ZnO:Al Thin Film Gas Sensor for Detection of Ethanol Vapor
Abstract
:1. Introduction
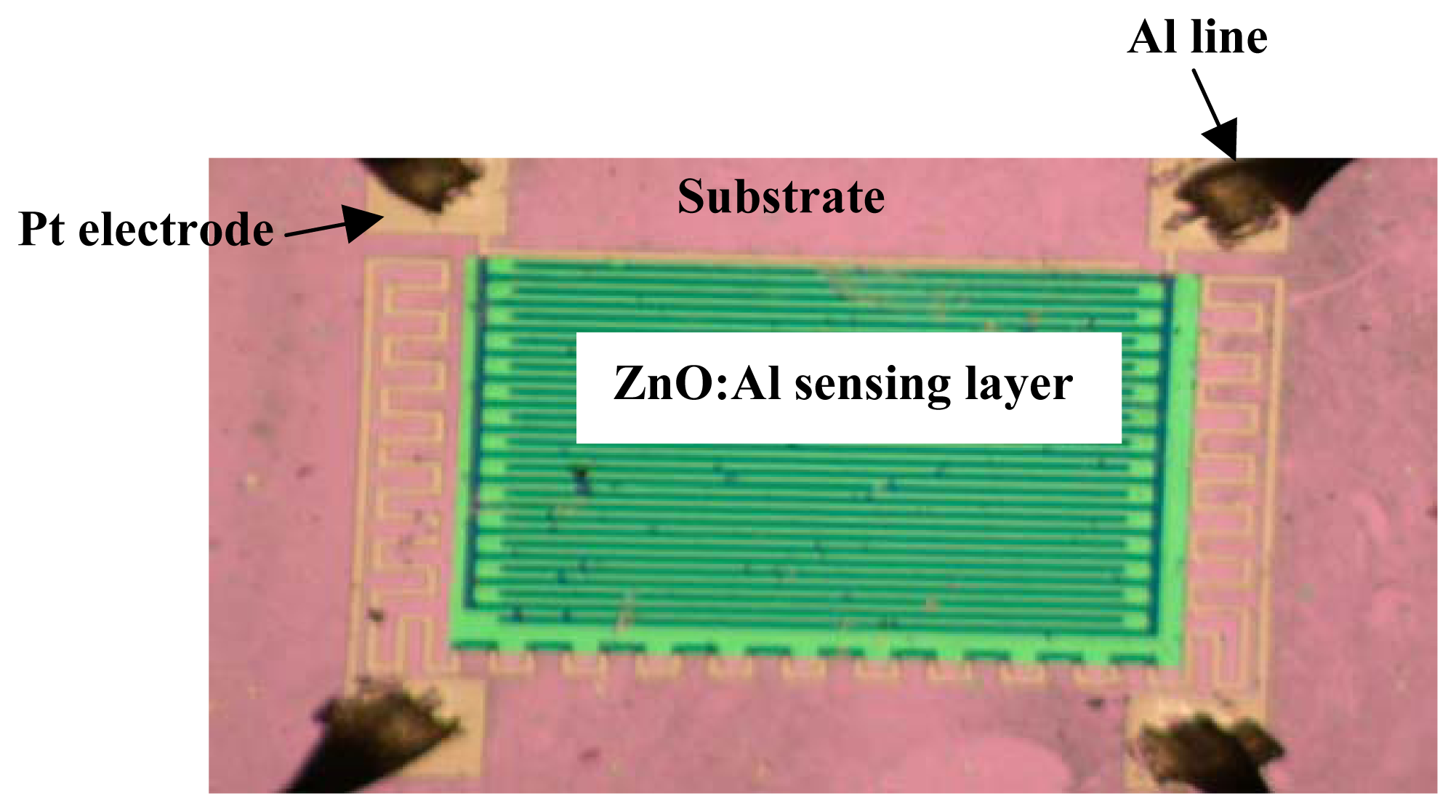
2. Experimental
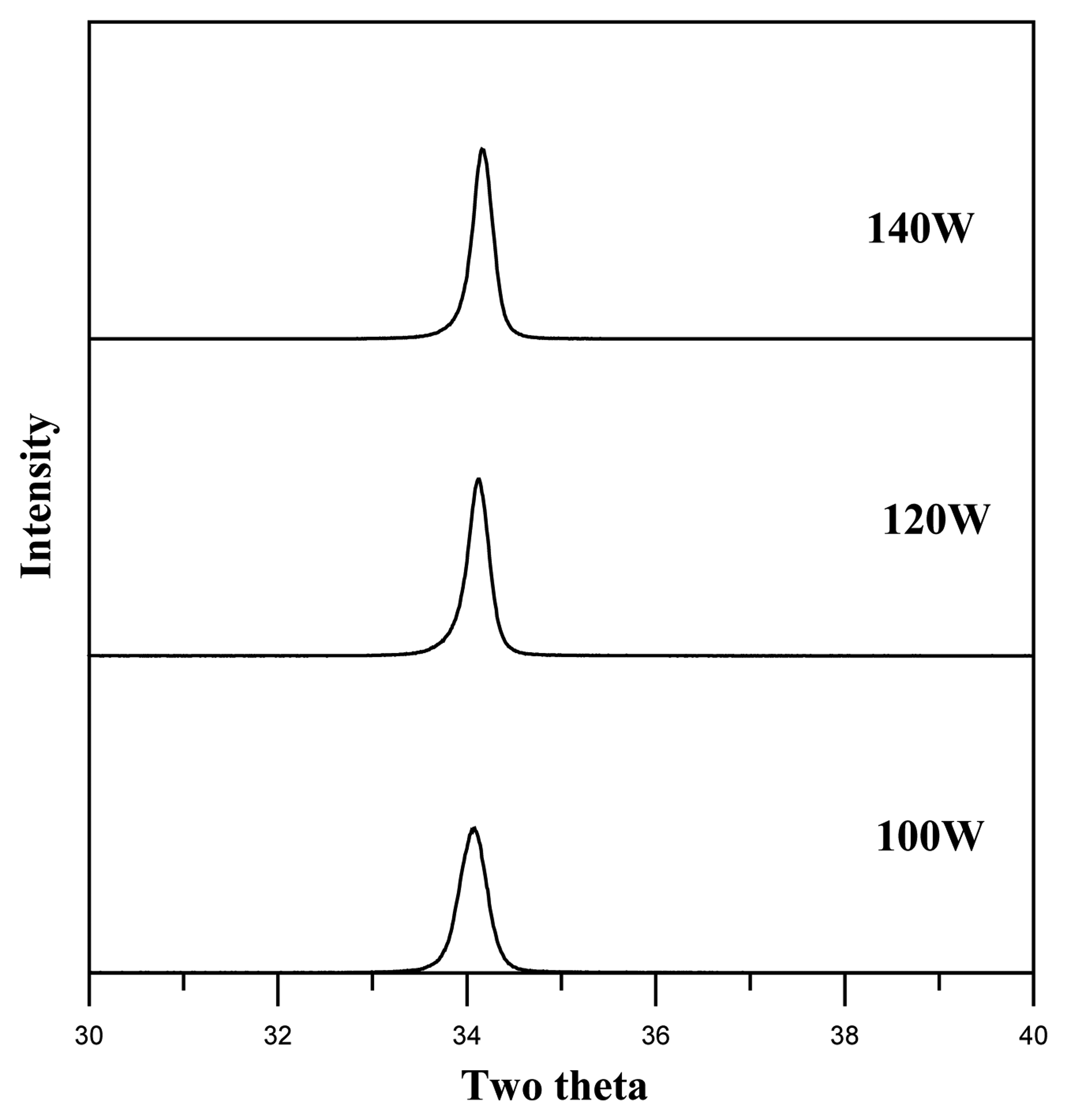
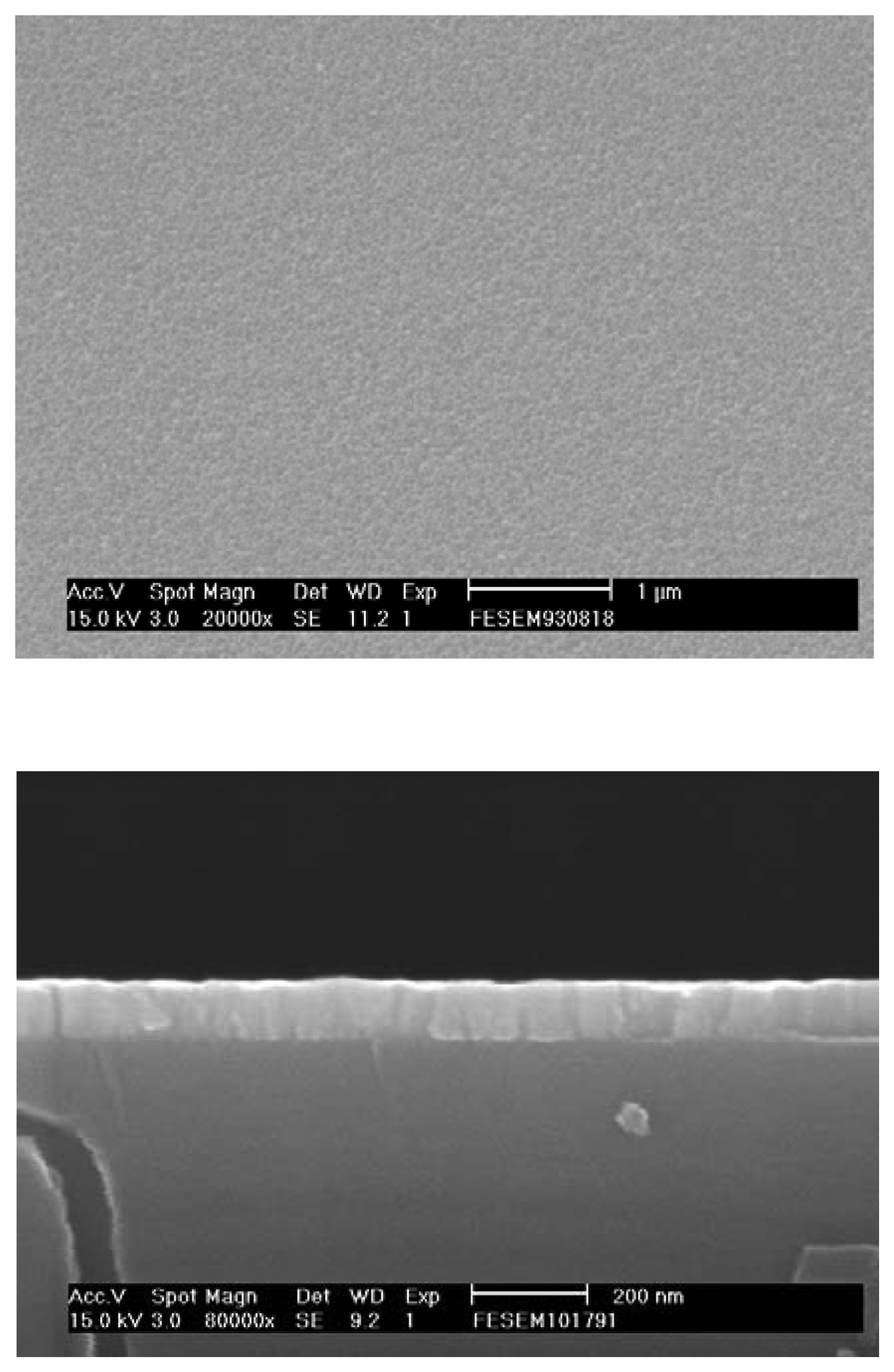
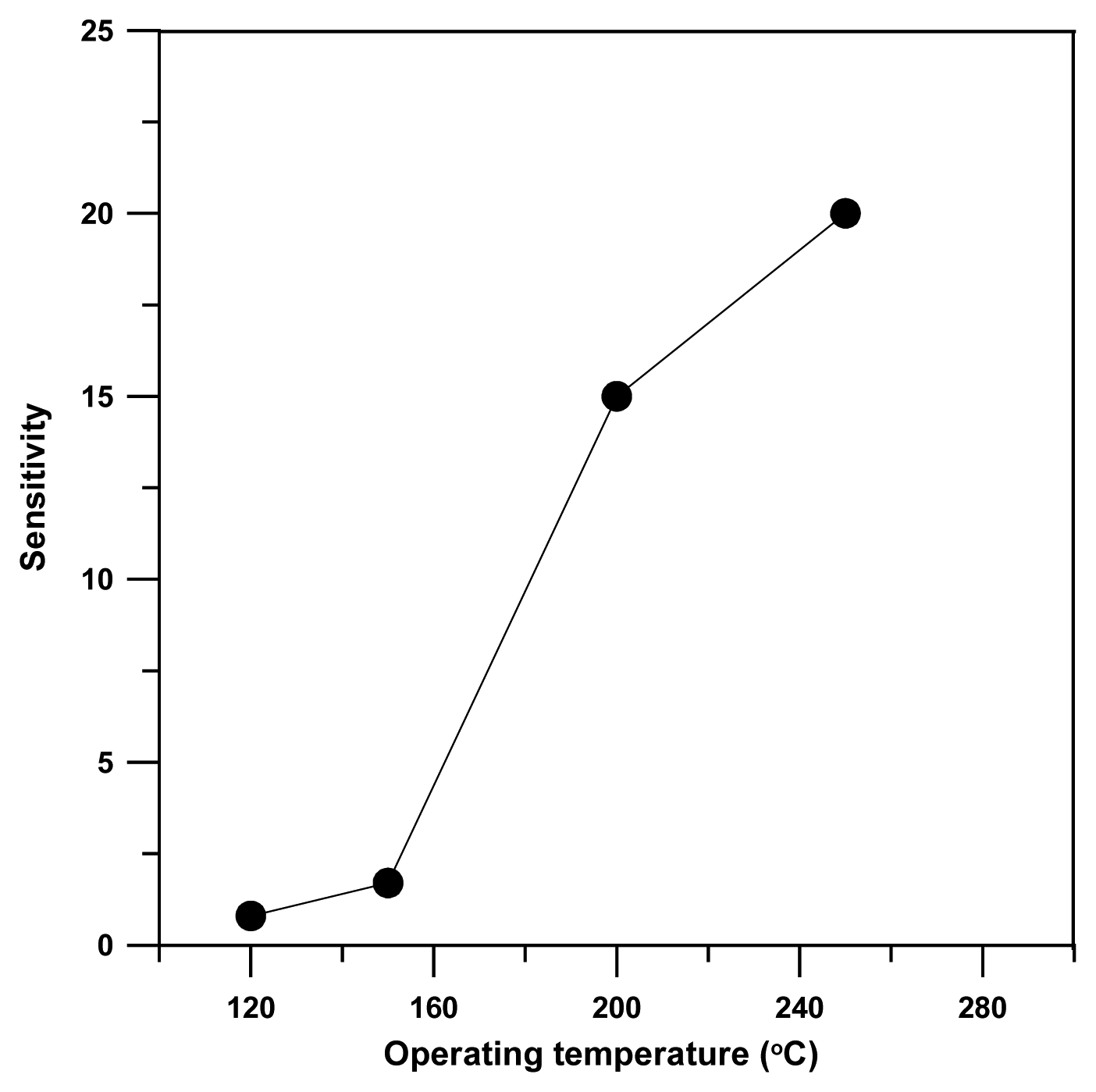
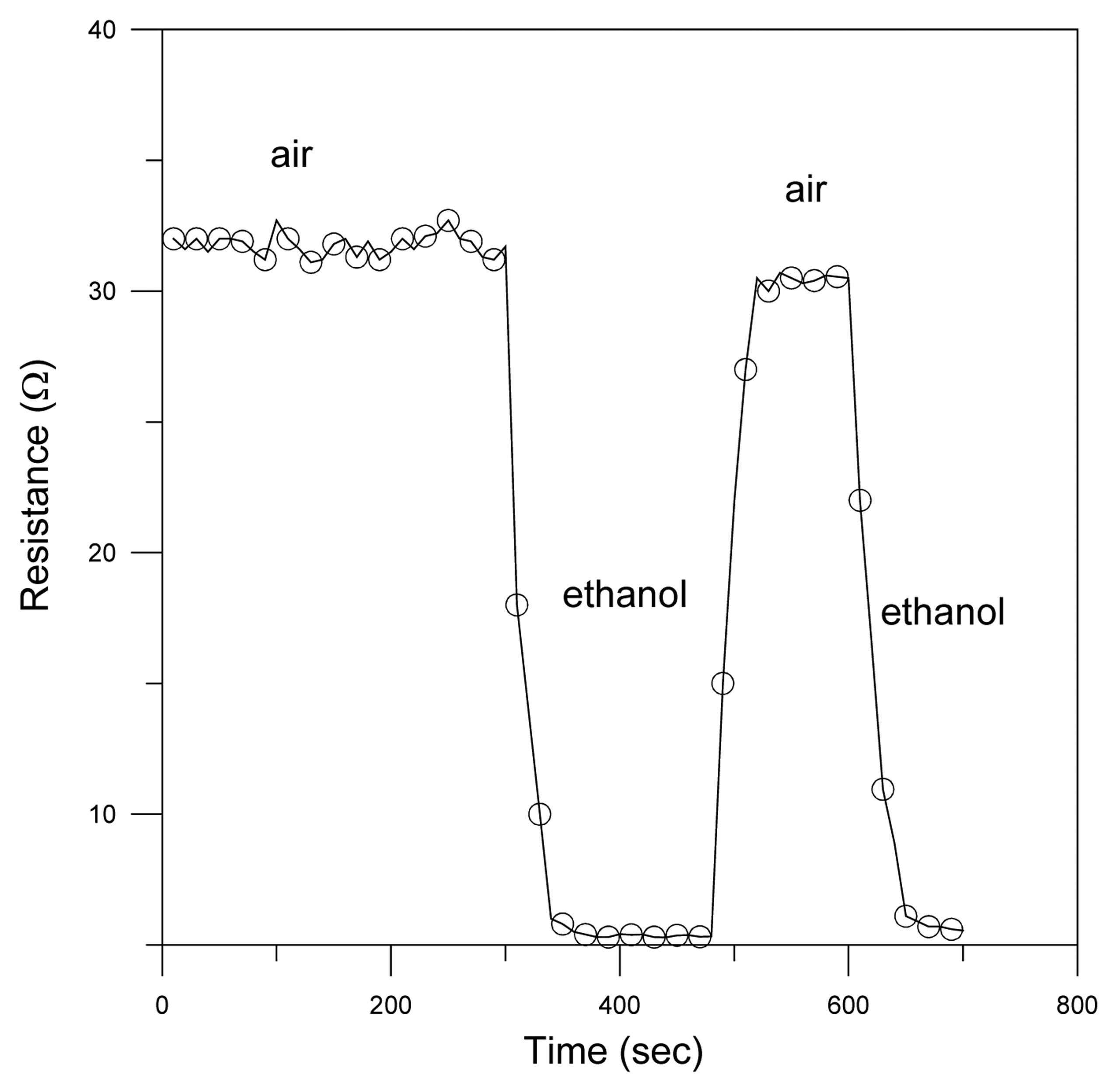
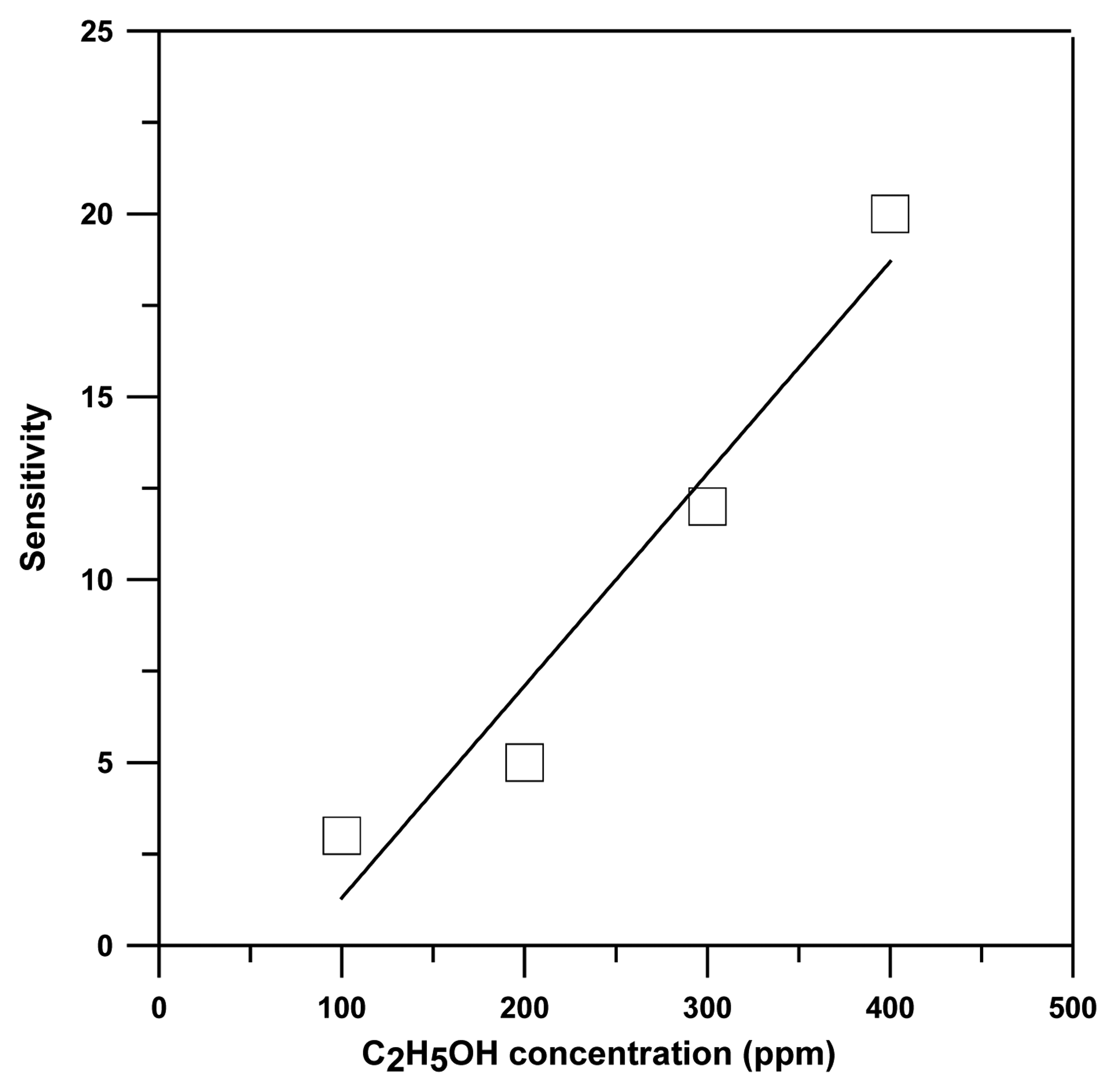
3. Results and discussion
4. Conclusions
Acknowledgments
References
- Costello, B.P.J.D.; Ewen, R.J.; Guernion, N.; Ratcliffe, N.M. Highly sensitive mixed oxide sensors for the detection of ethanol. Sens. Actuators B 2002, 87, 207–210. [Google Scholar]
- Paraguay, D.F.; Miki-Yoshida, M.; Morales, J.; Solis, J.; Estrada, L.W. Influence of Al, In, Cu, Fe and Sn dopants on the response of thin film ZnO gas sensor to ethanol vapor. Thin Solid Films 2000, 373, 137–140. [Google Scholar]
- Ivanov, P.; Llobet, E.; Vilanova, X.; Brezmes, J.; Hubalek, J.; Correig, X. Development of high sensitivity ethanol gas sensors based on Pt-doped SnO2 surfaces. Sens. Actuators B 2004, 99, 201–206. [Google Scholar]
- Cheng, X.L.; Zhao, H.; Huo, L.H.; Gao, S.; Zhao, J.G. ZnO nanoparticulate thin film: preparation, characterization and gas-sensing property. Sens. Actuators B 2004, 102, 248–252. [Google Scholar]
- Tamaki, J.; Maekawa, T.; Matsushima, S.; Miura, N.; Yamazoe, N. Ethanol gas sensing properties of Pd-La2O3-In2O3 thick film element. Chem. Lett. 1990, 3, 477–480. [Google Scholar]
- Sberveglieri, G.; Groppelli, S.; Nelli, P.; Quaranta, F.; Valentini, A.; Vasanelli, L. Oxygen gas-sensing characteristics for ZnO(Li) sputtered thin films. Sens. Actuators B 1992, 7, 747–751. [Google Scholar]
- Yamazaki, T.; Wada, S.; Noma, T.; Suzuki, T. Gas-sensing properties of ultrathin zinc oxide films. Sens. Actuators B 1993, 14, 594–595. [Google Scholar]
- Muller, J.; Weissenrieder, S. ZnO-Thin film chemical sensors. Fres. J. Anal. Chem. 1994, 349, 380–384. [Google Scholar]
- Stambolova, I.; Konstantinov, K.; Vassilev, S.; Peshev, P.; Tsacheva, Ts. Lanthanum doped SnO2 and ZnO thin films sensitive to ethanol and humidity. Materials Chemistry and Physics 2000, 63, 104–108. [Google Scholar]
- Nanto, H.; Minami, T.; Takata, S. Zinc-oxide thin film ammonia gas sensor with high sensitivity and excellent selectivity. J. Appl. Phys. 1986, 60, 482–484. [Google Scholar]
- Hu, J.; Gordon, R. G. Textured aluminum-doped zinc oxide thin films from atmospheric pressure chemical-vapor deposition. J. Appl. Phys. 1992, 71, 880–890. [Google Scholar]
- Takai, O.; Futsuhara, M.; Shimizu, G.; Lungu, C.P.; Nozue, J. Nanostructure of ZnO thin films prepared by reactive rf magnetron sputtering. Thin Solid Films 1998, 318, 117–119. [Google Scholar]
- Ohyama, M.; Kouzuka, H.; Yoko, T. Sol-gel preparation of ZnO films with extremely preferred orientation along (002) plane from zinc acetate solution. Thin Solid Films 1997, 306, 78–85. [Google Scholar]
- Van Heerden, J.L.; Swanepoel, R. XRD analysis of ZnO thin films prepared by spray pyrolysis. Thin Solid Films 1997, 299, 72–77. [Google Scholar]
- Lee, Y.E.; Kim, Y.J.; Kim, H.J. Thickness dependence of microstructural evolution of ZnO films deposited by rf magnetron sputtering. J. Mater. Res. 1998, 13, 1260–1265. [Google Scholar]
- Shinoki, F.; Itoh, A. Mechanism of rf reactive sputtering. J. Appl. Phys. 1975, 46, 3381–3384. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction, Second ed.; Addison-Wesley: Reading MA, 1978; p. 162. [Google Scholar]
- Islam, M.R.; Kumazawa, N.; Takeuchi, M. Chemical sensor based on titanium dioxide thick film:Enhancement of selectivity by surface coating. Appl. Surf. Sci. 1999, 142, 262–266. [Google Scholar]
- Matsushima, S.; Maekawa, T.; Tamaki, J.; Miura, N.; Yamazoe, N. Role of Additives on Alcohol Sensing by Semiconductor Gas Sensor. Chem. Lett. 1989, 18, 845–848. [Google Scholar]
- Kohl, D. Surface processes in the detection of reducing gases with SnO2-based devices. Sens. Actuators B 1989, 18, 71–113. [Google Scholar]
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Chou, S.M.; Teoh, L.G.; Lai, W.H.; Su, Y.H.; Hon, M.H. ZnO:Al Thin Film Gas Sensor for Detection of Ethanol Vapor. Sensors 2006, 6, 1420-1427. https://doi.org/10.3390/s6101420
Chou SM, Teoh LG, Lai WH, Su YH, Hon MH. ZnO:Al Thin Film Gas Sensor for Detection of Ethanol Vapor. Sensors. 2006; 6(10):1420-1427. https://doi.org/10.3390/s6101420
Chicago/Turabian StyleChou, Shih Min, Lay Gaik Teoh, Wei Hao Lai, Yen Hsun Su, and Min Hsiung Hon. 2006. "ZnO:Al Thin Film Gas Sensor for Detection of Ethanol Vapor" Sensors 6, no. 10: 1420-1427. https://doi.org/10.3390/s6101420
APA StyleChou, S. M., Teoh, L. G., Lai, W. H., Su, Y. H., & Hon, M. H. (2006). ZnO:Al Thin Film Gas Sensor for Detection of Ethanol Vapor. Sensors, 6(10), 1420-1427. https://doi.org/10.3390/s6101420



