A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide
Abstract
:1. Introduction
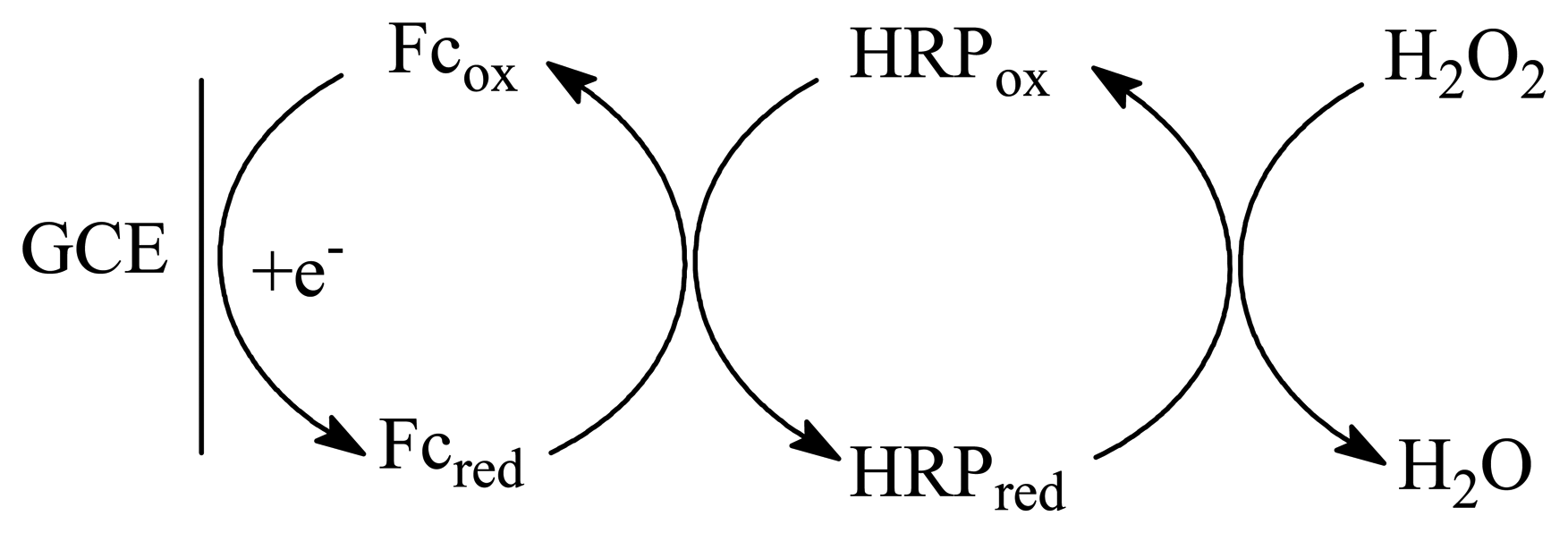
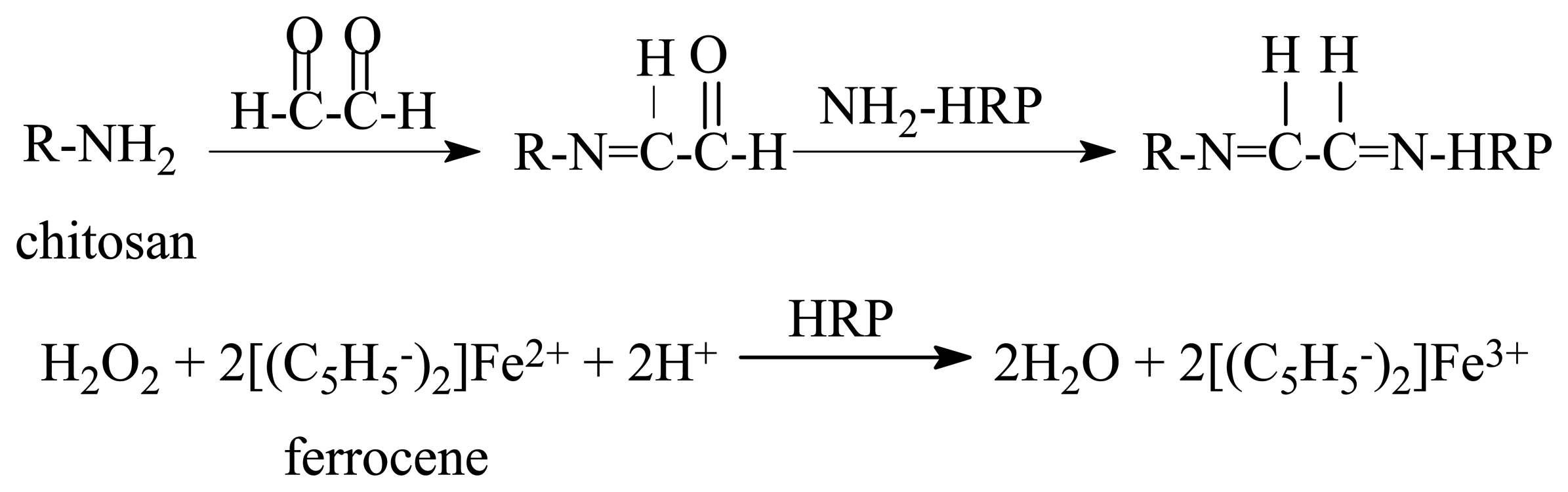
2. Experimental
2.1 Apparatus and reagents
2.2. Procedures
2.2.1. Preparation of the enzyme electrode
2.2.2 Measuring procedure
3. Results and discussion
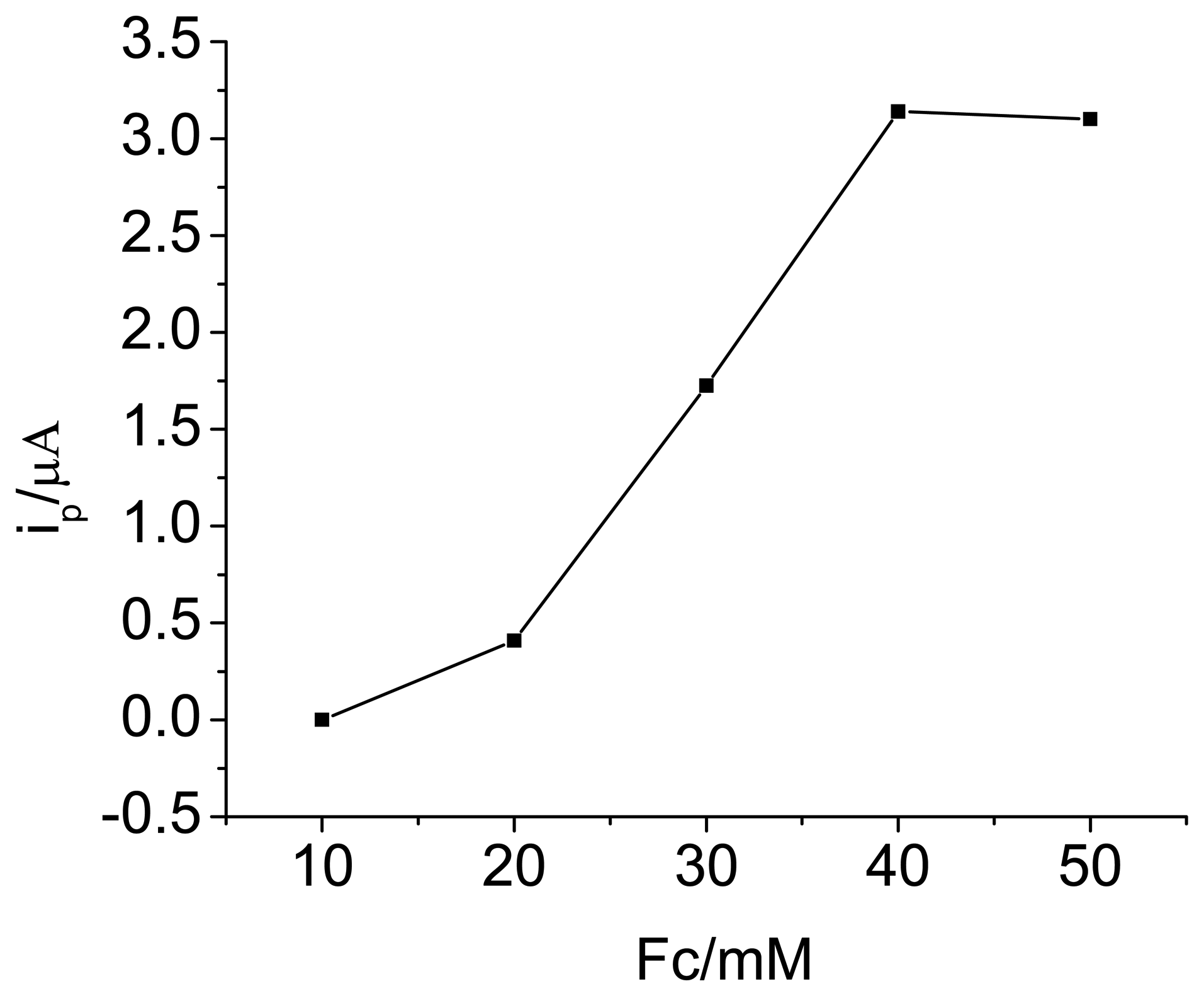
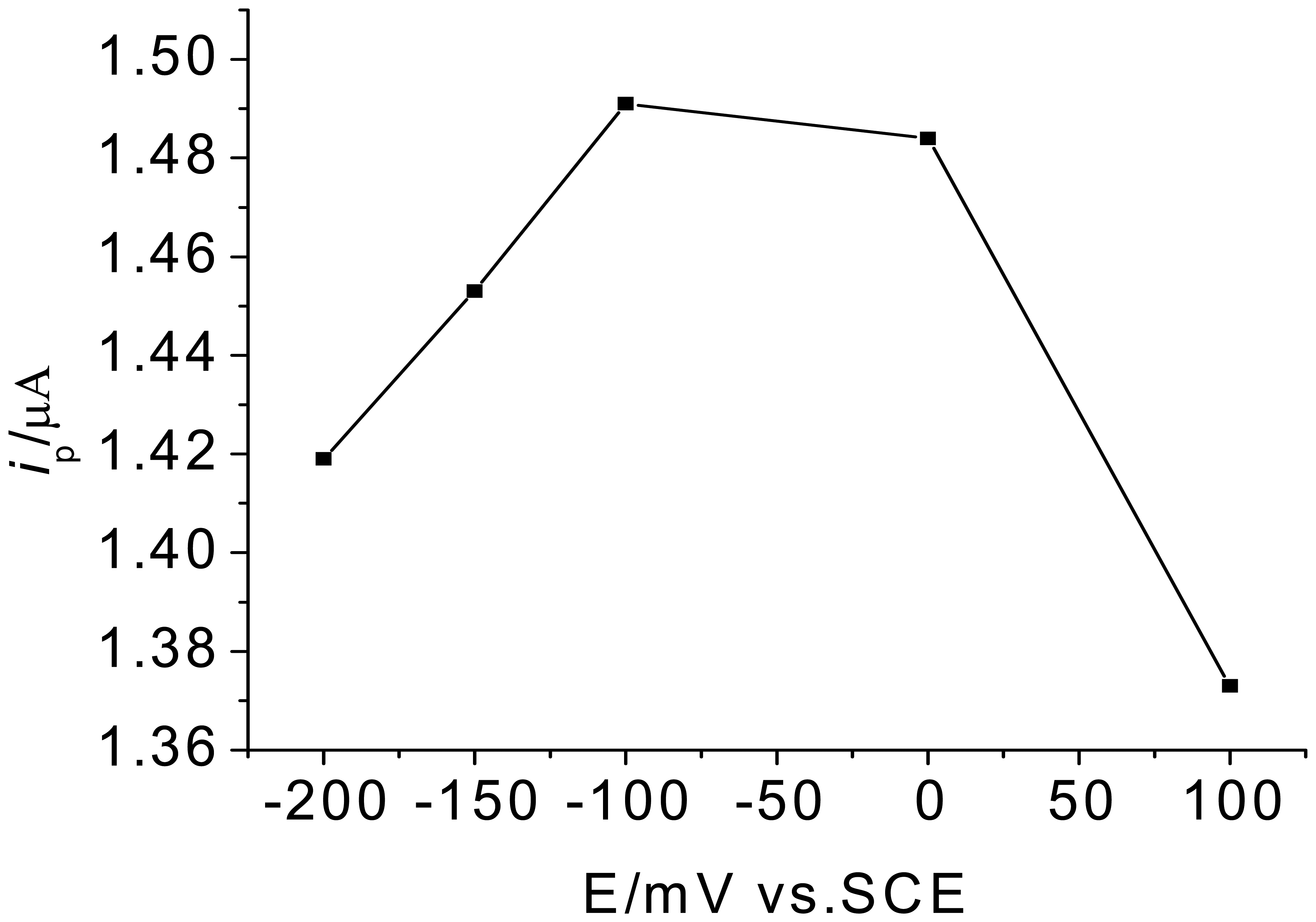
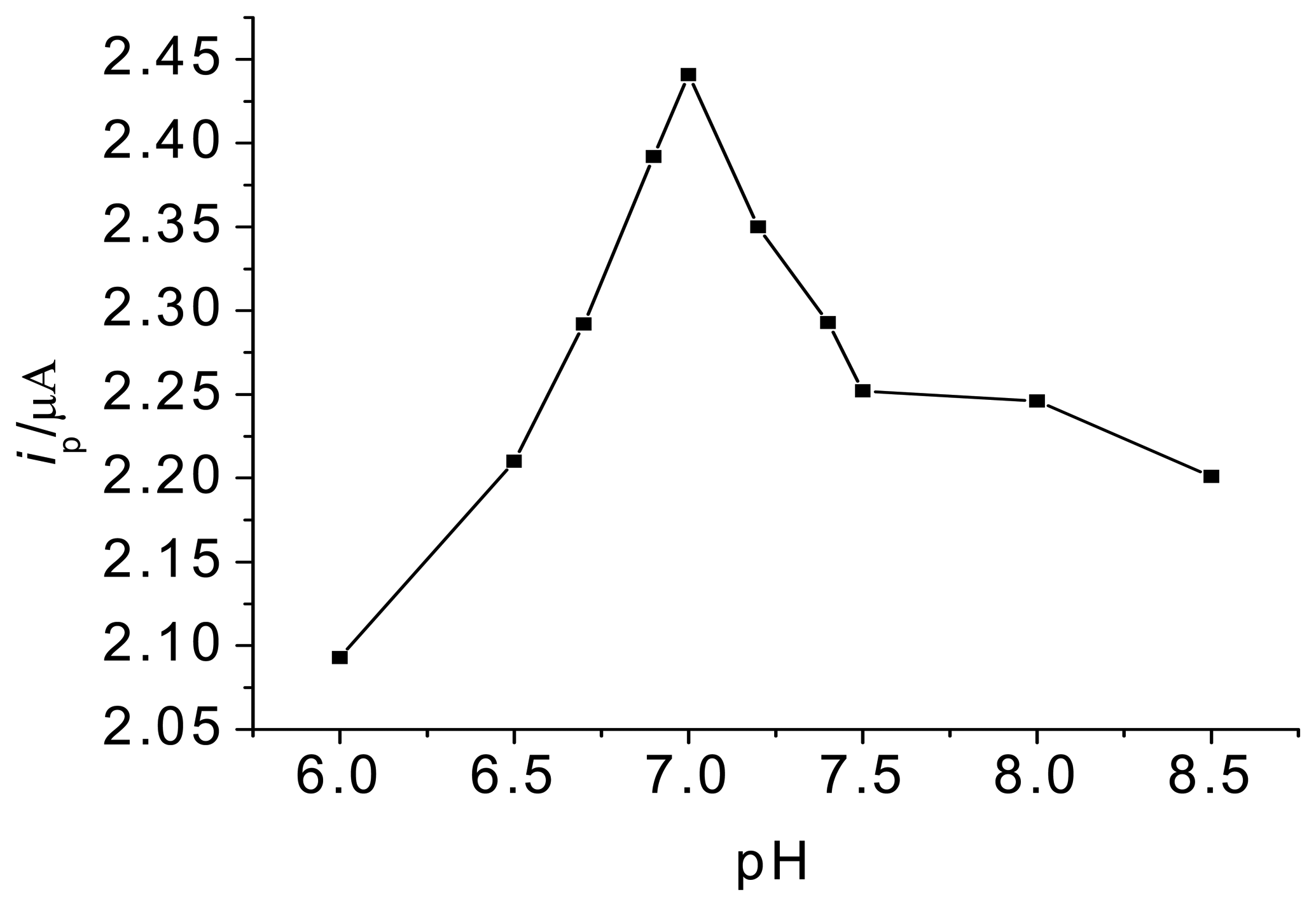
3.1. Optimization of experimental variables
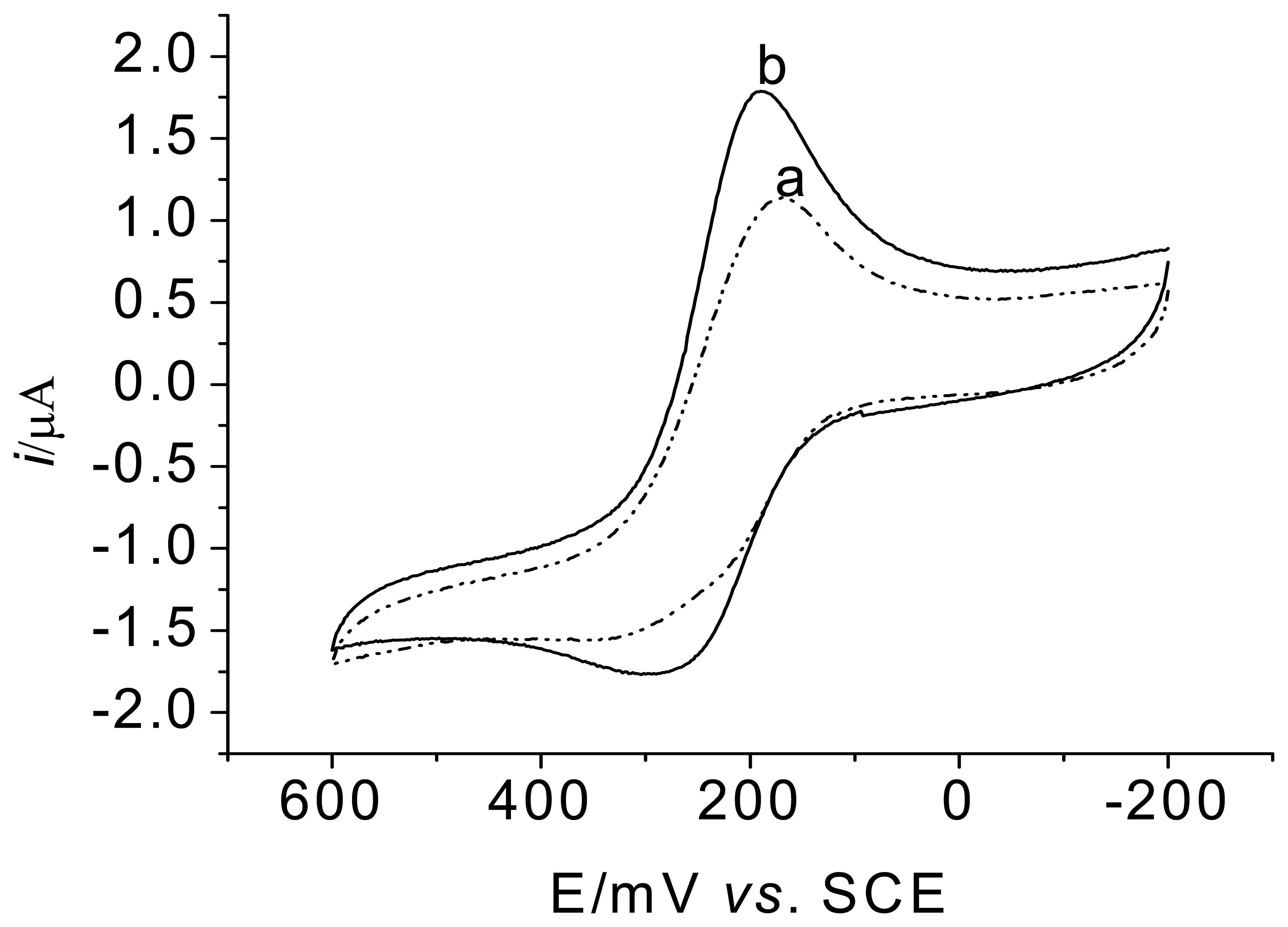
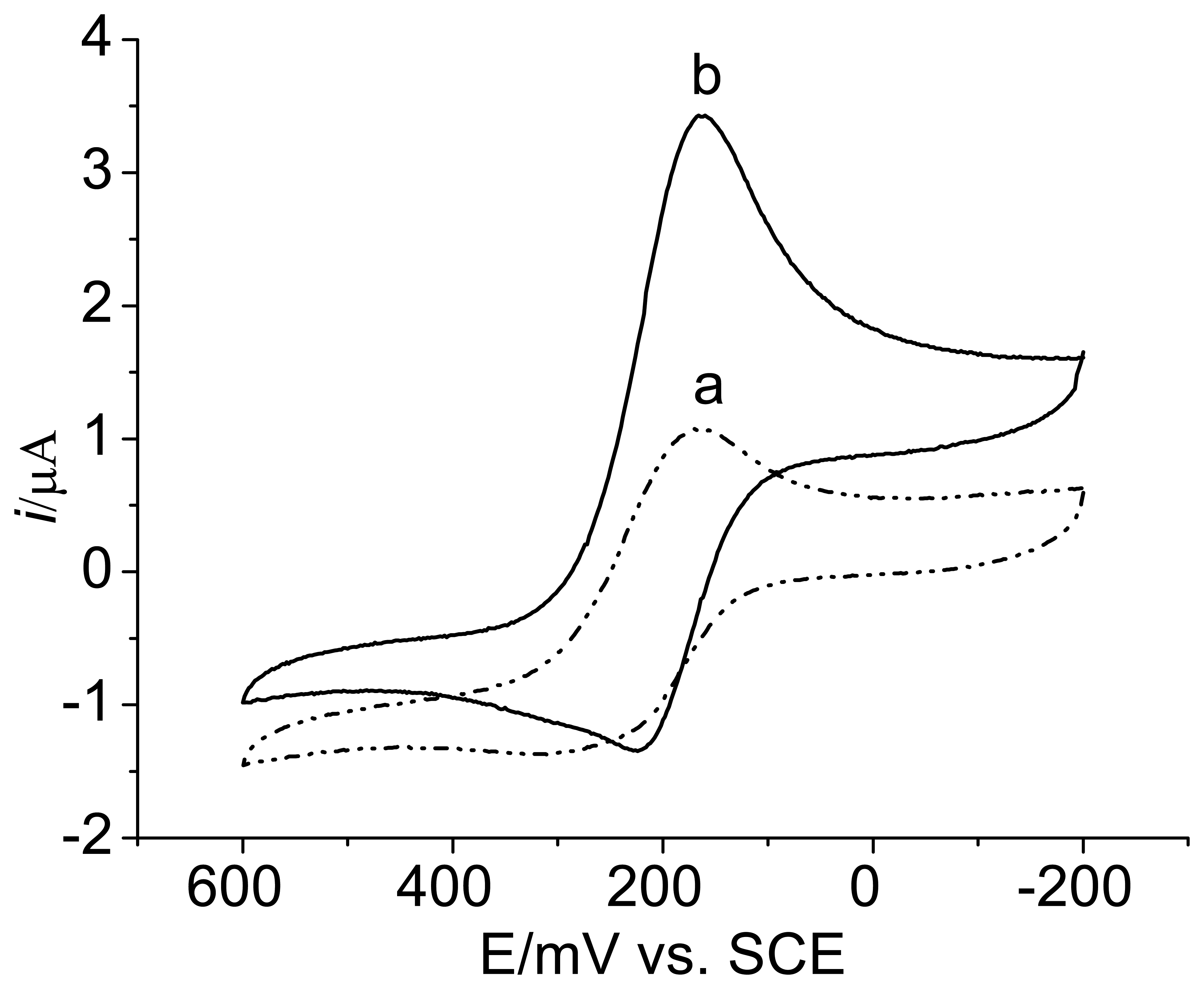
3.2. Voltammetric behavior of the enzyme electrode
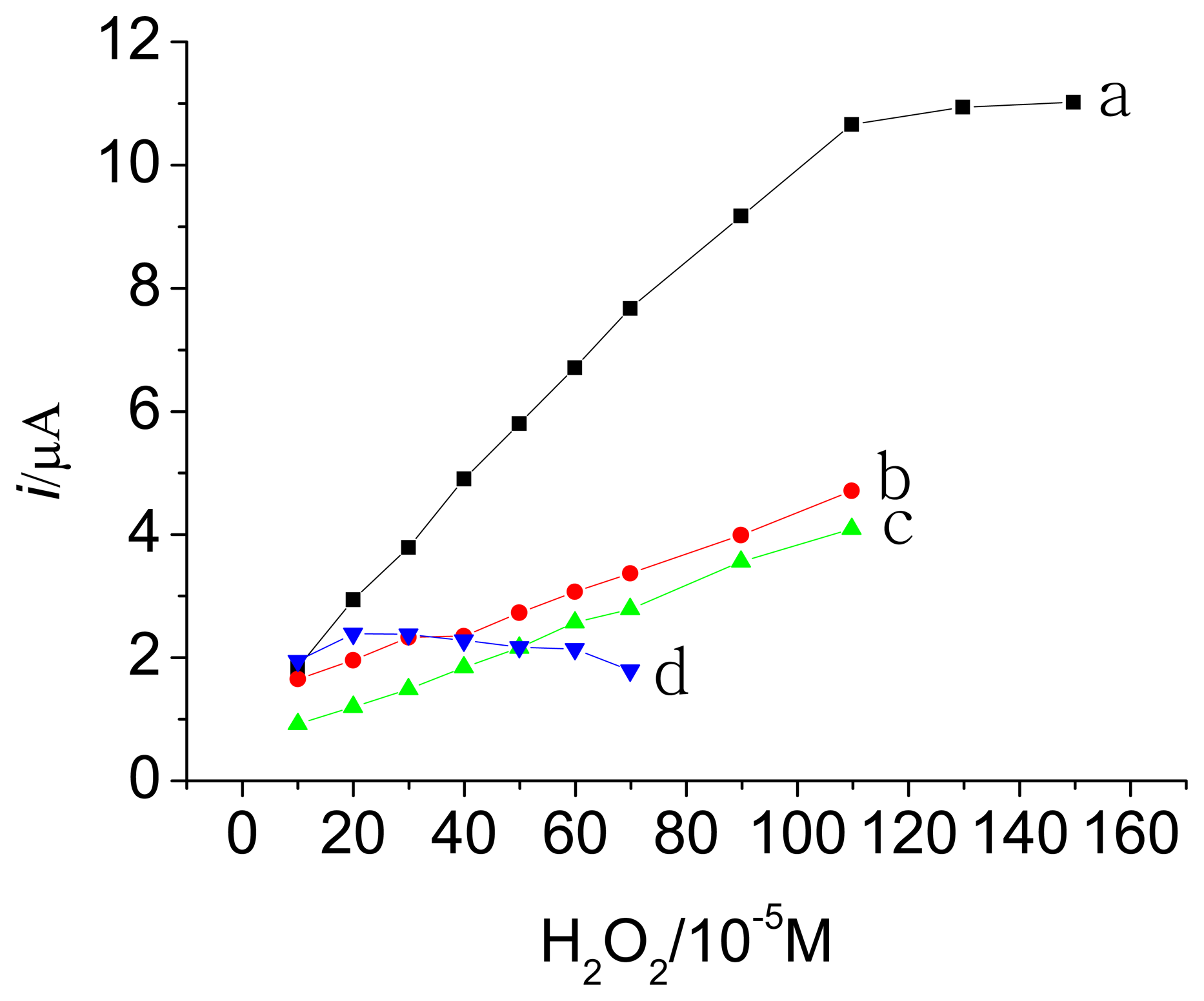
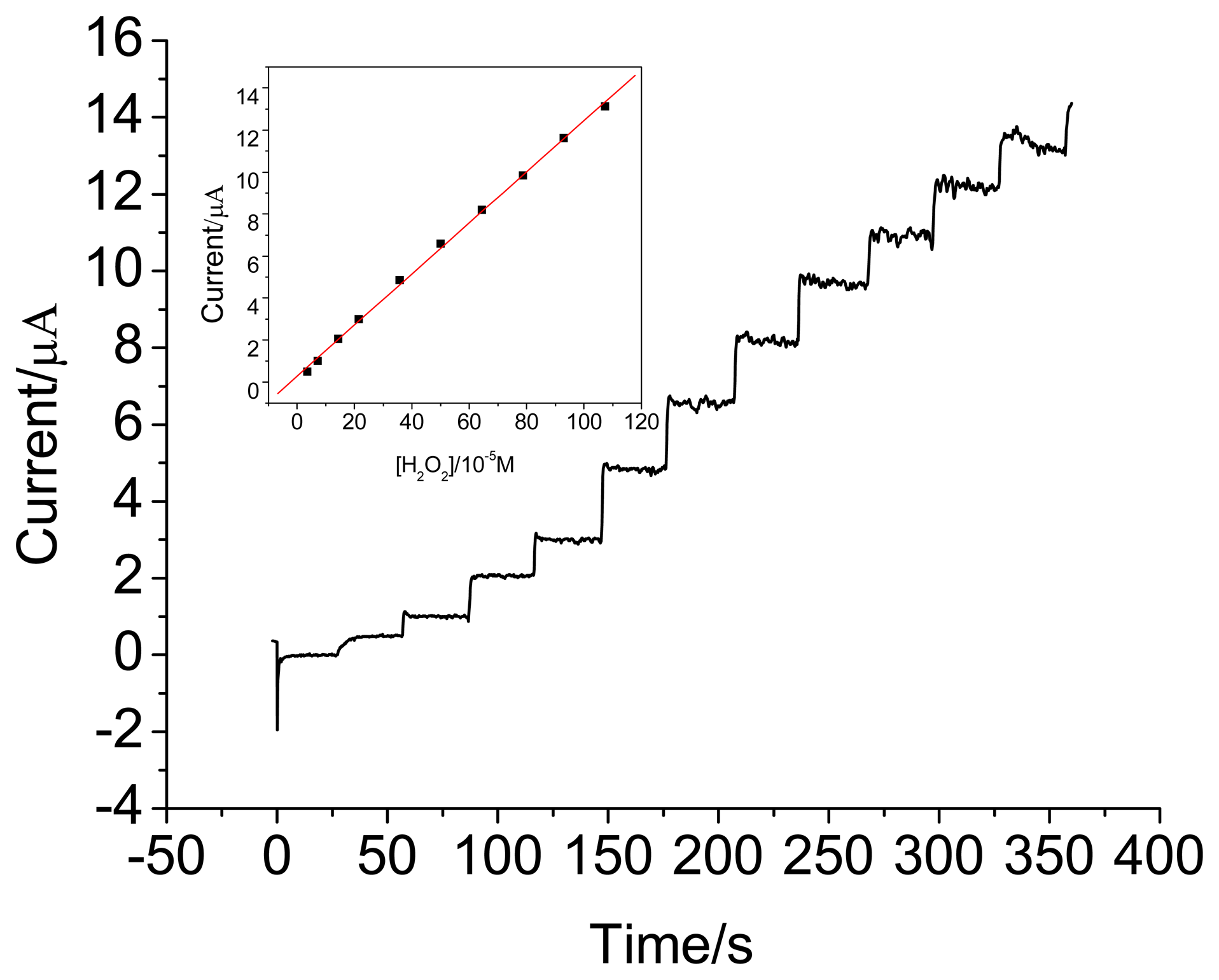
3.3. Amperometric response of the HRP electrodes
3.4. Selectivity against interference and storage stability
4. Conclusions
References
- Wang, G.; Xu, J.J.; Chen, H.Y. Amperometric Hydrogen Peroxide Biosensor with Sol-Gel/Chitosan Network-like Film as Immobilization Matrix. Biosens. Bioelectron. 2003, 18, 335–343. [Google Scholar]
- Ziegler, W.; Gaburjakova, J.; Gaburjakova, M. Agar-Supported Lipid Bilayers- Basic Structures for Biosensor Design. Electrical and mechanical properties. Colloid. Surf. A. 1998, 140, 357–367. [Google Scholar]
- Mousty, C.; Lepellec, A.; Cosnier, S.; Novoa, A. Fabrication of Organic Phase Biosensor Based on Multilayered Polyphenol Oxidase Protected by an Alginate Coating. Electrochem. Commun. 2001, 3, 727–732. [Google Scholar]
- Tziboula, A.; Horne, D.S. Influence of Whey Protein Denaturation on k-Carrageenan Gelation. Colloid. Surf. B. 1999, 12, 299–308. [Google Scholar]
- Sugawara, K.; Fukushi, H.; Hoshi, S.; Akatsuka, K. Electrochemical Sensing of Glucose at a Platinum Electrode with a Chitin/Glucose Oxidase Film. Anal. Sci. 2000, 16, 1139–1143. [Google Scholar]
- Miao, Y.; Tan, S.N. Amperometric Hydrogen Peroxide Biosensor Based on Immobilization of Peroxidase in Chitosan Matrix Crosslinked with Glutaraldehyde. Analyst 2000, 125, 1591–1594. [Google Scholar]
- Wan, Y.; Creber, K.A. M.; Peppley, B. Synthesis, Characterization and Ionic Conductive Properties of Phosphorylated Chitosan Membranes. Macromol. Chem. Phys. 2003, 204, 850–858. [Google Scholar]
- Genta, I.; Constantini, M.; Asti, A. Influence of Glutaraldehyde on Drug Release and Mucoadhesive Properties of Chitosan Microspheres. Carbohydr. Polym. 1998, 36, 81–88. [Google Scholar]
- Baba, Y.; Noma, H.; Nakayama, R.; Matsushita, Y. Preparation of Chitosan Derivatives Containing Methylthiocarbamoyl and Phenylthiocarbamoyl Groups and Their Selective Adsorption of Copper (II) over Iron (III). Anal. Sci. 2002, 18, 359–361. [Google Scholar]
- Modrzejewska, Z.; Kaminski, W. Separation of Cr (VI) on Chitosan Membranes. Ind. Eng. Chem. Res. 1999, 38, 4946–4950. [Google Scholar]
- Oshita, K.; Oshima, M.; Gao, Y.H.; Lee, K.H.; Motomizu, S. Adsorption Behavior of Mercury and Precious Metals on Cross-Linked Chitosan and the Removal of Ultratrace Amounts of Mercury in Concentrated Hydrochloric Acid by a Column Treatment with Cross-Linked Chitosan. Anal. Sci. 2002, 18, 1121–1125. [Google Scholar]
- Gao, Y.H.; Lee, K.H.; Oshima, M.; Motomizu, S. Adsorption Behavior of MetalIons on Cross-linked Chitosan and the Determination of Oxoanions after Pretreatment with a Chitosan Column. Anal. Sci. 2000, 16, 1303–1308. [Google Scholar]
- Baba, Y.; Noma, H.; Nakayama, R.; Matsushita, Y. Selective Adsorption of Mercury (II) on Chitosan Derivatives from Hydrochloric Acid. Anal. Sci. 1998, 14, 687–690. [Google Scholar]
- Yao, X.; Lu, G.H.; Wu, X.G.; Zhan, T. Studies on Electrochemical Behavior of Bromide at a Chitosan-Modified Glassy Carbon Electrode. Electroanalysis 2001, 13, 923–926. [Google Scholar]
- Rhee, J.S.; Jung, M.W.; Paeng, K.J. Evaluation of Chitin and Chitosan as a Sorbent for the Preconcentration of Phenol and Chlorophenols in Water. Anal. Sci. 1998, 14, 1089–1092. [Google Scholar]
- Magalhaes, J.M.C.S.; Machado, A.A.S.C. Urea Potentiometric Biosensor Based on Urease Immobilized on Chitosan Membranes. Talanta 1998, 47, 183–191. [Google Scholar]
- Miao, Y.; Tan, S.N. Amperometric Hydrogen Peroxide Biosensor with Silica Sol-Gel/Chitosan Film as Immobilization Matrix. Anal. Chim. Acta. 2001, 437, 87–93. [Google Scholar]
- Miao, Y.; Chia, L.S.; Goh, N.K.; Tan, S.N. Amperometric Glucose Biosensor Based on Immobilization of Glucose Oxidase in Chitosan Matrix Cross-Linked With Glutaraldehyde. Electroanalysis 2001, 13, 347–349. [Google Scholar]
- Chen, X.; Jia, J.B.; Dong, S.J. Organically Modified Sol-Gel/Chitosan Composite Based Glucose Biosensor. Electroanalysis 2003, 15, 608–612. [Google Scholar]
- Cruz, J.; Kawasaki, M.; Gorski, W. Electrode Coatings Based on Chitosan Scaffolds. Anal. Chem. 2000, 72, 680–686. [Google Scholar]
- Zhou, G.J.; Wang, G.; Xu, J.J.; Chen, H.Y. Reagentless Chemiluminescence Biosensor for Determination of Hydrogen Peroxide Based on the Immobilization of Horseradish Peroxidase on Biocompatible Chitosan Membrane. Sens. Actuat. B. 2002, 81, 334–339. [Google Scholar]
- Bao, S.; Nomura, T. Silver-selective Sensor Using an Electrode-Separated Piezoelectric Quartz Crystal Modified with a Chitosan Derivative. Anal. Sci. 2002, 18, 881–885. [Google Scholar]
- Haymond, S.; Babcock, G.T.; Swain, G. M. Electron Transfer Kinetics of Ferrocene at Microcrystalline Boron-Doped Diamond Electrodes: Effect of Solvent and Electrolyte. Electroanalysis 2003, 15, 249–253. [Google Scholar]
- Pandey, P.C.; Upadhyay, S.; Shukla, N.K.; Sharma, S. Studies on the Electrochemical Performance of Glucose Biosensor Based on Ferrocene Encapsulated ORMOSIL and Glucose Oxidase Modified Graphite Paste Electrode. Biosens. Bioelectron. 2003, 18, 1257–1268. [Google Scholar]
- Pandey, P.C.; Upadhyay, S. Bioelectrochemistry of Glucose Oxidase Immobilized on Ferrocene Encapsulated Ormosil Modified Electrode. Sens. Actuat. B. 2001, 76, 193–198. [Google Scholar]
- Nakabayashi, Y.; Wakuda, M.; Imai, H. Amperometric Glucose Sensors Fabricated by Electrochemical Polymerization of Phenols on Carbon Paste Electrodes Containing Ferrocene as an Electron Transfer Mediator. Anal. Sci. 1998, 14, 1069–1076. [Google Scholar]
- Nakabayashi, Y.; Yoshikawa, H. Amperometric Biosensors for Sensing of Hydrogen Peroxide Based on Electron Transfer between Horseradish Peroxidase and Ferrocene as a Mediator. Anal. Sci. 2000, 16, 609–613. [Google Scholar]
- Pandey, P.C.; Upadhyay, S.; Tiwari, I.; Singh, G. A Novel Ferrocene Encapsulated Palladium-Linked Ormosil-Based Electrocatalytic Dopamine Biosensor. Sens. Actuat. B. 2001, 75, 48–55. [Google Scholar]
- Armada, M.P.G.; Losada, J.; Cuadrado, I. A Siloxane Homopolymer with Interacting Ferrocenes as a New Material for the Preparation of Sensors Based on the Detection of Hydrogen Peroxide. Electroanalysis 2003, 15, 1109–1114. [Google Scholar]
- Yabuki, S.; Mizutani, F.; Hirata, Y. Glucose-Sensing Electrode Based on Glucose Oxidase-Attached Polyion Complex Membrane Containing Peroxidase and Ferrocene. Electroanalysis 2001, 13, 380–383. [Google Scholar]
- Maehly, A.C. Plant Peroxidases: Methods in Enzymology; Academic Press: New York, 1955; Vol. 11, p. p. 807. [Google Scholar]
- Zhang, J.Z.; Li, B.; Wang, Z.X.; Cheng, G.J.; Dong, S.J. Functionalized Inorganic-Organic Composite Material Derivated by Sol-Gel for Construction of Mediated Amperometric Hydrogen Peroxide Biosensor. Anal. Chim. Acta. 1999, 388, 71–78. [Google Scholar]
- Wang, B.Q.; Zhang, J.Z.; Cheng, G.J.; Dong, S.J. Amperometric Enzyme Electrode for the Determination of Hydrogen Peroxide Based on Sol-Gel/Hydrogel composite film. Anal. Chim. Acta. 2000, 407, 111–118. [Google Scholar]
| Possible interference | Current ratioa |
|---|---|
| Ascorbic acid | 0.21 |
| Cysteine | 0.86 |
| Sucrose | 0.97 |
| Citric acid | 0.98 |
| Nitrate | 0.99 |
| Glucose | 1.00 |
| Fluride | 1.00 |
| Oxalic acid | 1.00 |
© 2005 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Wang, H.-S.; Pan, Q.-X.; Wang, G.-X. A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide. Sensors 2005, 5, 266-276. https://doi.org/10.3390/s5040266
Wang H-S, Pan Q-X, Wang G-X. A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide. Sensors. 2005; 5(4):266-276. https://doi.org/10.3390/s5040266
Chicago/Turabian StyleWang, Huai-Sheng, Qian-Xiu Pan, and Gui-Xiang Wang. 2005. "A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide" Sensors 5, no. 4: 266-276. https://doi.org/10.3390/s5040266
APA StyleWang, H.-S., Pan, Q.-X., & Wang, G.-X. (2005). A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide. Sensors, 5(4), 266-276. https://doi.org/10.3390/s5040266




