Miniaturized, Planar Ion-selective Electrodes Fabricated by Means of Thick-film Technology
Abstract
:Introduction
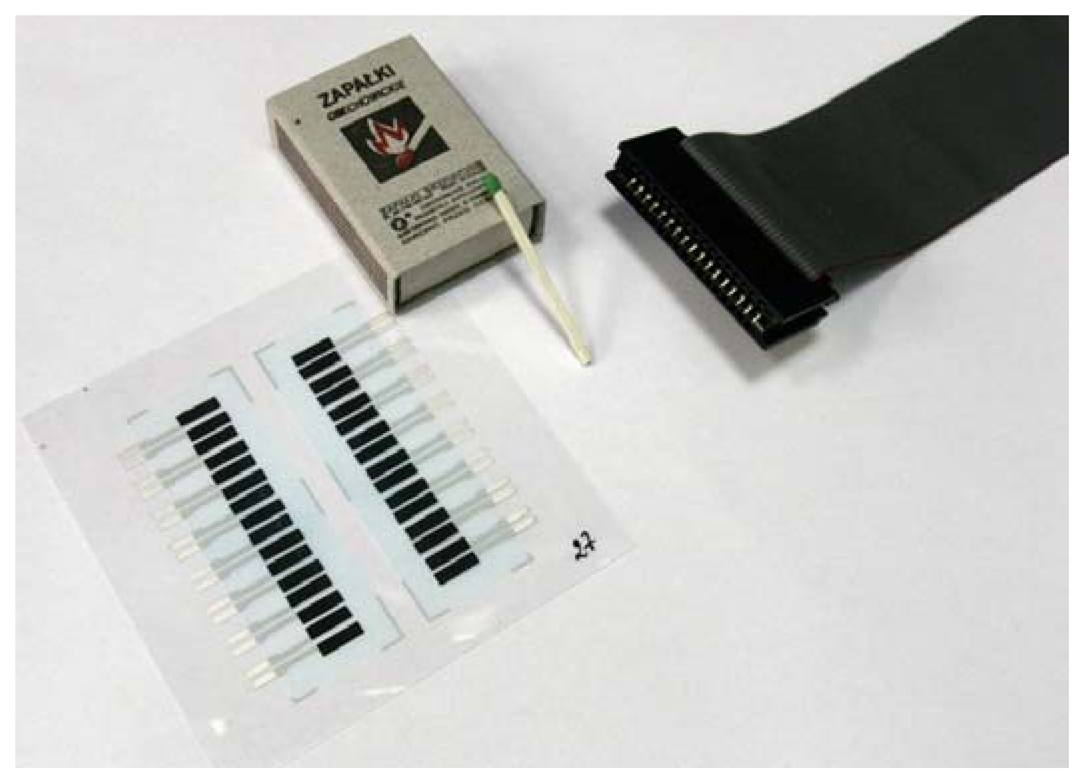
Thick-film technology
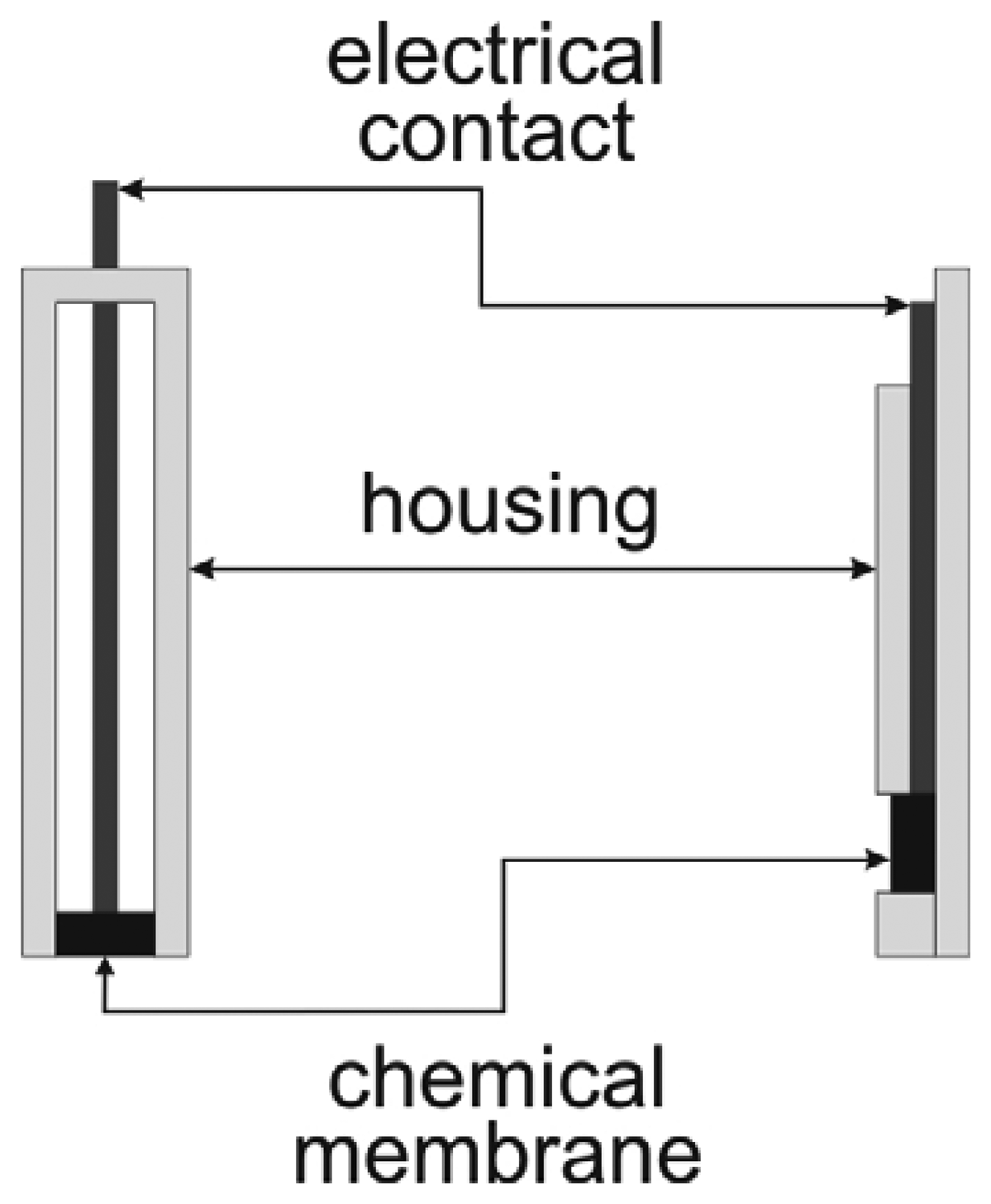
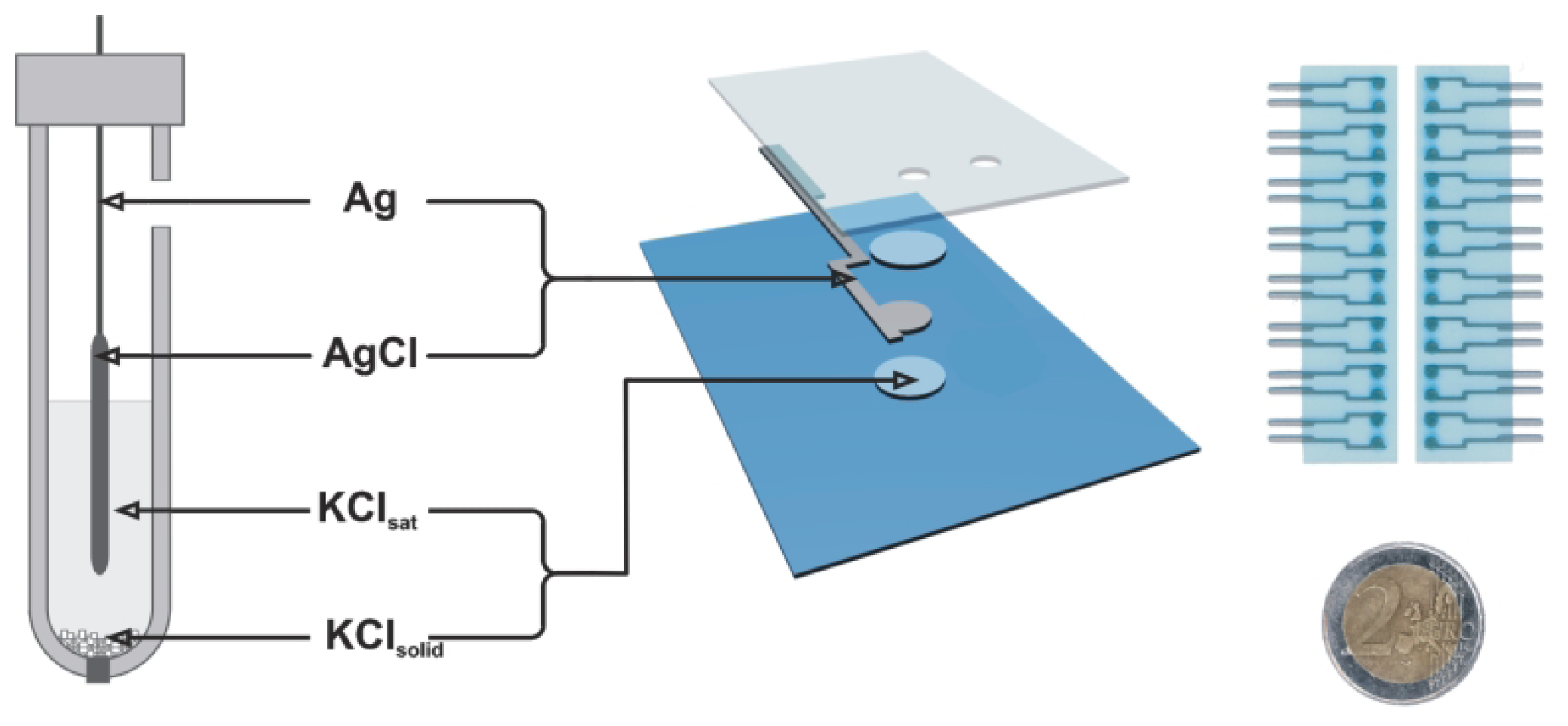
Thick-film electrodes
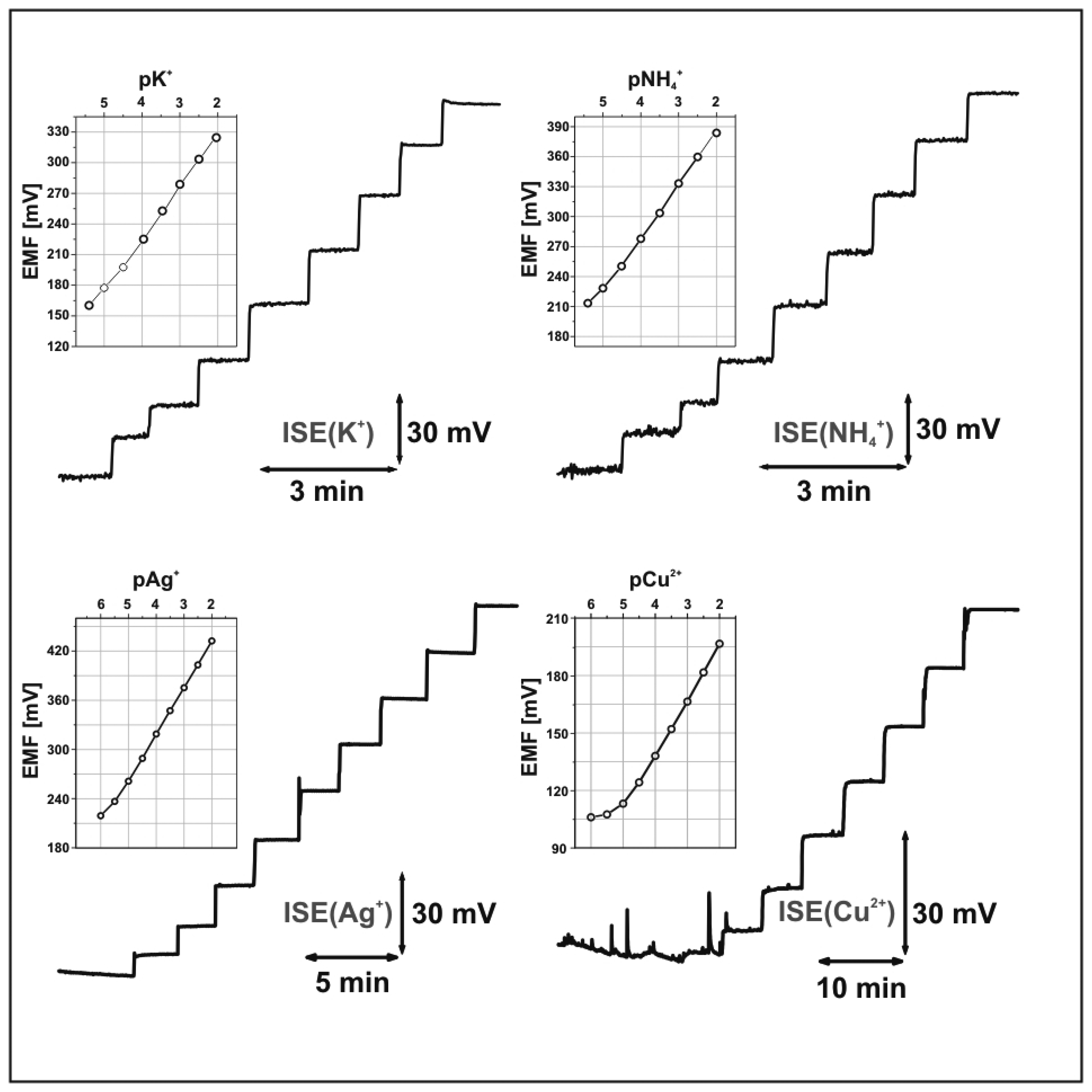
Thick-film ion-selective electrodes
Conclusions
References
- Galan-Vidal, C.A.; Munoz, J.; Dominguez, C.; Alegret, S. Chemical sensors, biosensors and thick-film technology. Trends in Analytical Chemistry 1995, 14, 225. [Google Scholar]
- Hart, J.P.; Wring, S.A. Recent developments in the design and application of screen-printed electrochemical sensors for biomedical, environmental and industrial analyses. Trends in Analytical Chemistry 1997, 16, 89. [Google Scholar]
- Honeychurch, K.C.; Hart, J.P. Screen-printed electrochemical sensors for monitoring metal pollutants. Trends in Analytical Chemistry 2003, 22, 456. [Google Scholar]
- Hart, J.P.; Crew, A.; Crouch, E.; Honeychurch, K.C.; Pembelton, R.M. Some recent designs and developments of screen-printed carbon electrochemical sensors/biosensors for biomedical, environmental, and industrial analyses. Analytical Letters 2004, 37, 789. [Google Scholar]
- Koncki, R.; Głąb, S.; Dziwulska, J.; Palchetti, I.; Mascini, M. Disposable strip potentiometric electrodes with solvent-polymeric ion-selective membranes fabricated using screen-printing technology. Analytica Chimica Acta 1999, 385, 451. [Google Scholar]
- Koncki, R.; Tymecki, Ł.; Zwierkowska, E.; Głąb, S. Screen-printed copper ion-selective electrodes. Fresenius Journal of Analytical Chemistry 2000, 367, 393. [Google Scholar]
- Tymecki, Ł.; Jakubowska, M.; Achmatowicz, S.; Koncki, R.; Głąb, S. Potentiometric thick-film graphite electrodes with improved response to copper ions. Analytical Letters 2001, 34, 71. [Google Scholar]
- Tymecki, Ł.; Zwierkowska, E.; Głąb, S.; Koncki, R. Strip thick-film silver ion-selective electrodes. Sensors and Actuators B 2003, 96, 482. [Google Scholar]
- Tymecki, Ł.; Zwierkowska, E.; Koncki, R. Screen-printed reference electrodes for potentiometric measurements. Analytica Chimica Acta 2004, 526, 3. [Google Scholar]


© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for non-commercial purposes.
Share and Cite
Tymecki, L.; Glab, S.; Koncki, R. Miniaturized, Planar Ion-selective Electrodes Fabricated by Means of Thick-film Technology. Sensors 2006, 6, 390-396. https://doi.org/10.3390/s6040390
Tymecki L, Glab S, Koncki R. Miniaturized, Planar Ion-selective Electrodes Fabricated by Means of Thick-film Technology. Sensors. 2006; 6(4):390-396. https://doi.org/10.3390/s6040390
Chicago/Turabian StyleTymecki, Lukasz, Stanisław Glab, and Robert Koncki. 2006. "Miniaturized, Planar Ion-selective Electrodes Fabricated by Means of Thick-film Technology" Sensors 6, no. 4: 390-396. https://doi.org/10.3390/s6040390



