Electrochemical Studies of the Inhibition and Activation Effects of Al (III) on the Activity of Bovine Liver Glutamate Dehydrogenase
Abstract
:1. Introduction
2. Materials and methods
3. Results and discussion
3.1. Differential pulse polarography of the GDH enzyme in the absence and presence of Al (III)
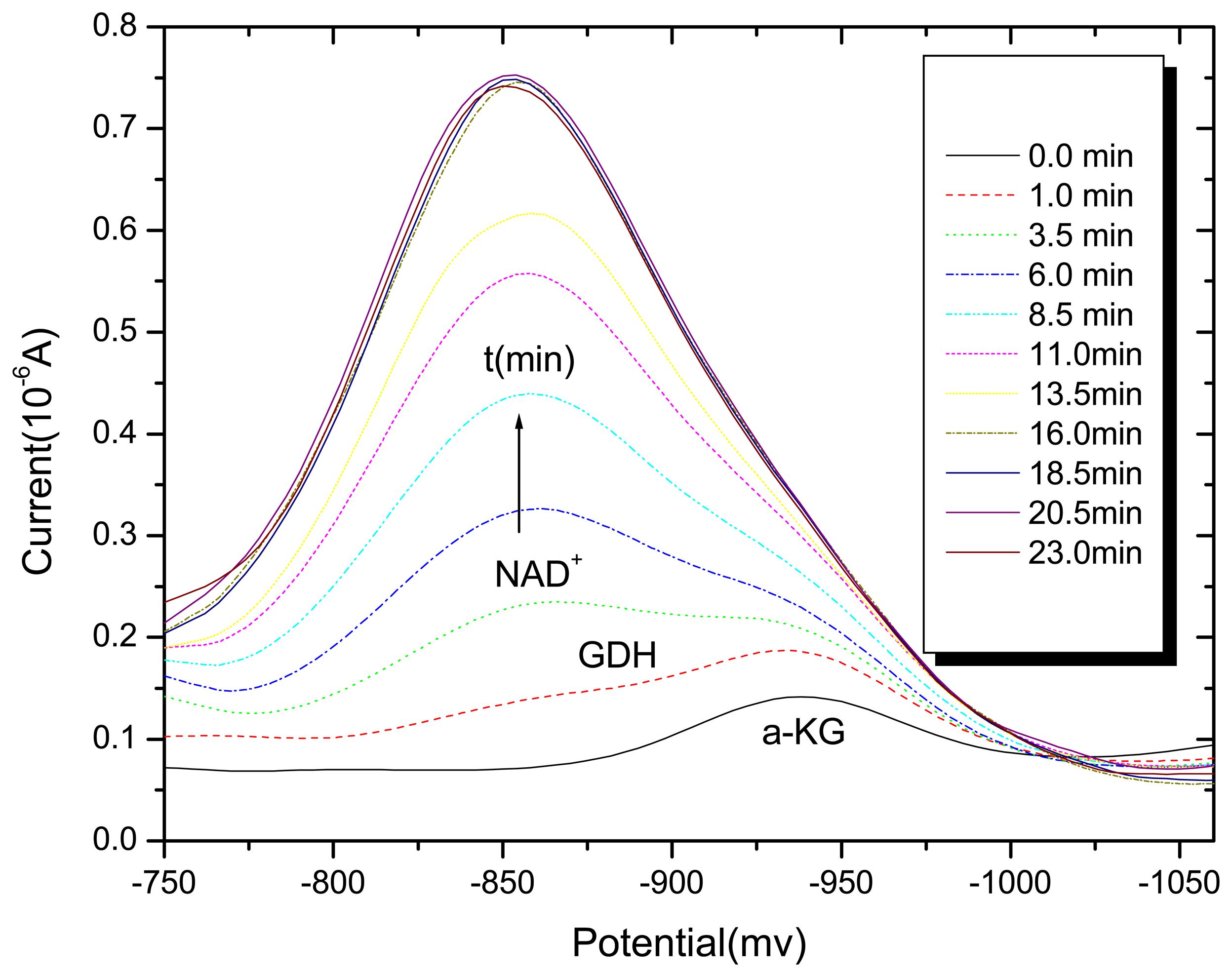
3.2. Voltammetry behaviors of Al (III) on the NAD+ and NAD+-GDH
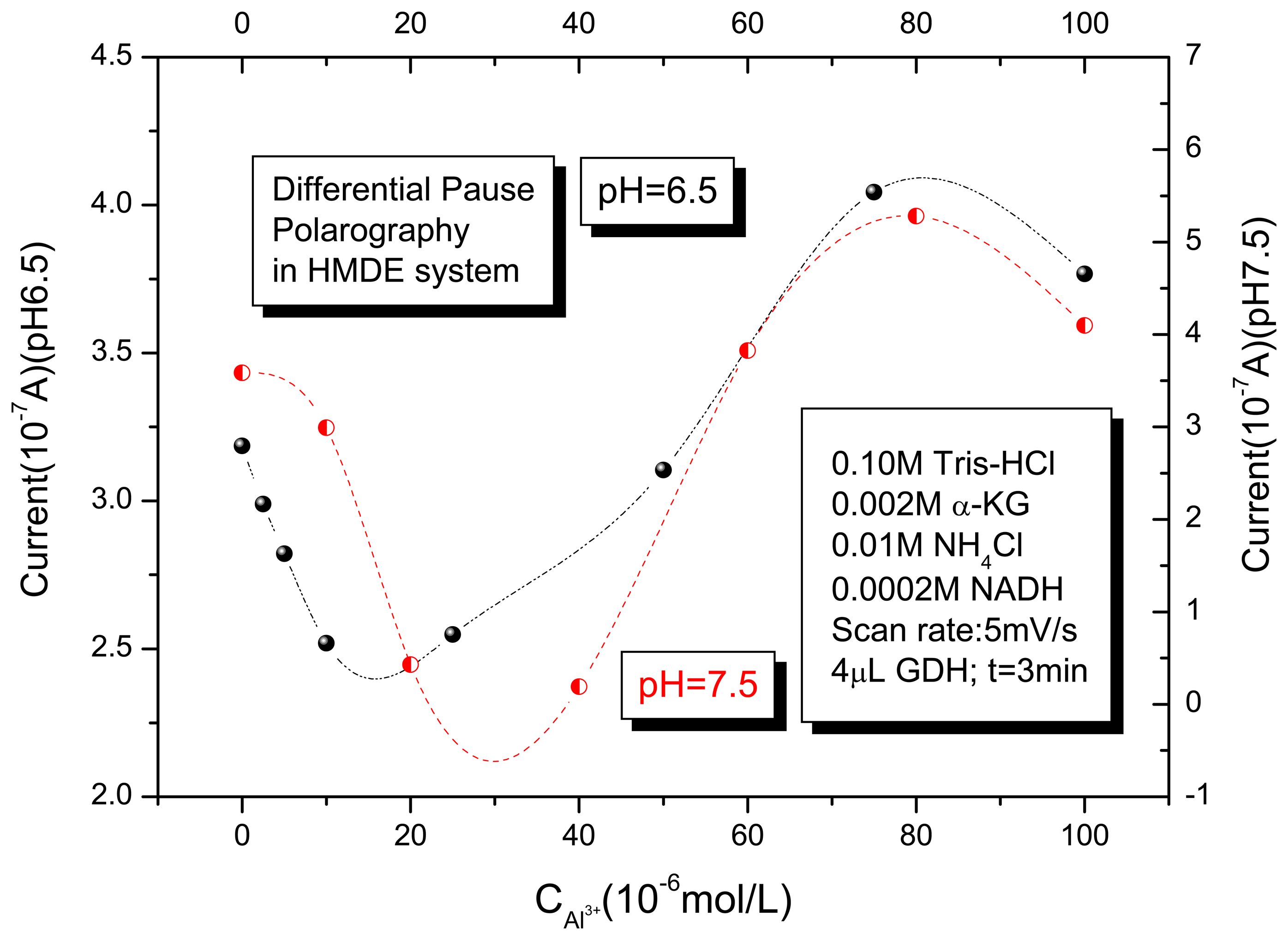
3.3. Proposal explanation of the inhibition and activation effects of Al (III) on GDH enzyme activities
4. Conclusions
Acknowledgments
References
- Peterson, P.E; Smith, T.J. The structure of bovine glutamate dehydrogenase provides insights into the mechanism of allostery. Structure 1999, 7, 769–782. [Google Scholar]
- Cho, S.W.; Cho, E.H.; Choi, S.Y. Activation of two types of brain glutamate dehydrogenase isoproteins by gabapetin. FEBS Lett. 1998, 426, 196–200. [Google Scholar]
- Pazhanisamy, S.; Maniscalco, S.J.; Singh, N.; Fisher, H.F. A kinetic mechanism of the allosteric control of enzyme-coenzyme binding: glutamate dehydrogenase-NADPH-phosphate acetate-hydrogen ion interactions. Biochemistry 1994, 33, 10381–10385. [Google Scholar]
- Jung, K.; Sokolowski, A.; Egger, E. Influence of calcium ions on the activity of human liver glutamate dehydrogenase. Hoppe-Seyler's Z. Physiol.Chem. 1973, 354, 101–103. [Google Scholar]
- McCarthy, A.D.; Tipton, K.F. The effects of magnesium ions on the interactions of ox brain and liver glutamate dehydrogenase with ATP and GTP. Biochem. J. 1984, 220, 853–856. [Google Scholar]
- Colman, R.F.; Foster, D.S.; Daniel, S. Absence of zinc in bovine liver glutamate dehydrogenase. J.Biol.Chem. 1971, 245, 6190–6195. [Google Scholar]
- Bell, E.T.; Stilwell, A.M.; Bell, J.E. Interaction of Zn2+ and Eu3+ with bovine liver glutamate dehydrogenase. Biochem.J. 1987, 246, 199–203. [Google Scholar]
- Zhuang, Q.K.; Dai, H.C.; Gao, X.X.; Xin, W.K. Electrochemical studies of the effect of lanthanide ions on the activity of glutamate dehydrogenase. Bioelectrochemistry 2000, 52, 37–41. [Google Scholar]
- Kiss, T.; Hollósi, M. Aluminum and Alzheimer's Disease The Science that Describes the Link; Exley, C., Ed.; Elivser: Amsterdam, 2001; p. p361. [Google Scholar]
- Louie, A.Y.; Meade, T.J. Metal complexes as enzyme inhibitors. Chem. Rev. 1999, 99, 2711–2734. [Google Scholar]
- Atwood, A.Y.; Yearwood, B.C. The future of aluminum chemistry. J. Organomet. Chem. 2000, 600, 186–197. [Google Scholar]
- Zatta, P.; Lain, E.; Cagnolini, C. Effects of aluminum on activity of Krebs cycle enzymes and glutamate dehydrogenase in rat brain homogenate. Eur. J. Biochem. 2000, 267, 3049–3055. [Google Scholar]
- Hamel, R.D; Appanna, V.D. Modulation of TCA cycle enzymes and aluminum stress in Pseudomonas fluorescens. J. Inorg. Biochem. 2001, 81, 1–8. [Google Scholar]
- Swegert, C.V.; Dave, K.R.; Katyare, S.S. Effects of aluminium-induced Alzheimer like condition on oxidative energy metabolism in rat liver,brain and heart mitochondria. Mech. Ageing Dev. 1999, 112, 27–42. [Google Scholar]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar]
- Xin, W.K.; Gao, X.X. Study of the effect of lanthanide ions on the kinetic of glutamate dehydrogenase by a chronoamoerometric method. Analyst 1996, 121, 687–690. [Google Scholar]
- Yang, X.D.; Bi, S.P.; Wang, X.L.; Liu, J.; Bai, Z.P. Multimethod characterization the interaction of aluminum ion with α-ketoglutaric acid in acidic aqueous solutions. Anal. Sci. 2003, 19, 273–279. [Google Scholar]
- Yang, X.D.; Tang, Y.Z.; Bi, S.P; Yang, G.S.; Hu, J. Multimethod study the complexation of aluminum (III) with L-glutamate in acidic aqueous solutions. Anal. Sci. 2003, 19, 133–138. [Google Scholar]
- Yang, X.D.; Bi, S.P.; Yang, X.L.; Yang, L.; Hu, J.; Liu, J. NMR spectra and potentiometry studies of aluminum (III) binding with coenzyme NAD+ in acidic aqueous solutions. Anal. Sci. 2003, 19, 815–821. [Google Scholar]
- Yang, X.D.; Bi, S.P.; Yang, L.; Zhu, Y.H.; Wang, X.L. Multi-NMR and fluorescence spectra study the effects of aluminum (III) on coenzyme NADH in aqueous solution. Spectrochim. Acta Part A. 2003, 58, 2561–2568. [Google Scholar]
- Jones, D.J.; Kochian, L.V. Aluminum interaction with plasma membrane lipids and enzyme metal binding sites and its potential role in Al cytotoxicity. FEBS Lett. 1997, 425, 51–57. [Google Scholar]
- Suhayda, C.G.; Haug, A. Organic acids prevent aluminum-induced conformational changes in calmodulin. Biochem. Biophy. Res. Comm. 1984, 119, 376–381. [Google Scholar]
- Wang, X.Q.; Gao, X.X. Voltammetric behavior studies on the interaction between lanthanide ions and NAD+,NAD-LDH complex. Chem. J. Chin. Univ. 1997, 18, 1027–1030. [Google Scholar]
- Šebela, M.; Studeničková, M.; Wimmerová, M. Differential pulse polarographic study of the redox centers in pea amine oxidase. Bioelectrochem. Bioenergetics 1996, 41, 173–179. [Google Scholar]
- Zatta, P.; Ibn-Lkhayat-Idrissi, M.; Zambenedetti, P.; Kilyen, M.; Kiss, T. In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Res. Bull. 2002, 59, 41–45. [Google Scholar]
- Yang, S.J.; Huh, J.W.; Lee, J.E.; Choi, S.Y.; Kim, T.U.; Cho, S.W. Inactivation of human glutamate dehydrogenase by aluminum. Cell. Molec. Life Sci. 2003, 60, 2538–2546. [Google Scholar]


© 2005 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yang, X.; Li, L.; Bi, S. Electrochemical Studies of the Inhibition and Activation Effects of Al (III) on the Activity of Bovine Liver Glutamate Dehydrogenase. Sensors 2005, 5, 235-244. https://doi.org/10.3390/s5040235
Yang X, Li L, Bi S. Electrochemical Studies of the Inhibition and Activation Effects of Al (III) on the Activity of Bovine Liver Glutamate Dehydrogenase. Sensors. 2005; 5(4):235-244. https://doi.org/10.3390/s5040235
Chicago/Turabian StyleYang, Xiaodi, Laifa Li, and Shuping Bi. 2005. "Electrochemical Studies of the Inhibition and Activation Effects of Al (III) on the Activity of Bovine Liver Glutamate Dehydrogenase" Sensors 5, no. 4: 235-244. https://doi.org/10.3390/s5040235



