla, G.; Harris, S.J. Symmetrical, unsymmetrical and bridged calix[4]arene derivatives as neutral carrier ionophores in poly (vinyl chloride) membrane sodium selective electrodes. Talanta 1994, 41, 1207–1217. [Google Scholar]
- Shibutani, Y.; Yoshinaga, H.; Yakabe, K.; Shono, T.; Tanaka, M. Polymeric membrane sodium-selective electrodes based on calix[4]arene ionophores. J. Inclus. Phenom. 1994, 19, 333–342. [Google Scholar]
- Kimura, K.; Tsujimura, Y.; Yokoyama, M. Silicone-rubber membrane sodium-ion sensors based on calix[4]arene neutral carriers. Pure & Appl. Chem. 1995, 67, 1085–1089. [Google Scholar]
- Tsujimura, Y.; Sunagawa, T.; Yokoyama, A.; Kimura, K. Sodium ion-selective electrodes based on silicone-rubber membranes covalently incorporating neutral carriers. Analyst 1996, 121, 1705–1709. [Google Scholar]
- Grady, T.; Cadogan, A.; McKittrick, T.; Harris, S.J.; Diamond, D.; McKervey, M.A. Sodium-selective electrodes based on triester monoacid derivatives of p-tert-butylcalix[4]arene. Comparison with tetraester calix[4]arene ionophores. Anal. Chem. Acta 1996, 336, 1–12. [Google Scholar]
- Lee, H.J.; Oh, H.J.; Cui, G.; Cha, G.S.; Nam, H. All-solid-state sodium-selective electrodes based on room temperature vulcanizing-type silicone rubber matrix. Anal. Sci. 1997, 13, 289–264. [Google Scholar]
- Kimura, K.; Tsujimura, Y.; Yokoham, M.; Maeda, T. Calix[4]arene Modified Oligosiloxane as a Quasi-Immobilized Neutral Carrier for Silicone-Rubber-Membrane Sodium Ion-Selective Electrodes. Bull. Chem. Soc. Jpn 1998, 71, 657–667. [Google Scholar]
- Muslinkina, L.A.; Evtugyn, G.A.; Kazakova, E.K.; Budnikov, H.C. Calix[4]resorcinarene ionophore in the ion-selective electrodes with plasticized poly(vinylchloride) membranes. J. Inclu. Phenom. 1999, 35, 361–367. [Google Scholar]
- Kim, Y.D.; Jeong, H.; Kang, S.O.; Nam, K.C.; Jeon, S. Polymeric membrane sodium ion-selective electrodes based on calix[4]arene trimesters. Bull. Korean Chem. Soc. 2001, 22, 405–408. [Google Scholar]
- Lugtenber, R.J.W.; Brzozka, Z.; Casnati, A.; Ungaro, R.; Engbersen, J.F.J.; Reinhoudt, D.N. Cesium-selective chemically-modified field-effect transistors with calix[4]arene-crown-6 derivatives. Anal. Chem. Acta 1995, 310, 263–267. [Google Scholar]
- Casnati, A.; Pochini, A.; Ungaro, R.; Ugozzoli, F.; Arnaud, F.; Fanini, S.; Schwing, M.J.; Egberink, R.J.M.; Dejong, F.; Reinhoudt, D.N. Synthesis, Complexation, and membrane-transport studies of 1,3-alternate calix[4]arene-crown-6 conformers-a new class of cesium selective ionophores. J. Am. Chem. Soc. 1995, 117, 2767–2777. [Google Scholar]
- Oh, H.; Choi, E.M.; Jeong, H.; Nam, K.C.; Jeon, S. Poly(vinyl chloride) membrane cesium ion-selective electrodes based on lipophilic calix[6]arene tetraester derivatives. Talanta 2000, 53, 535–542. [Google Scholar]
- Lee, S.H.; Ko, S.W.; Cho, E.J.; Chun, J.C.; Nam, K.C. Phenylene bridged calix[6]arenes: Cesium selective ionophores. Bull. Korean Chem. Soc. 2001, 22, 100–102. [Google Scholar]
- Nam, K.C.; Ko, S.W.; Kang, S.O.; Lee, S.H.; Kim, D.S. Synthesis and ion binding properties of cesium selective quadruply bridged calix[6]arenas. J. Inclus. Phenom. 2001, 40, 285–289. [Google Scholar]
- Chen, L.X.; Ju, H.F.; Zeng, X.S.; He, X.W.; Zhang, Z.Z. Cesium selective electrodes based on novel double flexible spacers bridged biscalix[4]arenas. Anal. Chem. Acta 2001, 447, 41–46. [Google Scholar]
- Choi, E.M.; Oh, H.; Ko, S.W.; Choi, Y.K.; Nam, K.C.; Jeon, S. Polymeric membrane cesium-selective electrodes based on quadruply-bridged calix[6]arenas. Bull. Korean Chem. Soc. 2001, 22, 1345–1349. [Google Scholar]
- Ko, S.W.; Yang, Y.S.; Mun, J.H.; Park, K.M.; Lee, S.S.; Nam, K.C. Highly cesium selective calix[6]arene receptors: Synthesis, structure and cesium binding properties of calix[6]arene biscrown. Bull. Korean Chem. Soc. 2002, 23, 1379–1380. [Google Scholar]
- Mahajan, R.K.; Kumar, M.; Sharma, V.; Kaur, I. Cesium ion selective electrode based on calix[4]crown ether-ester. Talanta 2002, 58, 445–450. [Google Scholar]
- Arora, V.; Chawla, H.M.; Francis, T.; Nanda, M.; Singh, S.P. Synthesis of a new cesium selective calix[4]arene based chromoionophore. Ind. J. Chem. section a-inorganic bio-inorganic physical theoretical and analytical chemistry 2003, 42, 3041–3043. [Google Scholar]
- Jeon, S.W.; Yeo, H.Y.; Lee, H.K.; Ko, S.W.; Nam, K.C. Novel cesium-selective electrodes based on lipophilic 1,3-bisbridged cofacial-calix[6]crowns. Electroanalysis 2004, 16, 472–477. [Google Scholar]
- Yajima, S.; Yoshioka, N.; Tanaka, M.; Kimura, K. Soft Metal Ion-Selective Electrodes Based on π-Coordinate Calixarene Derivatives. Electroanalysis 2003, 15, 1319–1326. [Google Scholar]
- Kimura, K.; Tatsumi, K.; Yokoyama, M.; Ouchi, M.; Mocerino, M. Remarkable thallium(I) selectivity for ion sensors based on π-coordination of calix[4]arene neutral carriers. Anal. Comm 1999, 36, 229–230. [Google Scholar]
- Park, K.S.; Jung, S.O.; Lee, S.S.; Kim, J.S. Thallium(l)-Selective Electrodes Based on Calix[4]pyrroles. Bull. Korean Chem. Soc. 2000, 21, 909–912. [Google Scholar]
- Malinowska, E.; Brzózka, Z.; Kasiura, K.; Egberink, J.M.; David, N. Reinhoudt Lead selective electrodes based on thioamide functionalized calix[4]arenes as ionophores. Anal. Chim. Acta 1994, 298, 253–258. [Google Scholar]
- Lugtenberg, R.J.W.; Egberink, R.J.M.; Engbersen, J.F.J.; Reinhoudt, D. N. Pb2+and Cd2+selective chemically modified field effect transistors based on thioamide functionalized 1,3-alternate calix[4]arenas. J. Chem. Soc. Perkin Trans. 1997, 2, 1353–1357. [Google Scholar]
- Ceresa, A.; Bakker, E.; Hattendorf, B.; Gunther, D.; Pretsch, E. Potentiometric polymeric membrane electrodes for measurement of environmental samples at trace levels: New requirements for selectivities and measuring protocols, and comparison with ICPMS. Anal. Chem. 2001, 73, 343–351. [Google Scholar]
- Memon, S.; Yilmaz, M. An exchange approach towards the designing of a Schiff-base type oligocalix[4]arene, selective for the toxic metal ions. J. Macromol. Sci. Pure Appl. Chem. 2002, 39, 63–73. [Google Scholar]
- Liu, Y.; Zhao, B.T.; Zhang, H.Y.; Ju, H.F.; Chen, L.X.; He, X.W. Molecular Design of Calixarene, Part 4, Synthesis of Novel Double-Armed p-(tert-Butyl)calix[4]arene-Derived Amides and Their Lead(II)(Pb2+)-Selective-Electrode Properties. Helv. Chim. Acta 2001, 84, 1969–1975. [Google Scholar]
- Gupta, V.K.; Mangla, R.; Agarwal, S. Pb(II) Selective Potentiometric Sensor Based on 4-tert-Butylcalix[4]arene in PVC Matrix. Electroanalysis 2002, 14, 1127–1132. [Google Scholar]
- Lu, J.Q.; Chen, R.; He, X. A lead ion-selective electrode based on a calixarene carboxyphenyl azo derivative. J. Electroanal. Chem. 2002, 528, 33–38. [Google Scholar]
- Ju, H.F.; Chen, L.X.; He, X.W.; Zhao, B.T.; Liu, Y. Lead ion selective electrode based on novel calix[4]arene derivative. Chinese J. Anal. Chem. 2001, 29, 1121–1124. [Google Scholar]
- Vaishali, S.; Ijeri, V.S.; Ashwini, K.; Srivastava, K. Coated wire lead(II) selective potentiometric sensor based on 4-tert-butylcalix[6]arene. Sens. Actuators B. 2004, 99, 98–105. [Google Scholar]
- Katsu, T.; Akagi, M.; Hiramatsu, T.; Tsuchiya, T. Determination of the pH difference across a cell membrane using a methylammonium-selective membrane electrode. Analyst 1998, 123, 1369–1372. [Google Scholar]
- Katsu, T.; Matsumoto, M. Discrimination of methylammonium from organic ammonium ions using ion-selective electrodes based on calix[4]arene-crown-6 conjugates. Anal. Sci. 2001, 17, 721–725. [Google Scholar]
- Katsu, T.; Ido, K. Ethylammonium-selective membrane electrode using p-tert-butylcalix[6]arene derivatives. Anal. Sci. 2002, 18, 473–476. [Google Scholar]
- Katsu, T.; Okaki, N.; Watanabe, K.; Takaishi, K.; Yokosu, H. Comparative study of the response of membrane electrodes based on calix[6]arene and calix[8]arene derivatives to organic ammonium ions. Anal. Sci. 2003, 19, 771–774. [Google Scholar]
- Park, S.J.; Shon, O.J.; Rim, J.A.; Lee, J.K.; Kim, J.S.; Nam, H.; Kim, H. Calixazacrown ethers for copper(II) ion-selective electrode. Talanta 2001, 55, 297–304. [Google Scholar]
- Chen, L.; He, X.; Zhao, B.; Liu, Y. Calixarene derivative as the neutral carrier in silver ion-selective electrode and liquid membrane transport. Anal. Chim. Acta 2000, 417, 51–56. [Google Scholar]
- Chen, L.; Zeng, X.; Ju, H.; He, X.; Zhang, Z. Calixarene derivatives as the sensory molecules for silver ion-selective electrode. Microchem. J. 2000, 65, 129–135. [Google Scholar]
- O'Connor, K.M.; Henderson, W.; O'Neill, E.; Arrigan, D.W.M.; Harris, S.J.; Mckervey, M.A.; Svehla, G. Calix[4]Arenes in the partial cone conformation as ionophores in silver ion-selective electrodes. Electroanalysis 1997, 9, 311–315. [Google Scholar]
- Liu, Y.; Zhao, B.T.; Chen, L.X.; He, X.W. Liquid membrane transport and silver selective electrode based on novel bis(3-pyridinecarboxylate) calix[4]arene as ionophore. Microchem. J 2000, 65, 75–79. [Google Scholar]
- Zeng, X.S.; Weng, L.H.; Chen, L.X.; Leng, X.B.; Zhang, Z.Z.; He, X.W. Improved silver ion-selective electrodes using novel 1,3-bis(2-benzothiazolyl)thioalkoxy-p-tert-butylcalix[4]arenas. Tetrahedron Lett. 2000, 41, 4917–4921. [Google Scholar]
- Chen, L.X.; Zeng, X.S.; He, X.W.; Zhang, Z. Selective electrodes for silver based on polymeric membranes containing calix[4]arene derivatives. J. Anal. Chem. 2000, 367, 535–538. [Google Scholar]
- Kimura, K.; Yajima, S.; Tatsumi, K.; Yokoyama, M.; Oue, M. Silver ion-selective electrodes using pi-coordinate calix[4]arene derivatives as soft neutral carriers. Anal. Chem. 2000, 72, 5290–5294. [Google Scholar]
- Chen, L.; Ju, H.; Zeng, X.; He, X.; Zhang, Z. Silver ion-selective electrodes based on novel containing benzothiazolyl calix[4]arene. Anal. Chem. Acta 2001, 437, 191–197. [Google Scholar]
- Mahajan, R.K.; Kumar, M.; Sharma, V.; Kaur, I. Silver(I) ion-selective membrane based on Schiff base–p-tert-butylcalix[4]arene. Analyst 2001, 126, 505–507. [Google Scholar]
- Shinohara, T.; Higuchi, H.; Senba, Y.; Ohto, K.; Yoshizuka, K.; Inoue, K. Silver Ion Selective Electrode Based on Calix[4]arene Methyl Ketonic Derivative. Anal. Sci. 2001, 17, 889–892. [Google Scholar]
- Lim, S.M.; Chung, H.J.; Paeng, K.J.; Lee, C.H.; Choi, H.N.; Lee, W.Y. Calix[2]furano[2]pyrrole and related compounds as the neutral carrier in silver ion-selective electrode. Anal. Chim. Acta 2002, 453, 81–88. [Google Scholar]
- Park, K.S.; Jung, S.O.; Yoon, I.; Park, K.M.; Kim, J.; Lee, S.S.; Kim, J.S. Structural Selectivity of Calix[m]pyrroles[n]furans (m + n = 4) as Ionophores in Ag(I) Ion-Selective Electrodes. J. Inclus. Phenom. 2001, 39, 295–301. [Google Scholar]
- Chen, L.X.; He, X.W.; Zhang, H.Y.; Liu, Y.; Hu, X.B.; Sheng, Y. Selective electrode for silver based on polymer membranes containing exocyclic chalcogen atoms calix[4]arene and crown ether. Anal. Lett. 2001, 34, 2237–2248. [Google Scholar]
- Kumar, M.; Mahajan, R.K.; Sharma, V.; Singh, H.; Sharma, N.; Kaur, I. Synthesis of new bis-calix[4]arenes with imine linkages. A search for new silver-selective sensors. Tetrahedron Lett. 2001, 42, 5315–5318. [Google Scholar]
- Zeng, X.S.; Weng, L.H.; Chen, L.X.; Leng, X.B.; Ju, H.F.; He, X.W.; Zhang, Z.Z. The synthesis of some pyridyl functionalized calix[4]arenes as the sensor molecule for silver ion-selective electrodes. J. Chem. Soc. Perkin Trans. 2 2001, 2, 545–549. [Google Scholar]
- Ceresa, A.; Radu, A.; Peper, S.; Bakker, E.; Pretsch, E. Rational Design of Potentiometric Trace Level Ion Sensors. A Ag+-Selective Electrode with a 100 ppt Detection Limit. Anal. Chem. 2002, 74, 4027–4036. [Google Scholar]
- Sun, H.; Zeng, X.S.; Lu, J.Q.; Leng, X.B.; Chen, Q.F.; Xu, F.B.; Li, Q.S.; He, X.W.; Zhang, W.Q.; Zhang, Z.Z. Synthesis of a tweezer-like bis(phenylthiapropoxy)calix[4]arene as a cation/pi enhanced sensor for ion-selective electrodes. Chin. J. Chem. 2002, 20, 1109–1114. [Google Scholar]
- Zeng, X.S.; Weng, L.H.; Chen, L.X.; Xu, F.B.; Li, Q.S.; Leng, X.B.; He, X.W.; Zhang, Z.Z. The syntheses and Ag+-selective electrode properties of benzothiazolylthiaalkoxy functionalized calix[4]arenes: an investigation of the structure-selectivity relationship in the ionophore-based ISEs. Tetrahedron 2002, 58, 2647–2658. [Google Scholar]
- Mazloum Ardakani, M.; Ensafi, A.A.; Salavati Niasari, M.; Mirhoseini, H. Silver(I)-selective Coated-wire Electrode Based on an Octahydroxycalix[4]arene Derivative. Anal. Sci. 2003, 19, 1187–1190. [Google Scholar]
- Mahajan, R.K.; Kaur, I.; Kumar, M. Silver ion-selective electrodes employing Schiff's base p-tert-butyl calix[4]arene derivatives as neutral carriers. Sens. Actuators B. 2003, 91, 26–31. [Google Scholar]
- Zeng, X.S.; Sun, H.; Chen, L.X.; Leng, X.B.; Xu, F.B.; Li, Q.S.; He, X.W.; Zhang, Q.W.; Zhang, Z.Z.; Zhang, Z. Zhang, Synthesis of a tweezer-like bis(arylthiaalkoxy)calix[4]arene as a cation sensor for ion-selective electrodes: an investigation of the influence of neighboring halogen atoms on cation selectivity. Org. Bio. Chem. 2003, 1, 1073–1079. [Google Scholar]
- Ganjali, M.R.; Hajiagha Babaei, L.; Taghvaei Ganjali, S.; Modjallal, A.; Shamsipur, M.; Hosseini, M.; Javanbakht, M. A new cone shaped asymmetrically substituted calix[4]arene as an excellentIonophore in construction of Ag(I) ion-selective membrane electrode. Bull. Korean Chem. Soc. 2004, 25, 177–181. [Google Scholar]
- Lu, J.Q.; Pang, D.W.; Zeng, X.S.; He, X.W. A new solid-state silver ion-selective electrode based on a novel tweezer-type calixarene derivative. J. Electroanal. Chem. 2004, 568, 37–43. [Google Scholar]
- Lu, J.Q.; Tong, X.O.; He, X.W. A mercury ion-selective electrode based on a calixarene derivative containing the thiazole azo group. J. Anal. Chem. 2003, 540, 111–117. [Google Scholar]
- Mahajan, R.K.; Kaur, R.; Kaur, I.; Sharma, V.; Kumar, M. Mercury(II) ion-selective electrodes based on p-tert-butyl calix[4]crowns with imine units. Anal. Sci. 2004, 20, 811–814. [Google Scholar]
- Grady, T.; Maskula, S.; Diamond, D.; Marrs, D.J.; Mckervey, M.A.; Ohagan, P. Haracteristics of a europium-selective electrode based on a calix[4]arene tetraphosphine oxide. Anal. Proceed 1995, 32, 471–473. [Google Scholar]
- Gupta, V.K.; Mangla, R.; Khurana, U.; Kumar, P. Determination of Uranyl Ions Using Polyvinyl chloride) Based 4-tert-Butylcalix[6]arene Membrane Sensor. Electroanalysis 1999, 11, 573–576. [Google Scholar]
- Amemiya, S.; Bu1hlmann, P.; Umezawa, Y. An ion-selective electrode for acetate based on a urea-functionalized Porphyrin as a Hydrogen-Bonding Ionophore. Anal. Chem. 1999, 71, 1049–1054. [Google Scholar]
- Daunert, S.; Wallace, S.; Florido, A.; Bachas, G.L. Anion-Selective Electrodes Based on Electropolymerized Porphyrin Films. Anal. Chem. 1991, 63, 1676–1679. [Google Scholar]
- Khorasani, J.H.; Amini, M.K.; Motaghi, H.; Tangestaninejad, S.; Moghadam, M. Manganese porphyrin derivatives as ionophores for thiocyanate-selective electrodes: the influence of porphyrin substituents and additives on the response properties. Sens. Actuators B. 2002, 87, 448–456. [Google Scholar]
- Vlascici, D.; Fagadar-Cosma, E.; Spiridon-Bizerea, O.; Pascariu, A. Chiriac, The characterization of the membrane material of a tiocyanate-selective electrode based on rhodium (III) [5,10,15,20-tetraphenyl-21H, 23H-porphyrinato-N21,N22,N23,N24] chloride). Rev. Chim 2005, 56, 224–227. [Google Scholar]
- Chaniotakis, N.A.; Park, S.B.; Meyerhoff, M.E. Anion-Selective Electrodes Based on Electropolymerized Porphyrin Films. Anal. Chem. 1989, 61, 566–570. [Google Scholar]
- Malinowska, E.; Niedzióka, J.; Roźniecka, E.; Meyerhoff, M.E. Salicylate-selective membrane electrodes based on Sn(IV)- and O=Mo(V)- porphyrins: differences in response mechanism and analytical performance. J. Electroanal. Chem. 2001, 514, 109–117. [Google Scholar]
- Shahrokhian, S.; Hamzehloei, A.; Bagherzadeh, M. Chromium(III) Porphyrin as a Selective Ionophore in a Salicylate-Selective Membrane Electrode. Anal. Chem. 2002, 74, 3312–3320. [Google Scholar]
- Messick, M.S.; Krishnan, S.K.; Hulvey, M.K.; Steinle, E.D. Development of anion selective polymer membrane electrodes based on lutetium(III) porphyrins. Anal. Chim. Acta 2005, 539, 223–228. [Google Scholar]
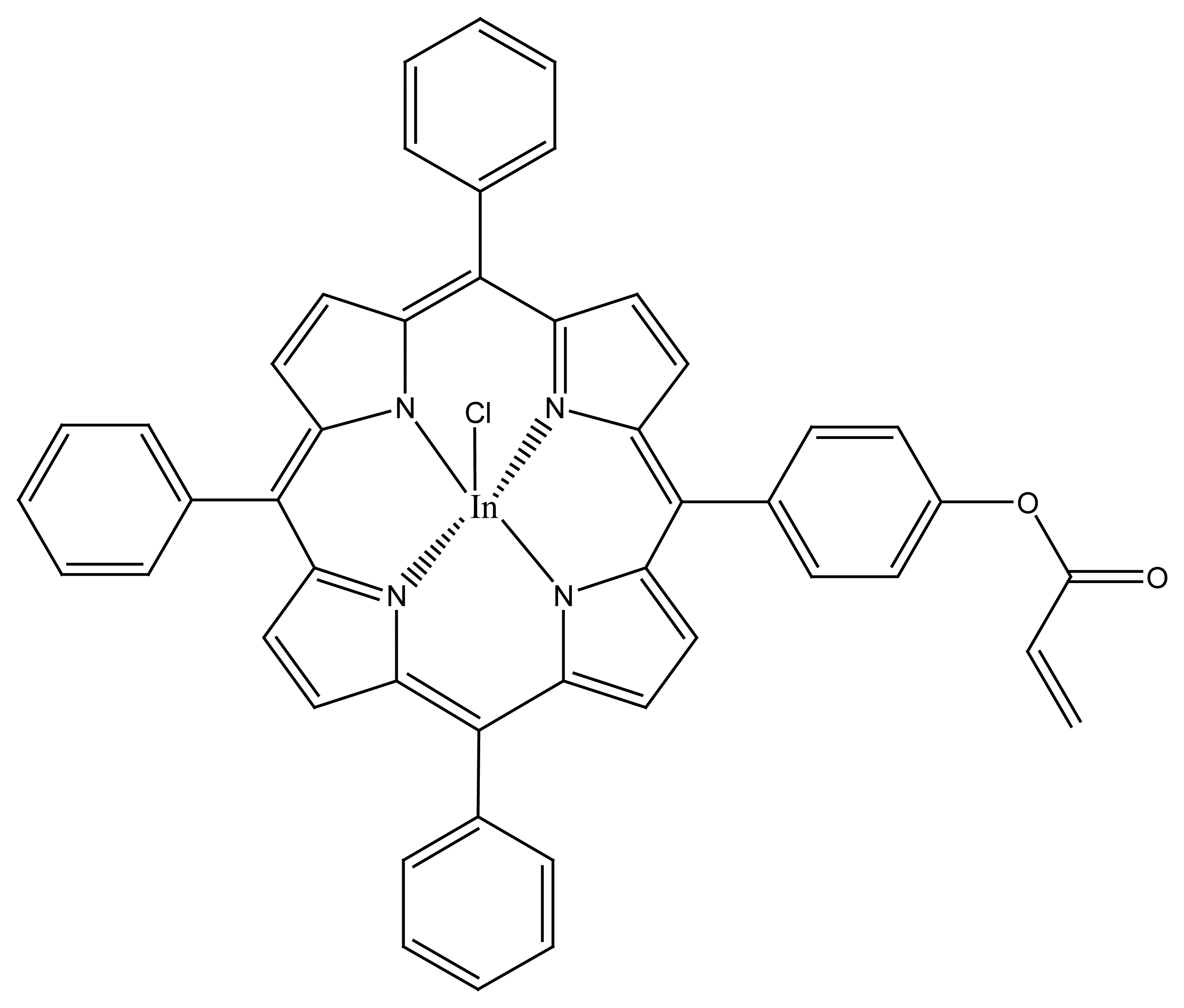
- Pimenta, A.M.; Araújo, A.N.; Conceição, M.; Montenegro, B.S.M.; Pasquini, C.; Rohwedder, J.J.R.; Raimundo, I.M. Chloride-selective membrane electrodes and optodes based on an indium(III) porphyrin for the determination of chloride in a sequential injection analysis system. J. Pharm. Biomed. Analysis 2004, 36, 49–55. [Google Scholar]
- Qin, Y.; Bakker, E. Elimination of Dimer Formation in In(III) Porphyrin-Based Anion-Selective Membranes by Covalent Attachment of the Ionophore. Anal. Chem. 2004, 76, 4379–4386. [Google Scholar]
- Jagessar, R.C.; Shang, M.; Scheidt, W.R.; Burns, D.H. Neutral Ligands for Selective Chloride Anion Complexation:(R,R,R,R)-5,10,15,20-Tetrakis(2-(arylurea)phenyl)porphyrins. J. Am. Chem. Soc. 1998, 120, 11684–11692. [Google Scholar]
- Malinowska, E.; Górski, L.; Meyerhoff, M.E. Zirconium(IV)-porphyrins as novel ionophores for fluoride-selective polymeric membrane electrodes. Anal. Chim. Acta 2002, 468, 133–141. [Google Scholar]
- Gorski, L.; Meyerhoff, M.E.; Malinowska, E. Polymeric membrane electrodes with enhanced fluoride selectivity using Zr(IV)-porphyrins functioning as neutral carriers. Talanta 2004, 63, 101–107. [Google Scholar]
- Mitchell-Koch, J.T.; Pietrzak, M.; Malinowska, E.; Meyerhoff, M.E. Aluminum(III) Porphyrins as Ionophores for Fluoride Selective Polymeric Membrane Electrodes. Electroanalysis 2006, 18, 551–557. [Google Scholar]
- Sun, C.; Zhao, J.; Xu, H.; Sun, Y.; Zhang, X.; Shen, J. Fabrication of a multilayer film electrode containing porphyrin and its application as a potentiometric sensor of iodide ion. Talanta 1998, 46, 15–21. [Google Scholar]
- Farhadi, K.; Maleki, R.; Hosseinzadeh Yamchi, R.; Sharghi, H.; Shamsipur, M. [Tetrakis(4-N,N-dimethylaminobenzene)porphyrinato]-manganese(III) Acetate as a Novel Carrier for a Selective Iodide PVC Membrane Electrode. Anal. Sci. 2004, 20, 805–809. [Google Scholar]
- Farhadi, K.; Shaikhlouei, H.; Maleki, R.; Sharghi, H.; Shamsipur, M. Highly Selective Triiodide Polymeric Membrane Electrode Based on Tetra(p-chlorophenyl)porphyrinato Manganese (III) Acetate. Bull. Korean Chem. Soc. 2002, 23, 1635–1639. [Google Scholar]
- Gupta, V.K.; Agarwal, S. PVC based 5,10,15,20-tetrakis (4-methoxyphenyl) porphyrinatocobalt (II) membrane potentiometric sensor for arsenite. Talanta 2005, 65, 730–734. [Google Scholar]
- Seo, H.R.; Lee, H.K.; Jeon, S. Improved Thiocyanate-Selective Electrode Based on Tetra(trimethylphenyl)-porphyrinato Manganese(III) Chloride: The Electronic and pH Effects. Bull. Korean Chem. Soc. 2004, 25, 1484–1488. [Google Scholar]
- Lin, X.M.; Umezawa, K.; Tohda, K.; Furuta, H.; Sessler, J.L.; Umezawa, Y. Potentiometric Responses of Expanded Porphyrin Incorporated Liquid Membrane Electrodes toward a Series of Inorganic and Organic Anions. Anal. Sci. 1998, 14, 99–108. [Google Scholar]
- Amini, M.K.; Shahrokhian, S.; Tangestaninejad, S. PVC-Based Mn(III) Porphyrin Membrane-Coated Graphite Electrode for Determination of Histidine. Anal. Chem. 1999, 71, 2502–2505. [Google Scholar]
- Fagadar-Cosma, E.; Vlascici, D.; Fagadar-Cosma, G.; Bizerea, O.; Chiriac, A. The study of the electrochemical behaviour of metalo-porphyrins with Co(II) and Co(Ill). Nitrite-selective electrode based on [5,10,15,20-tetraphenyl-21H,23H-porphyrinato-N21, N22, N23, N24]Co(III) chloride. Rev. Chim 2004, 55, 882–885. [Google Scholar]
- Lee, H.K.; Song, K.; Seo, H.R.; Jeon, S. Nitrate-selective electrodes based on meso-tetrakis[(2-arylphenylurea)-phenyl] porphyrins as neutral lipophilic ionophores. Talanta 2004, 62, 293–297. [Google Scholar]
- Fakhari, A.R.; Alaghemand, M.; Shamsipur, M. Iron(III)-Selective Membrane Potentiometric Sensor Based on 5,10,15,20-Tetrakis-(pentafluorophenyl)-21H,23H-Porphyrin. Anal. Lett. 2001, 34, 1097–1106. [Google Scholar]
- Malinski, T.; Ciszewski, A.; Fish, J.R. Conductive Polymeric Tetrakis(3-methoxy-4-hydroxyphenyl)porphyrin Film Electrode for Trace Determination of Nickel. Anal. Chem. 1990, 62, 909–914. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Singh, L.P.; Khurana, U. Porphyrins as carrier in PVC based membrane potentiometric sensors for nickel(II). Anal. Chim. Acta 1997, 355, 33–41. [Google Scholar]
- Gupta, V.K.; Jain, A.K.; Maheshwari, G.; Lang, H.; Ishtaiwi, Z. Copper(II)-selective potentiometric sensors based on porphyrins in PVC matrix. Sens. Actuators B. 2006, 117, 99–106. [Google Scholar]
- Mazloum Ardakani, M.; Dehghani, H.; Jalayer, M.; Zare, H.R. Potentiometric Determination of Silver(I) by Selective Membrane Electrode Based on Derivative of Porphyrin. Anal. Sci. 2004, 20, 1667–1672. [Google Scholar]
- Fakhari, A.R.; Shamsipur, M.; Ghanbari, Kh. Zn(II)-selective membrane electrode based on tetra(2-aminophenyl) porphyrin. Anal. Chim. Acta 2002, 460, 177–183. [Google Scholar]
- Galowski, J.; Rieckemann, B.; Umland, F. Potassium-selective membrane electrodes based on the cryptand [2b2b2]. Fresenius J. Anal. Chem. 1981, 309, 343–346. [Google Scholar]
- Cross, G.G.; Fyles, T.M.; Suresh, V.V. Coated-wire electrodes containing polymer immpbilized ionophores blended with poly(vinyl chloride). Talanta 1994, 41, 1589–1595. [Google Scholar]
- Lukyanenk, N.G.; Titova, N.Y.; Nesterenko, N.L.; Kirichenko, T.I.; Scherbakov, S.V. Cation selective of poly(vinyl chloride) membrane based on cryptands with thiourea fragments. Anal. Chim. Acta 1992, 263, 169–173. [Google Scholar]
- Amini, M.K.; Mazloum, M.; Ensafi, A.A. Lead selective membrane electrode using cryptand(222) neutral carrier. Fresenius J. Anal. Chem. 1999, 364, 690–693. [Google Scholar]
- Srivastava, S.K.; Gupta, V.K.; Jain, S. PVC-based 2,2,2-cryptand sensor for zinc ions. Anal. Chem. 1996, 68, 1272–1275. [Google Scholar]
- Pouretedal, H.R.; Shamsipur, M. A PVC-based cryptand C2(B)22 membrane potentiometric sensor for zinc(II). Fresenius J. Anal. Chem. 1998, 362, 415–418. [Google Scholar]
- Alexandratos, S.D.; Stine, C.L. Synthesis of ion-selective polymer-supported crown ethers:a review. React. Funct. Polym 2004, 60, 3–16. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements.; Pergamon Press Inc.: Tarrytown, 1984; pp. 105–110. [Google Scholar]
- Fabre, B.; Simonet, J. Electroactive polymers containing crown ether or polyether ligands as cation-responsive materials. Coor. Chem. Rev 1998, 178, 1211–1250. [Google Scholar]
- Saalfrank, R.W.; Low, N.; Kareth, S.; Seitz, V.; Hampel, F.; Stalke, D.; Teichert, M. Crown ethers, double-decker, and sandwich complexes: Cation-mediated formation of metallatopomer coronates. Angew. Chem. Int. Ed 1998, 37, 172–175. [Google Scholar]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar]
- Pearson, R.G. Acids and Bases. Science 1966, 151, 172–177. [Google Scholar]
- Alexandratos, S.D. Polymer-supported reagents with enhanced ionic recognition. Sep. Purif. Meth 1992, 21, 1–22. [Google Scholar]
- Zong, Z.; Dong, S.; Hu, Y.; Xu; Liu, W. Synthesis and complexation properties of polystyrene supported polymeric thiacrown ether. Eur. Polym. J 1998, 34, 761–766. [Google Scholar]
- Attiyat, A.S.; Christian, G.D.; Xie, R.Y.; Wen, X.W.; Bartsch, R. A. Comparative-evaluation of neutral and proton-ionizable crown ether compounds as lithium ionophores in ion-selective electrodes and in solvent-extraction. Anal. Chem. 1988, 60, 2561–2564. [Google Scholar]
- Kataky, R.; Nicholson, P.E.; Parker, D.; Covington, A.K. Comparative performance of 14-crown-4 derivatives as lithium-selective electrodes. Analyst 1991, 116, 135–140. [Google Scholar]
- Suzuki, K.; Yamada, H.; Sato, K.; Watanabe, K.; Hisamoto, H.; Tobe, Y.; Kobiro, K. Design and synthesis of highly selective ionophores for lithium ion based on 14-crown-4 derivatives for an ion-selective electrode. Anal. Chem. 1993, 65, 3404–3410. [Google Scholar]
- Kobiro, K. New class of lithium ion selective crown ethers with bulky decalin subunits. Coord. Chem. Rev 1996, 148, 135–149. [Google Scholar]
- Zhou, X.Y.; Luo, Y.; Uo; Wu, C.T.; Zou, Z.F.; Hu, Q.Z. Sodium ion-selective membrane-electrode based on a new crown ether. Anal. Chim. Acta 1988, 212, 325–329. [Google Scholar]
- Suzuki, K.; Hayashi, K.; Tohda, K.; Watanabe, K.; Ouchi, M.; Hakushi, T.; Inoue, Y. Sodium-ion selective electrodes based on lipophilic 16-crown-5 derivatives. Anal. Lett. 1991, 24, 1085–1091. [Google Scholar]
- Shibata, Y.; Miyagi, H. Anion interference in response of sodium ion-selective electrodes based on a bis(crown ether) compound. Nippon Kagaku Kaishi 1992, 1, 33–39. [Google Scholar]
- Lukyanenko, N.G.; Titova, N.Y.; Karpinchik, O.S.; Melnik, O.T. Sodium-selective electrodes based on PVC membranes containing bis[(3N+1)-crown-N] ether derivatives. Anal. Chim. Acta 1992, 259, 145–148. [Google Scholar]
- Kimura, K.; Yoshinaga, M.; Funaki, K.; Shibutani, Y.; Yakabe, K.; Shono, T.; Kasai, M.; Mizufune, H.; Tanaka, M. Effects of alpha-substituents on ion selectivity of bis(12-crown-4-methyl) malonates as neutral carriers for sodium ion-selective electrodes. Anal. Sci. 1996, 12, 67–70. [Google Scholar]
- Kimura, K.; Yajima, S.; Takase, H.; Yokoyama, M.; Sakurai, Y. Sol-gel modification of pH electrode glass membranes for sensing anions and metal ions. Anal. Chem. 2001, 73, 1605–1609. [Google Scholar]
- Moriuchi-Kawakami, T.; Aoki, R.; Morita, K.; Tsujioka, H.; Fujimori, k.; Shibutani, Y.; Shono, T. Conformational analysis of 12-crown-3 and sodium ion selectivity of electrodes based on bis(12-crown-3) derivatives with malonate spacers. Anal. Chim. Acta 2003, 480, 291–298. [Google Scholar]
- Tavakkoli, N. Sodium ion-selective membrane electrode based on dibenzopyridino-18-crown-6. Bull. Korean Chem. Soc. 2004, 25, 1474–1476. [Google Scholar]
- Willis, J.P.; Young, C.C.; Olsonmank, L.; Radle, L. The Clinical-evaluation of bis crown ether based potassium-ion selective electrodes. Clin. Chem. 1983, 29, 1193–1193. [Google Scholar]
- Ogata, T.; Sakaki, T.; Yamagami, H.; Kawano, K. Determination of sodium and potassium in biological-fluids by ion-selective electrodes based on crown ether compounds. Clin. Chem. 1988, 34, 1236–1236. [Google Scholar]
- An, H.Y.; Wu, Y.J.; Zhang, Z.J.; Izatt, R.M.; Bradshaw, J.S. The synthesis of bis(benzo-crown ether)s and their incorporation into potassium-selective PVC membrane electrodes. J. Inclus. Phenom. 1991, 11, 303–311. [Google Scholar]
- Luboch, E.; Cygan, A.; Biernat, J.F. Bis(benzocrown ether)s with polymethylene bridges and their application in ion-selective electrodes. Tetrahedron 1991, 47, 4101–4112. [Google Scholar]
- Allen, J.R.; Cynkowski, T.; Desai, J.; Baghas, L.G. Crown-ether derivatives of anthraquinone as ionophores in ion-selective electrodes. Electroanalysis 1992, 4, 533–537. [Google Scholar]
- Li, A.G.; Zhang, Z.J.; Wu, Y.J.; An, H.Y.; Izatt, R.M.; Bradshaw, J.S. The synthesis of bis(benzo-15-crown-5) derivatives and their use in potassium PVC membrane electrodes. J. Inclus. Phenom. 1993, 15, 317–327. [Google Scholar]
- Brzozka, Z.; Lammerink, B.; Reinhoudt, D.N.; Ghidini, E.; Ungaro, R. Transduction of selective recognition by preorganized ionophores-K+ selective of the different 1,3-diethoxycalix[4]arene crown-ether conformers. J. Chem. Soc. Perkin Trans. 2 1993, 2, 1037–1040. [Google Scholar]
- Ganjali, M.R.; Moghimi, A.; Buchanan, G.W.; Shamsipur, M. The synthesis of styrene/4 ′ -vinyl-benzo-24-crown-8 copolymer and its use in potassium-ion selective electrodes. J. Inclus. Phenom. 1998, 30, 29–43. [Google Scholar]
- Gyurcsanyi, R.E.; Nyback, A.S.; Toth, K.; Nagy, G.; Ivaska, A. Novel polypyrrole based all-solid-state potassium-selective microelectrodes. Analyst 1998, 123, 1339–1344. [Google Scholar]
- Xia, Z.R.; Badr, I.H.A.; Plummer, S.L.; Cullen, L.; Bachas, L.G. Synthesis and evaluation of a bis(crown ether) ionophore with a conformationally constrained bridge in ion-selective electrodes. Anal. Sci. 1998, 14, 169–173. [Google Scholar]
- Cho, H.Y.; Rha, S.G.; Chang, S.K.; Chung, T.D.; Cho, K.C.; Kim, H. New potassium- and cesium-selective ionophoric bis(crown ether)s derived from xanthene-4,5-dicarboxylic acid. J. Inclus. Phenom. 1998, 31, 119–129. [Google Scholar]
- Yanagi, H.; Sakaki, T.; Ogata, T. Development of high-performance ion sensors based on the functions of crown ethers and synthetic bilayer membranes. Nippon Kagaku Kaishi 1999, 10, 629–636. [Google Scholar]
- Heng, L.Y.; Hall, E.A.H. Taking the plasticizer out of methacrylic-acrylic membranes for K+-selective electrodes. Electroanalysis 2000, 12, 187–193. [Google Scholar]
- Heng, L.Y.; Hall, E.A.H. One-step synthesis of K+-selective methacrylic-acrylic copolymers containing grafted ionophore and requiring no plasticizer. Electroanalysis 2000, 12, 178–186. [Google Scholar]
- Kimura, K.; Yajima, S.; Okamoto, K.; Yokoyama, M. Supported sol-gel-derived membranes for neutral carrier-type ion-selective electrodes. J. Mater. Chem. 2000, 10, 1819–1824. [Google Scholar]
- Bereczki, R.; Gyurcsanyi, R.; Agai, B.; Toth, K. Synthesis and characterization of covalently immobilized bis-crown ether based potassium ionophore. Analyst 2005, 130, 63–70. [Google Scholar]
- Sugihara, H.; Hiratani, K. 1,10-phenanthroline derivatives as ionophores for alkali metal ions. Coord. Chem. Rev 1996, 148, 285–299. [Google Scholar]
- Hyun, m.H.; Piao, M.H.; Cho, Y.J.; Shim, Y.B. Ionophores in rubidium ion-selective membrane electrodes. Electroanalysis 2004, 16, 1785–1790. [Google Scholar]
- Kim, J.S.; Ohki, A.; Ueki, R.; Ishizuka, T.; Shimotashiro, T.; Maeda, S. Cesium-ion selective electrodes based on calix[4]arene dibenzocrown ethers. Talanta 1999, 43, 705–710. [Google Scholar]
- Mahajan, R.K.; Kumar, M.; Sharma, V.; Kaur, I. Cesium ion selective electrode based on calix[4]crown ether-ester. Talanta 2003, 61, 757–757. [Google Scholar]
- Sheu, H.C.; Shih, J.S. Organic ammonium ion selective electrodes based on 18-crown-6-phosphotungstic acid precipitates. Anal. Chim. Acta 1996, 324, 125–134. [Google Scholar]
- Shvedene, N.V.; Shishkanova, T.V.; Torocheshnikova, I.I.; Nazarova, I.A.; Baulin, V.E.; Zolotov, Y.A.; Pletnev, I.V. Phosphoryl containing podands as carriers in plasticized membrane electrodes selective to quaternary ammonium surfactants. J. Inclus. Phenom. 1998, 32, 9–21. [Google Scholar]
- Moriuchi-Kawakami, T.; Nakazawa, S.; Ota, M.; Nishihira, M.; Hayashi, H.; Shibutani, Y.; Shono, T. Pyrazole-containing crown ethers as ionophores for NH4+-selective electrodes. Anal. Sci. 1998, 14, 1065–1068. [Google Scholar]
- Kim, H.S.; Do, K.S.; Kim, K.S.; Shim, J.H.; Cha, G.S.; Nam, H. Ammonium ion binding property of naphtho-crown ethers containing thiazole as sub-cyclic unit. Bull. Korean Chem. Soc. 2004, 25, 1465–1470. [Google Scholar]
- Ganjali, M.R.; Moghimi, A.; Shamsipur, M. Beryllium-selective membrane electrode based on benzo-9-crown-3. Anal. Chem. 1998, 70, 5259–5263. [Google Scholar]
- Ganjali, M.R.; Daftari, A.; Pourjavid, M.R.; Rastegar, M.F.; Moghimi, A. Novel Be(II) membrane electrode-based on a derivative of benzo-9-crown-3. Main Group Metal Chem. 2002, 25, 669–676. [Google Scholar]
- Ganjali, M.R.; Daftari, A.; Faal-Rastegar, M.; Moghimi, A. Novel potentiometric sensor for monitoring beryllium based on naphto-9-crown-3. Anal. Sci. 2003, 19, 353–356. [Google Scholar]
- Ganjali, M.R.; Akbar, V.; Norouzi, P.; Moghimi, A.; Sepehrifard, A. Be(II) graphite coated membrane sensor based on a recently synthesized benzo-9-crown-3 derivative. Electroanalysis 2005, 17, 895–900. [Google Scholar]
- Ganjali, M.R.; Rahimi-Nasrabadi, M.; Maddah, B.; Moghimi, A.; Faal-Rastegar, M.; Borhany, S.; Namazian, M. Sub-micro level monitoring of beryllium ions with a novel beryllium sensor based on 2,6-diphenyl-4-benzo-9-crown-3-pyridine. Talanta 2004, 63, 899–906. [Google Scholar]
- Ganjali, M.R.; Kiani, R.; Yousefi, M.; Faal-Rastegar, M. Novel potentiometric strontium membrane sensor based on dibenzo-30-crown-10. Anal. Lett. 2003, 36, 2123–2137. [Google Scholar]
- Aghaie, H.; Giahi, A.; Monajjemi, M.; Arvand, A.; Nafissi, G.H.; Aghaie, M. Tin(II)-selective membrane potentiometric sensor using a crown ether as neutral carrier. Sens. Actuators B. 2005, 107, 756–761. [Google Scholar]
- Attiyat, A.S.; Christian, G.D.; Cason, C.V.; Bartsch, R.A. Benzo-18-crown-6 and its lariat ether derivatives as ionophores for potassium, strontium, and lead ion-selective electrodes. Electroanalysis 1992, 4, 51–56. [Google Scholar]
- Sheen, S.R.; Shin, J.S. Lead(II) ion-selective electrodes based on crown ethers. Analyst 1992, 117, 1691–1695. [Google Scholar]
- Srivastava, S.K.; Gupta, V.K.; Jain, S. Determination of lead using a poly(vinyl chloride)-based crown-ether membrane. Analyst 1995, 120, 495–498. [Google Scholar]
- Mousavi, M.F.; Sahari, S.; Alizadeh, N.; Shamsipur, M. Lead ion-selective membrane electrode based on 1,10-dibenzyl-1,10-diaza-18-crown-6. Anal. Chim. Acta 2000, 414, 189–194. [Google Scholar]
- Ganjali, M.R.; Roubollahi, A.; Mardan, A.R.; Hamzeloo, M.; Mogimi, A.; Shamsipur, M. Lead ion-selective electrode based on 4 ′-vinylbenzo-l5-crown-5 homopolymer. Microchem. J 1998, 60, 122–133. [Google Scholar]
- Shamsipur, M.; Ganjali, M.; Rouhollahi, A. Lead-selective membrane potentiometric sensor based on an 18-membered thiacrown derivative. Anal. Sci. 2001, 17, 935–938. [Google Scholar]
- Ganjali, M.R.; Hosseini, M.; Basiripour, F.; Javanbakht, M.; Hashemi, O.R.; Rastegar, M.F.; Shamsipur, M.; Buchanen, G.W. Novel coated-graphite membrane sensor based on N,N'-dimethylcyanodiaza-18-crown-6 for the determination of ultra-trace amounts of lead. Anal. Chim. Acta 2002, 464, 181–186. [Google Scholar]
- Saleh, M.B. Cationic surfactant ion-selective PVC membrane electrode containing macrocyclic diimine crown ether. Anal. Lett. 1999, 32, 2201–2215. [Google Scholar]
- Bobacka, J.; Alaviuhkola, T.; Hietapelto, V.; Koskinen, H.; Lewenstam, A.; Lamsa, M.; Pursiainen, J.; Ivaska, A. Solid-contact ion-selective electrodes for aromatic cations based on pi-coordinating soft carriers. Talanta 2002, 58, 341–349. [Google Scholar]
- Su, C.C.; Ueng, C.H.; Liu, L.K. Characteristics of lariat crown ether-copper(II) ion-selective electrodes. J. Chin. Chem. Soc. 2001, 48, 733–738. [Google Scholar]
- Kuckling, D.; Pareek, P. Synthesis of transition-metal-ion-selective poly(N-isopropylacrylamide) hydrogels by the incorporation of an aza crown ether. J. Polym. Sci. Part A: Polym. Chem. 2003, 41, 1594–1602. [Google Scholar]
- Casabo, J.; Flor, T.; Romero, m.I.; Teixidor, F.; Perezjimenez, C. Silver-selective membrane electrodes using acyclic dithia benzene drivative neutral carriers–comparision with related macrocyclic-compounds. Anal. Chim. Acta 1994, 294, 207–213. [Google Scholar]
- Casabo, J.; Teixidor, F.; Escriche, L.; Vinas, C.; Perezjimenez, C. Silver-selective electrodes based on supported liquid membranes. Adv. Mater 1995, 7, 238–243. [Google Scholar]
- Siswanta, D.; Nagatsuka, K.; Yamada, N.; Kumakura, K.; Hisamoto, H.; Shichi, Y.; Toshima, K.; Suzuki, K. Structural ion selectivity of thia crown ether compounds with a bulky block subunit and their application as an ion-sensing component for an ion-selective electrode. Anal. Chem. 1996, 68, 4166–4172. [Google Scholar]
- Su, C.C.; Chang, M.C.; Liu, L.K. New Ag+- and Pb2+-selective electrodes with lariat crown ethers as ionophores. Anal. Chim. Acta 2001, 432, 261–267. [Google Scholar]
- Gupta, V.K.; Al Khayat, M.; Minocha, A.; Kumar, P. Zinc(II)-selective sensors based on dibenzo-24-crown-8 in PVC matrix. Anal. Chim. Acta 2005, 532, 153–158. [Google Scholar]
- Gupta, V.K.; Chandra, S.; Mangla, R. Dicyclohexano-18-crown-6 as active material in PVC matrix membrane for the fabrication of cadmium selective potentiometric sensor. Electrochim. Acta 2002, 47, 1579–1586. [Google Scholar]
- Gupta, V.K.; Jain, S.; Khurana, U.A. PVC-based pentathia-15-crown-5 membrane potentiometric sensor for mercury(II). Electroanalysis 1997, 9, 478–480. [Google Scholar]
- Fakhari, A.R.; Ganjali, M.R.; Shamsipur, M. PVC-based hexathia-18-crown-6-tetraone sensor for mercury(II) ions. Anal. Chem. 1997, 69, 3693–3696. [Google Scholar]
- Javanbakht, M.; Ganjali, M.R.; Eshghi, H.; Sharghi, H.; Shamsipur, M. Mercury(II) ion-selective electrode based on dibenzodiazathia-18-crown-6-dione. Electroanalysis 1999, 11, 81–84. [Google Scholar]
- Lee, S.S.; Ahn, M.K.; Park, S.B. Silver(I)-selective membrane electrodes based on mono- to quadridentate podands. Analyst 1998, 123, 383–386. [Google Scholar]
- Hayashita, T.; Qing, D.; Minagawa, M.; Lee, J.C.; Ku, C.H.; Teramae, N. Highly selective recognition of lead ion in water by a podand fluoroionophore/g-cyclodextrin complex sensor. Chem. Commun 2003, 17, 2160–2161. [Google Scholar]
- Yan, Z.; Fan, Y.; Gao, Q.; Lu, H.; Hou, H. Tripodal compound 1,1,1-tris(N-ethyl-N-phenylamino-carboxylmethoxymethyl) propane as an ionophore for alkali and alkaline earth metal cations-selective electrode. Talanta 2002, 57, 81–88. [Google Scholar]
- Kim, Y.K.; Ha, J.; Cha, G.S.; Ahn, K.H. Synthesis of tripodal trifluoroacetophenone derivatives and their evaluation as ion-selective electrode membranes. Bull. Korean Chem. Soc. 2002, 23, 1420–1424. [Google Scholar]
- Berrocal, M.J.; Cruz, A.; Badr, H.A.; Bachas, L.G. Tripodal Ionophore with Sulfate Recognition Properties for Anion-Selective Electrodes. Anal. Chem. 2000, 72, 5295–5299. [Google Scholar]
- Alizadeh, N.; Ershad, S.; Naeimi, H.; Sharghi, H.; Shamsipur, M. Copper(II)-selective membrane electrode based on a recently synthesized naphthol-derivative, Schiff's base. Fresenius J. Anal. Chem. 1999, 365, 511–515. [Google Scholar]
- Gupta, V.K.; D'Arc, M.J. Performance evaluation of copper ion selective electrode based on cyanocopolymers. Sens. Actuators B. 2000, 62, 171–176. [Google Scholar]
- Poursaberi, T.; Hajiagha-Babaei, L.; Yousefi, M.; Rouhani, S.; Shamsipur, M.; Kargar-Razi, M.; Moghimi, A.; Aghabozorg, H.; Ganjali, M.R. The synthesis of a new thiophene-derivative Schiff's base and its use in preparation of copper-ion selective electrodes. Electroanalysis 2001, 13, 1513–1517. [Google Scholar]
- Ganjali, M.R.; Poursaberi, T.; Yousefi, M.; Rouhani, S.; Hajiagha-Babaei, L.; Kargar-Razi, M.; Moghimi, A.; Aghabozorg, H.; Shamsipur, M. Highly selective and sensitive copper(II) membrane coated graphite electrode based on a recently synthesized Schiff's base. Anal. Chim. Acta 2001, 440, 81–87. [Google Scholar]
- Mashhadizadeh, M.H.; Sheikhshoaie, I. Mercury(II) ion-selective polymeric membrane sensor based on a recently synthesized Schiff base. Talanta 2003, 60, 73–80. [Google Scholar]
- Ganjali, M.R.; Emami, M.; Rezapour, M.; Shamsipur, M.; Maddah, M.; Salavat-Niasari, M.; Hosseini, M.; Talebpour, Z. Novel gadolinium poly(vinyl chloride) membrane sensor based on a new S-N Schiff's base. Anal. Chim. Acta 2003, 495, 51–59. [Google Scholar]
- Ganjali, M.R.; Emami, M.; Salavat-Niasari, M.; Yousefi, M. Determination of trace amounts of Cr(III) in presence of Cr(VI) by a novel potentiometric membrane sensor based on a new tridentate S,N,O Schiff's base. Anal. Lett. 2003, 36, 2735–2747. [Google Scholar]
- Ganjali, M.R.; Daftari, A.; Norouzi, P.; Salavat-Niasari, M. Novel Y(III) PVC-based membrane microelectrode based on a new S-N Schiff's base. Anal. Lett. 2003, 36, 1511–1522. [Google Scholar]
- Ganjali, M.R.; Ravanshad, J.; Hosseini, M.; Salavati-Niasari, M.; Pourjavid, M.R.; Baezzat, M.R. Novel Dy(III) sensor based on a new bis-pyrolidene schiff's base. Electroanalysis 2004, 16, 1771–1776. [Google Scholar]
- Shamsipur, M.; Saeidi, M.; Yari, A.; Yaganeh-Faal, A.; Mashhadizadeh, M.H.; Azimi, G.; Naeimi, H.; Sharghi, H. UO22+ ion-selective membrane electrode based on a naphthol-derivative Schiff's base 2,2′-[1,2-ethandiylbis (nitriloethylidene)] bis(1-naphthalene). Bull. Korean Chem. Soc. 2004, 25, 629–633. [Google Scholar]
- Ganjali, M.R.; Qomi, M.; Daftari, A.; Norouzi, P.; Salavat-Niasari, M.; Rabbani, M. Novel lanthanum(III) membrane sensor based on a new N-S Schiff's base. Sens. Actuators B. 2004, 98, 92–96. [Google Scholar]
- Shamsipur, M.; Ershad, S.; Samadi, N.; Esmaeilbeig, A.R.; Kia, R.; Abdolmaleki, A. Polymeric membrane lanthanum(III)-selective electrode based on N,N'-adipylbis(5-plenylazo salicylaldehyde hydrazone). Electroanalysis 2005, 17, 1828–1834. [Google Scholar]
- Ganjali, M.R.; Matloobi, P.; Ghorbani, M.; Norouzi, P.; Salavat-Niasari, M. La(III) selective membrane sensor based on a new N-N Schiff's base. Bull. Korean Chem. Soc. 2005, 26, 38–42. [Google Scholar]
- Ganjali, M.R.; Ghesmi, A.; Hosseini, M.; Pourjavid, M.R.; Rezapour, M.; Shamsipur, M.; Salavati-Niasari, M. Novel terbium(III) sensor based on a new bis-pyrrolidene Schiff's base. Sens. Actuators B. 2005, 105, 334–339. [Google Scholar]
- Ganjali, M.R.; Dodangeh, M.; Ghorbani, H.; Norouzi, P.; Adib, M. PPb level monitoring of Dy(III) ions by a highly sensitive and selective Dy(III) sensor based on a new asymmetrical Schiff's bas. Anal. Lett. 2006, 39, 495–506. [Google Scholar]
- Saleh, M.B.; Soliman, E.M.; Gaber, A.A.A.; Ahmed, S.A. A novel Hg(II) PVC membrane sensor based on simple ionophore ethylenediamine bis-thiophenecarboxaldehyde. Anal. Lett. 2006, 39, 659–673. [Google Scholar]
- Ganjali, M.R.; Norouzi, P.; Daftari, A.; Faridbod, F.; Salavati-Niasari, M. Fabrication of a highly selective Eu(III) membrane sensor based on a new S–N hexadentates Schiff's base. Sens. Actuators B. 2006. in Press. [Google Scholar]
- Ganjali, M.R.; Tamaddon, A.; Norouzi, P.; Adib, M. Novel Lu(III) membrane sensor based on a new asymmetrically S–N Schiff's base. Sens. Actuators B. 2006, 120, 194–199. [Google Scholar]
- Sun, C.J.; Sun, X.X.; Aboul-Enein, H.Y. Potentiometric, enantioselective electrode responsive to propranolol enantiomers based on a lipophilic beta-cyclodextrin. Anal. Lett. 2004, 37, 2259–2266. [Google Scholar]
- Shamsipur, M.; Jalali, F.; Ershad, S. Preparation of a diclofenac potentiometric sensor and its application to pharmaceutical analysis and to drug recovery from biological fluids. J. Pharm. Biomed. Anal 2005, 37, 943–947. [Google Scholar]
- El-Kosasy, A.M.; Shehata, M.A.; Hassan, N.Y.; Fayed, A.S.; El-Zeany, B.A. Membrane electrodes for the determination of glutathione. Talanta 2005, 66, 746–754. [Google Scholar]
- Fukui, H.; Kaminaga, A.; Maeda, T.; Hayakawa, K. Preparation of surfactant anion-selective electrodes with different selectivity coefficients and a trial to determine each component in binary surfactant mixtures. Anal. Chim. Acta 2003, 481, 221–228. [Google Scholar]
- Lima, J.L.F.C.; Montenegro, M.C.B.S.M. Dopamine ion-selective electrode for potentiometry in pharmaceutical preparations. Mikrochim. Acta 1999, 131, 187–190. [Google Scholar]
- Li, X.W.; Yang, B.L.; Wu, Y.Y.; Lin, H.Y. Determination of atropine in injection with beta-cyclodextrin modified ion sensitive field effect transistor sensor. Sensors 2005, 5, 604–612. [Google Scholar]
- Kelani, K.M. Selective potentiometric determination of zolpidem hemitartrate in tablets and biological fluids by using polymeric membrane electrodes. JAOAC Intern. l 2004, 87, 1309–1318. [Google Scholar]
- Mahajan, R.K.; Kaur, I.; Bakshi, M.S. Cationic surfactant-selective potentiometric sensors based on neutral ion-pair carrier complexes. J. Surf. Deterg 2004, 7, 131–134. [Google Scholar]
- Missel, P.J. Coated-wire ion-selective electrodes for levobetaxolol. Langmuir 1999, 15, 7122–7124. [Google Scholar]
- Odashima, K.; Hashimoto, H.; Umezawa, Y. Potentiometric discrimination of organic amines by liquid membrane electrodes based on a long alkyl chain derivative of beta-cyclodextrin. Mikrochim. Acta 1994, 113, 223–238. [Google Scholar]
- Peyre, V.; Baillet, S.; Letellier, P. Ion-selective electrode for dodecyldimethylamine oxide: A “twice-Nernstian” slope for the determination of a neural component. Anal. Chem. 2000, 72, 2377–2382. [Google Scholar]
- Zhang, G.; Wang, X.; Shi, X.; Sun, T. b-Cyclodextrin–ferrocene inclusion complex modified carbon paste electrode for amperometric determination of ascorbic acid. Talanta 2000, 53, 1019–1025. [Google Scholar]
- Gong, F.Ch.; Zhang, X.B.; Guo, C.Ch.; Shen, G.L.; Yu, R.Q. Amperometric metronidazole sensor based on the supermolecular recognition by metalloporphyrin incorporated in carbon paste electrode. Sensors 2003, 3, 91–100. [Google Scholar]
- Damos, F.S.; Sotomayer, M.D.T.; Kubota, L.T.; Tanaka, S.M.C.N.; Tanaka, A.A. Iron(III) tetra-(N-methyl-4-pyridyl)-porphyrin as a biomimetic catalyst of horseradish peroxidase on the electrode surface: An amperometric sensor for phenolic compound eterminations. Analyst 2003, 128, 255–259. [Google Scholar]
- Quintino, M.S.M.; Winnischofer, H.; Araki, K.; Toma, H.; Angnes, E.L. Cobalt oxide/tetraruthenated cobalt-porphyrin composite for hydrogen peroxide amperometric sensors. Analyst 2005, 130, 221–226. [Google Scholar]
- Quintino, M.S.M.; Winnischofer, H.; Nakamura, M.; Araki, K.; Toma, H.; Angnes, E.L. Amperometric sensor for glucose based on electrochemically polymerized tetraruthenated nickel-porphyrin. Analytica Chimica Acta 2005, 539, 215–222. [Google Scholar]
- Yang, D.W.; Gong, F.C.; Cao, Z. Amperometric dimetridazole sensor using glycosylated metalloporphyrin as a recognition element. Sens. Actuators B. 2006, 114, 152–157. [Google Scholar]
- Santos, W.J.R.; Sousa, A.; Luz, L.R.C.S.; Damos, F.S.; Kubota, L.T.; Tanaka, A.A.; Tanaka, S.M.C.N. Amperometric sensor for nitrite using a glassy carbon electrode modified with alternating layers of iron(III) tetra-(N-methyl-4-pyridyl)-porphyrin and cobalt(II) tetrasulfonated phthalocyanine. Talanta 2006, 70, 588–594. [Google Scholar]
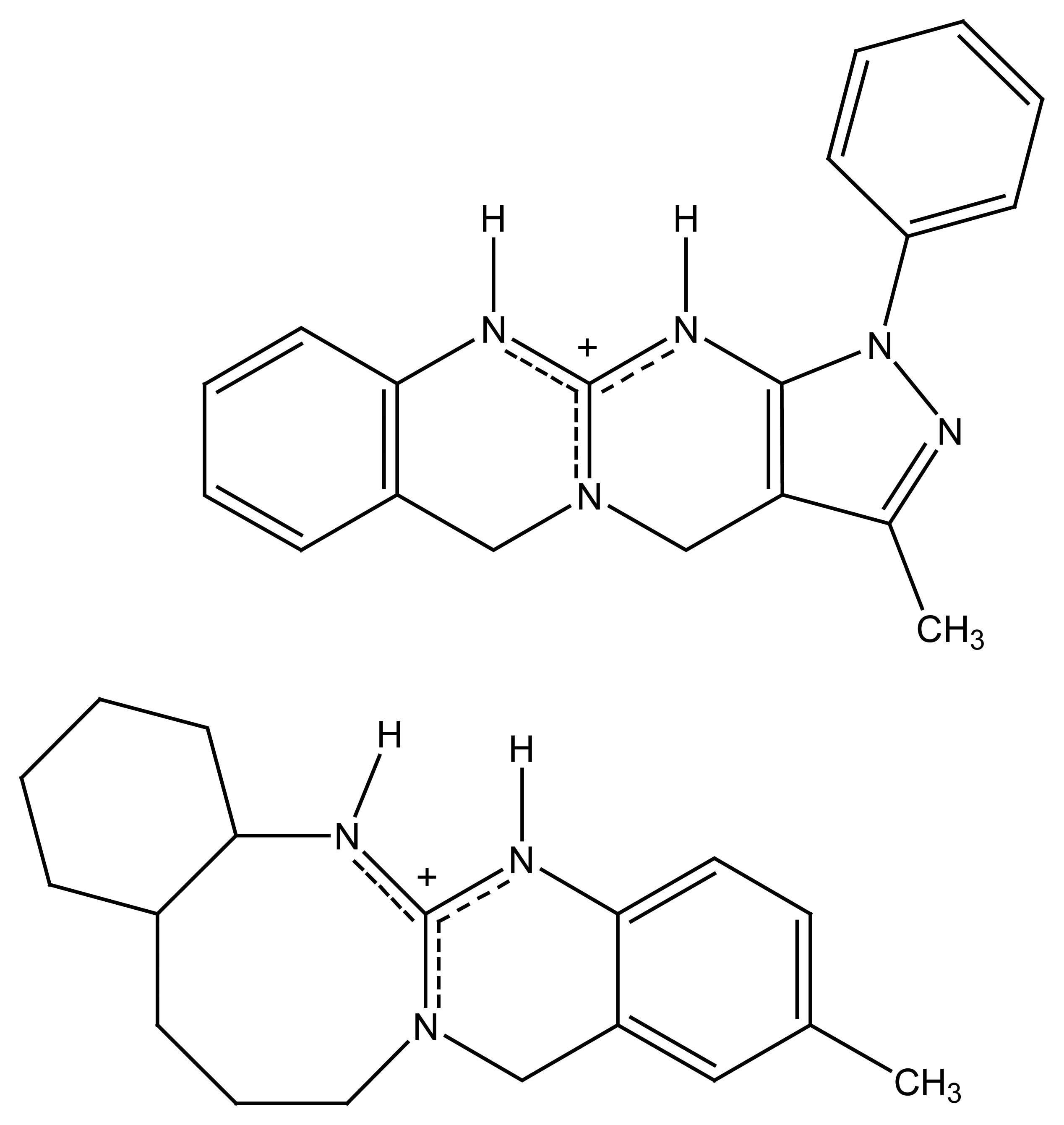
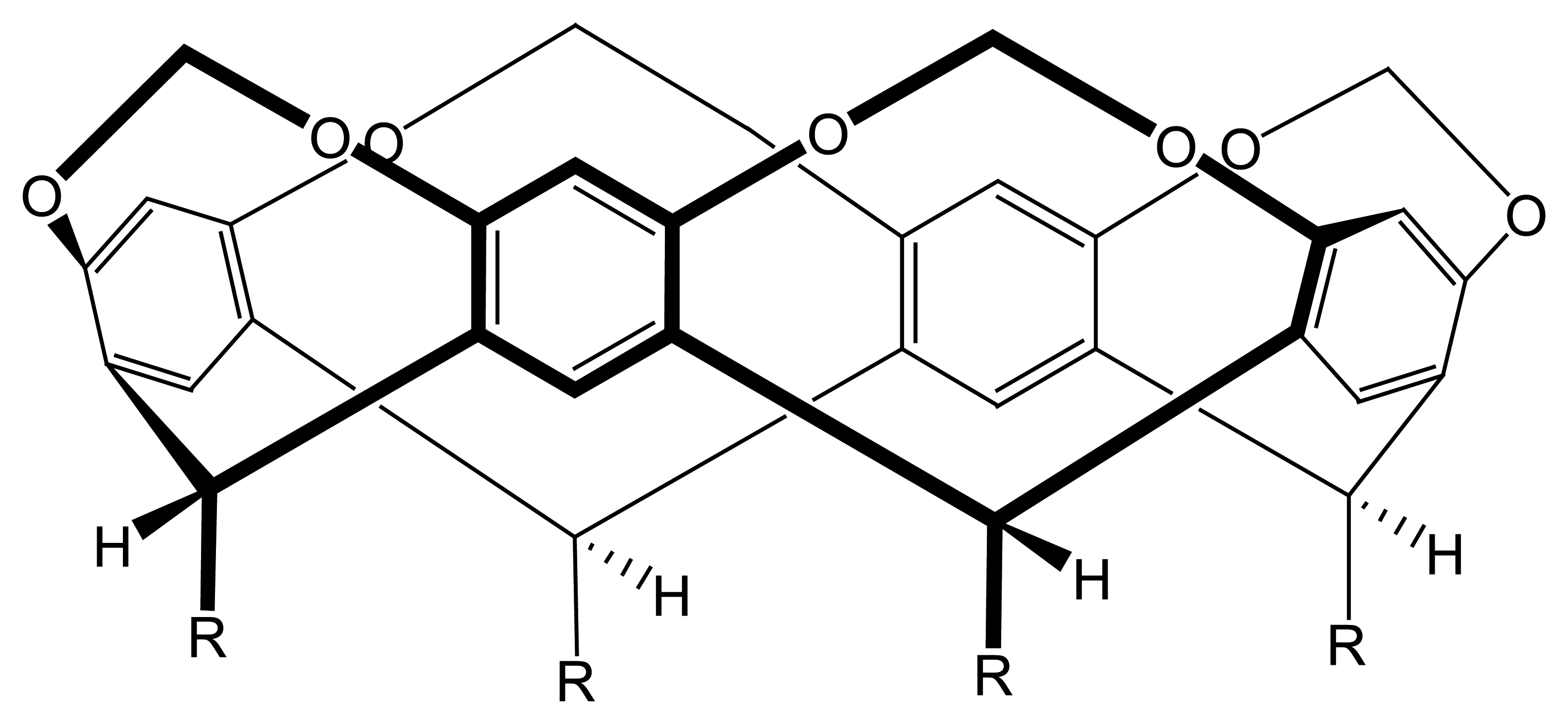
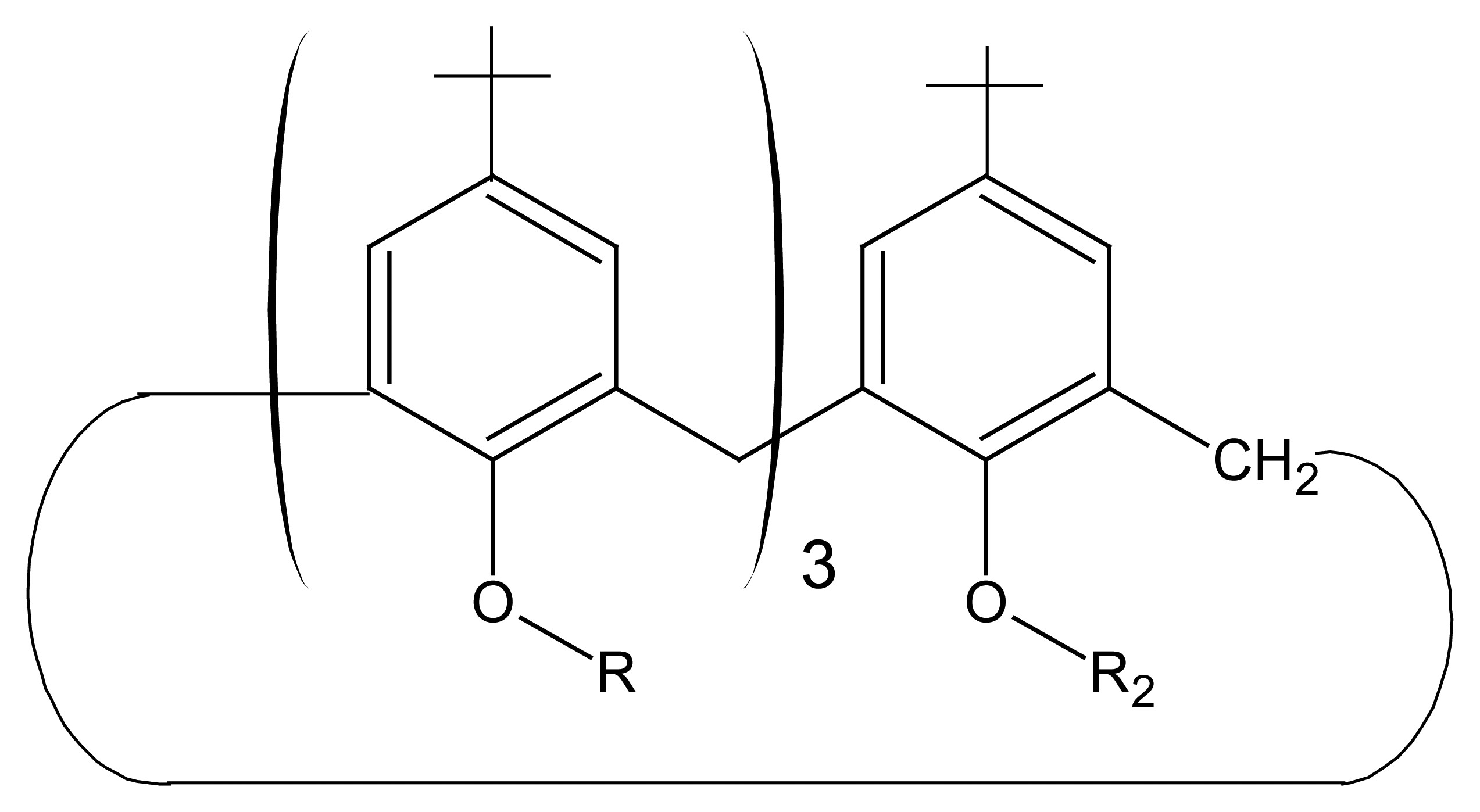
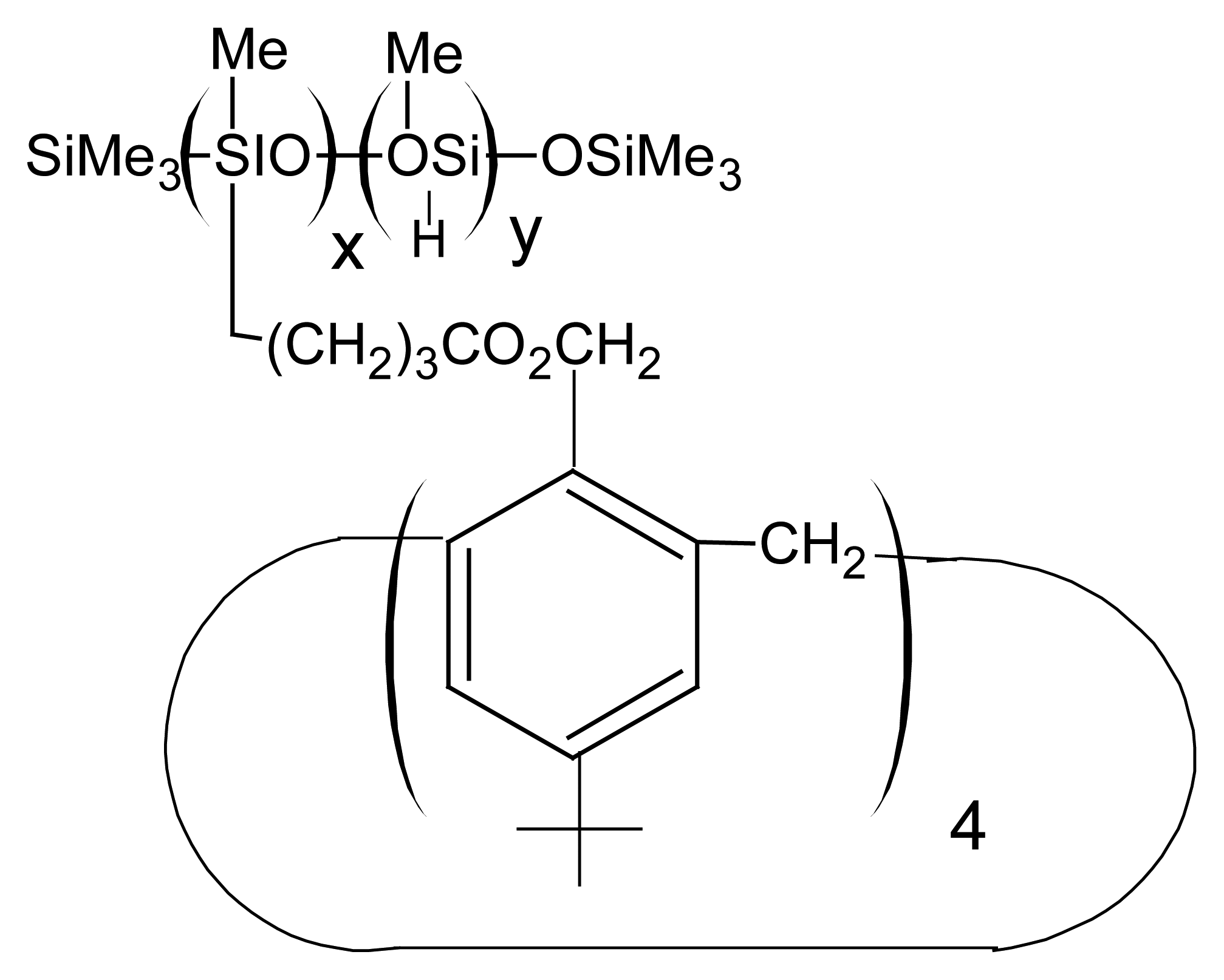
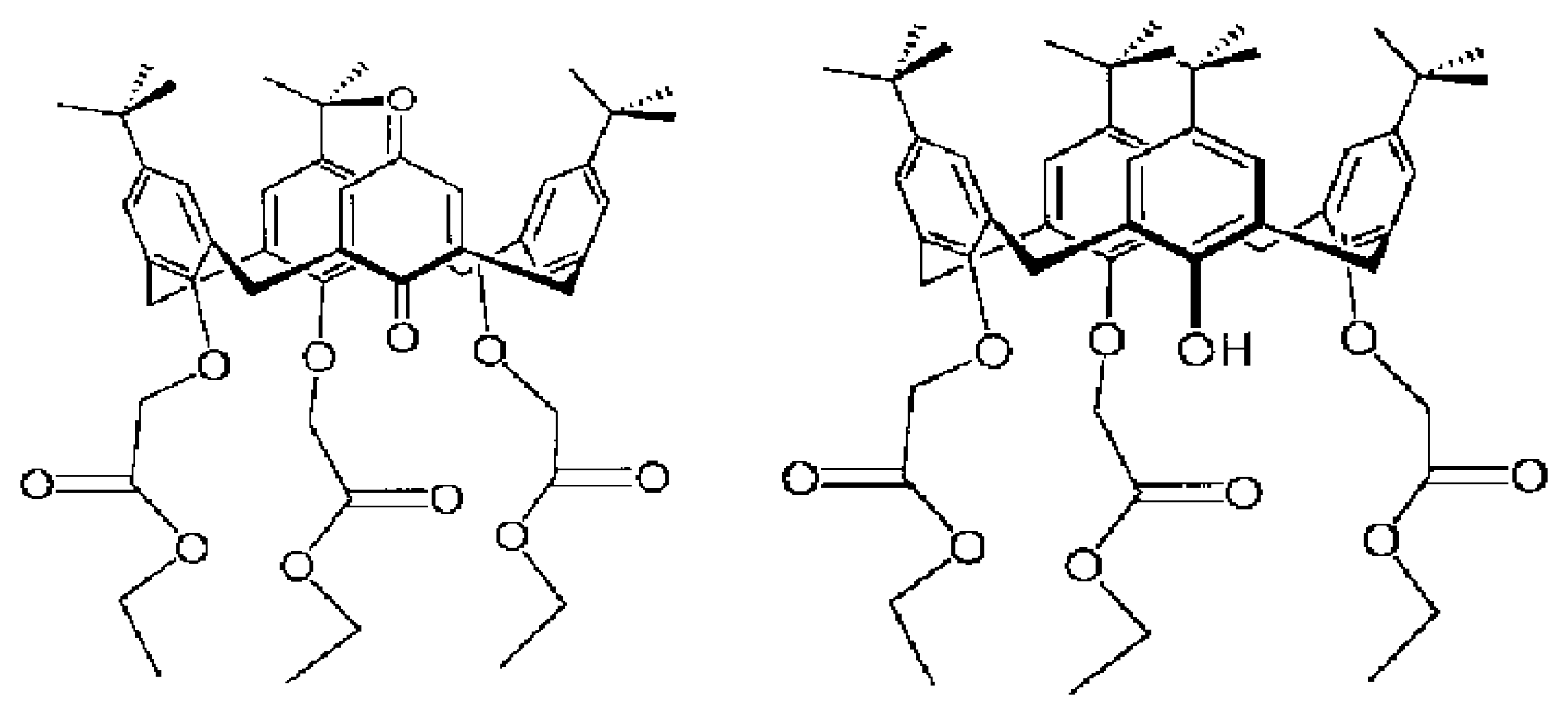

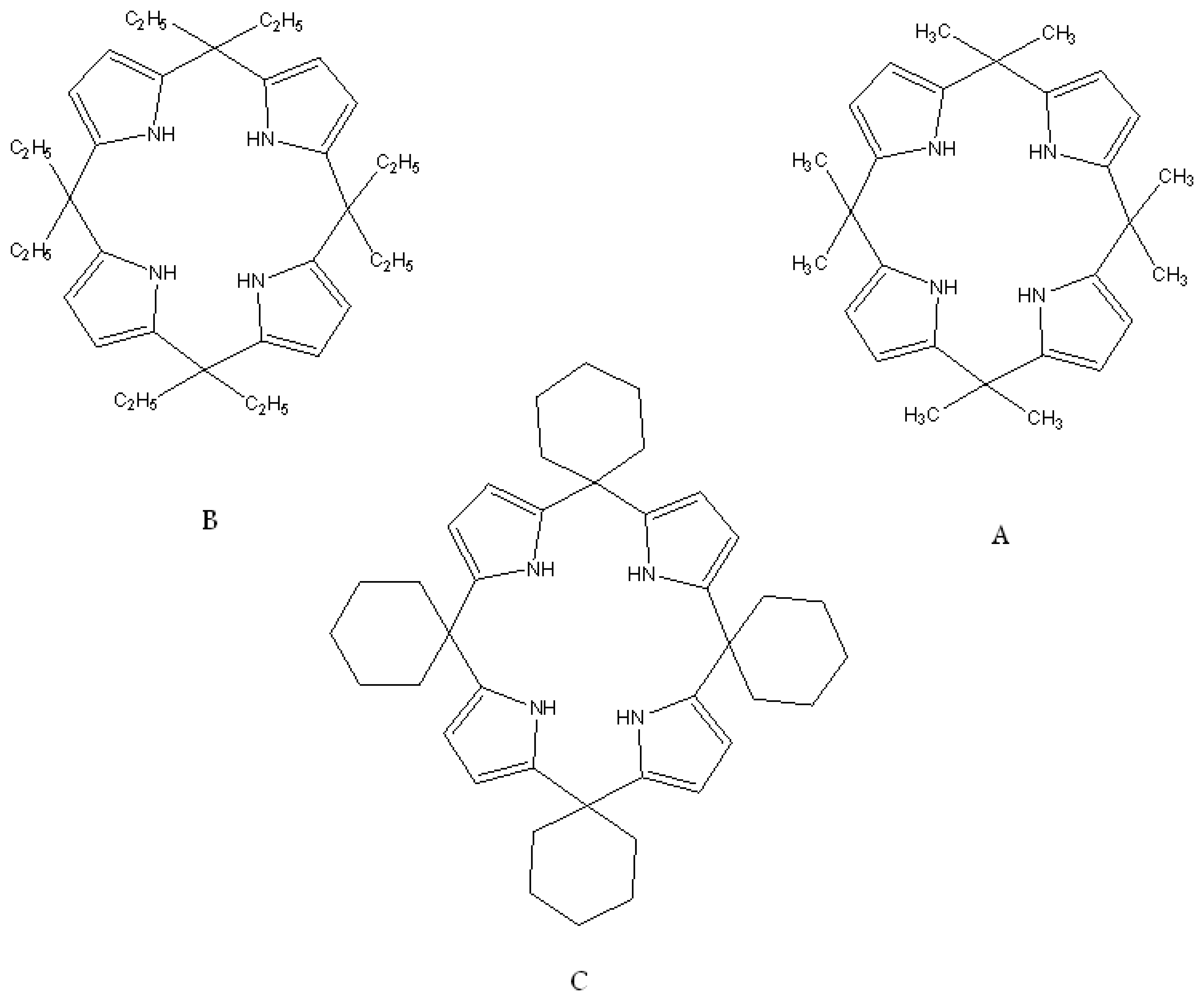

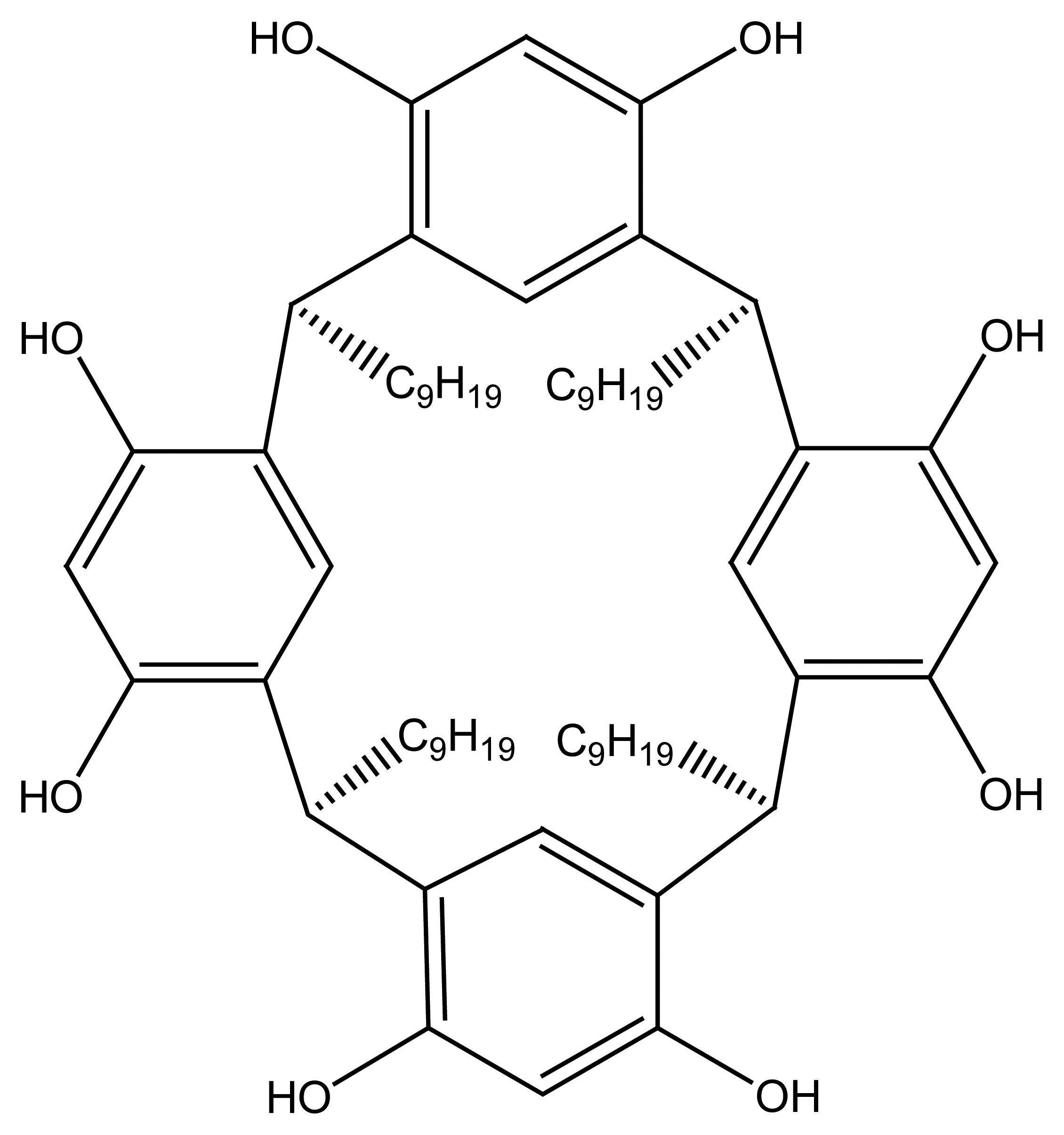
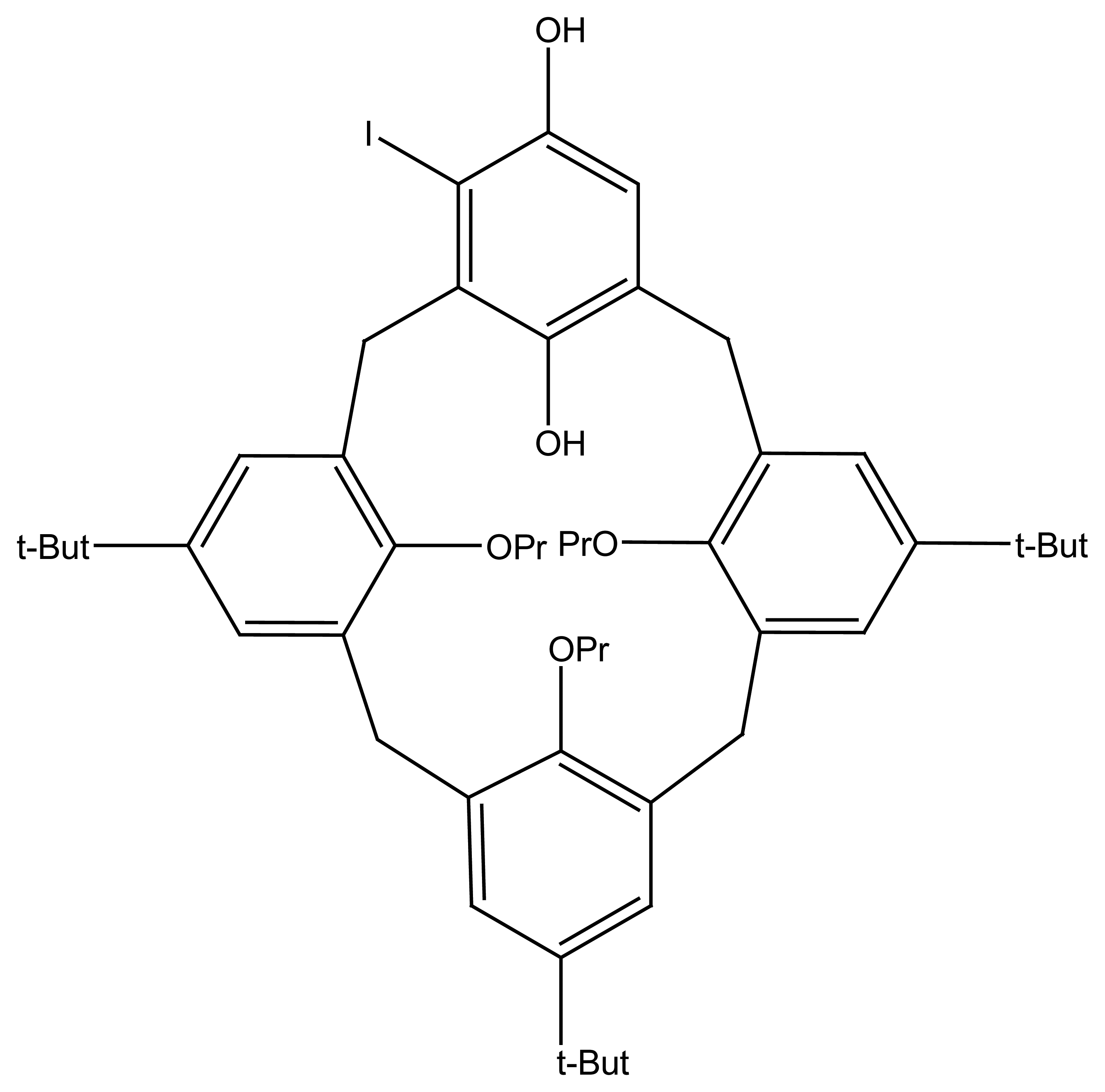
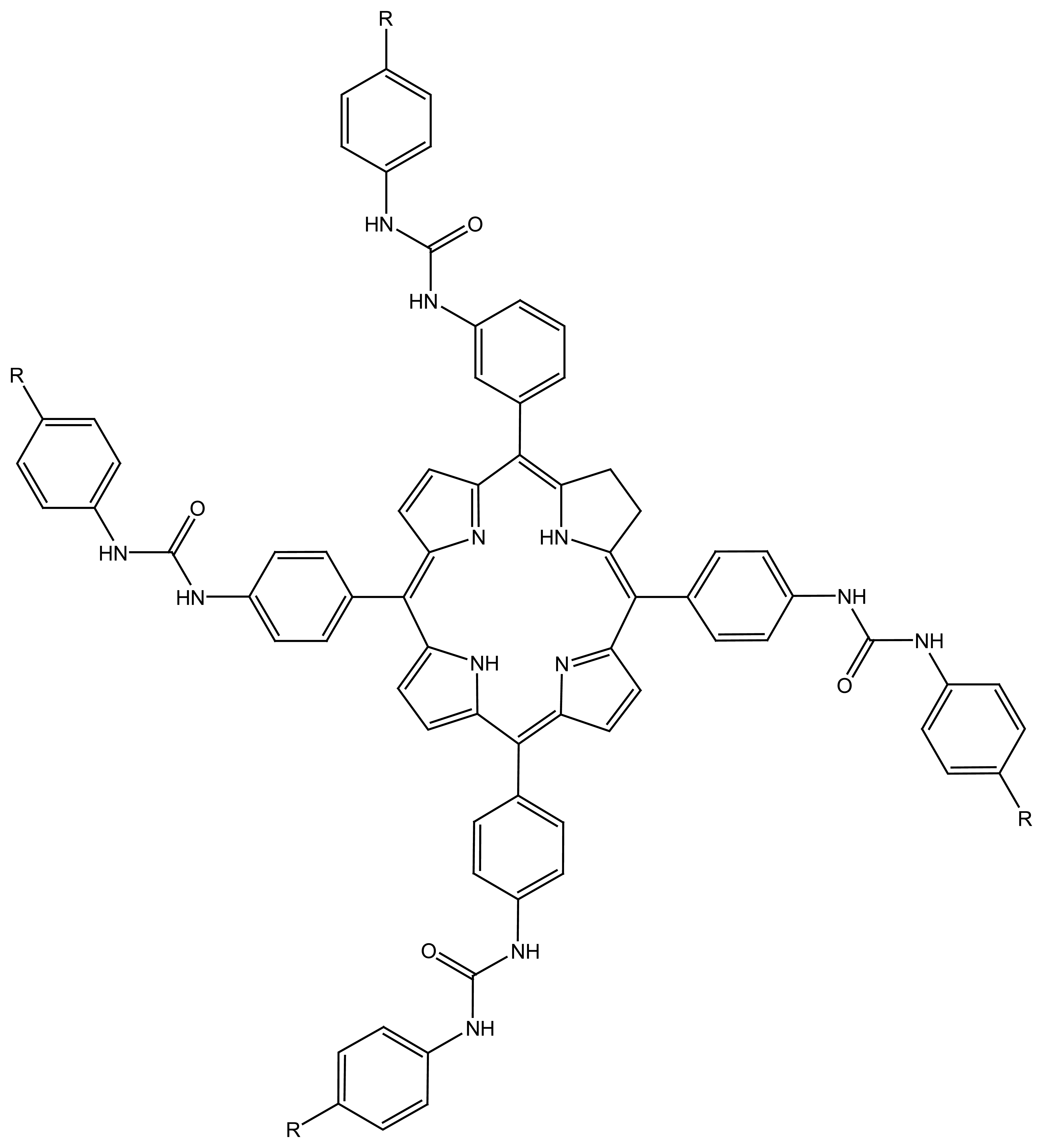
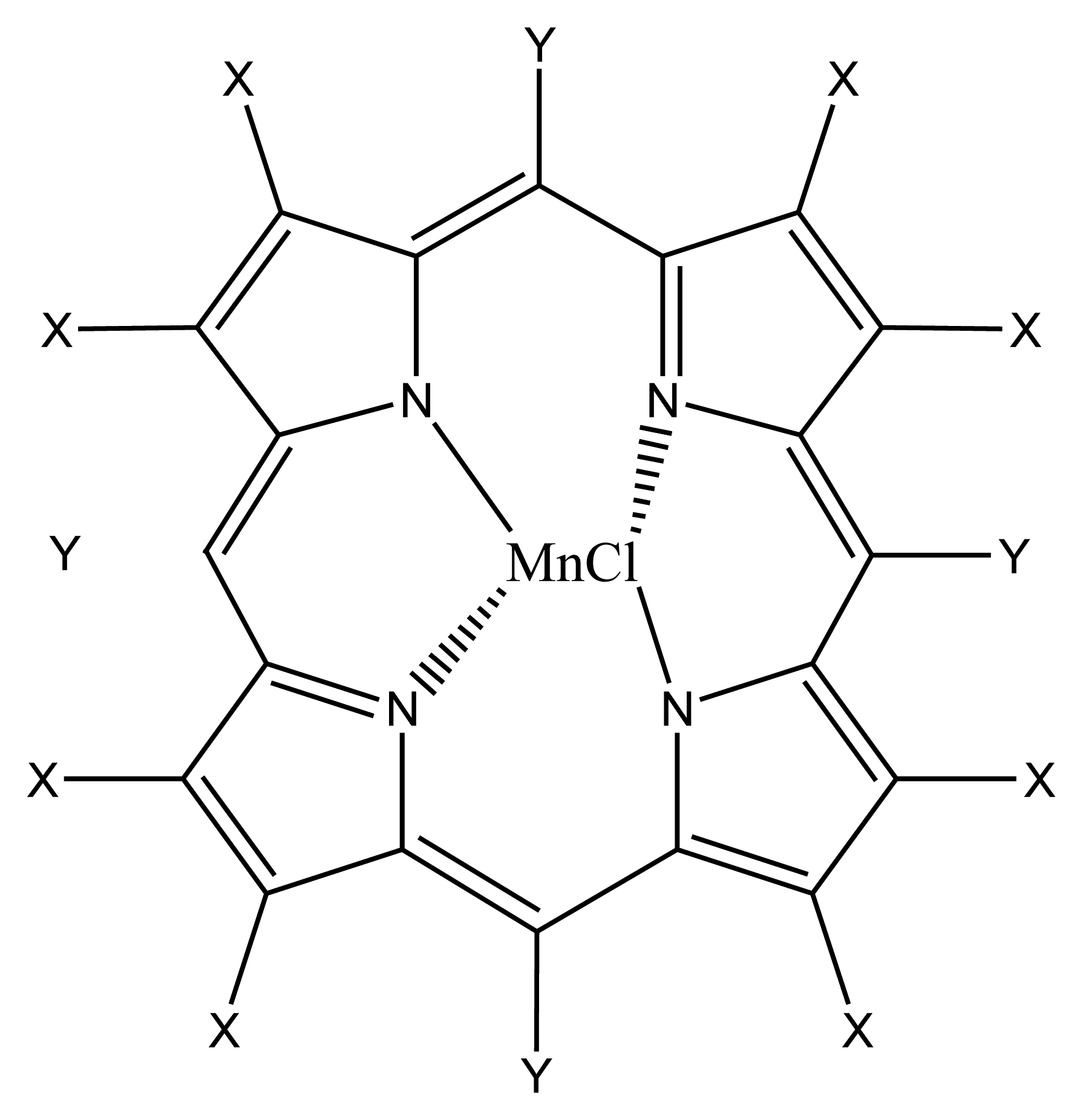
| R1=R2= CH3 | Mn(TMP)Cl (I) |
| R1= Cl, R2= H | Mn(Cl8TPP)Cl (II) |
| R1=R2= CH3 | Mn(TMP)Cl (I) |
| R1= Cl, R2= H | Mn(Cl8TPP)Cl (II) |








© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ganjali, M.R.; Norouzi, P.; Rezapour, M.; Faridbod, F.; Pourjavid, M.R. Supramolecular Based Membrane Sensors. Sensors 2006, 6, 1018-1086. https://doi.org/10.3390/s6081018
Ganjali MR, Norouzi P, Rezapour M, Faridbod F, Pourjavid MR. Supramolecular Based Membrane Sensors. Sensors. 2006; 6(8):1018-1086. https://doi.org/10.3390/s6081018
Chicago/Turabian StyleGanjali, Mohammad Reza, Parviz Norouzi, Morteza Rezapour, Farnoush Faridbod, and Mohammad Reza Pourjavid. 2006. "Supramolecular Based Membrane Sensors" Sensors 6, no. 8: 1018-1086. https://doi.org/10.3390/s6081018



