On Calibration of pH Meters
Abstract
:Introduction
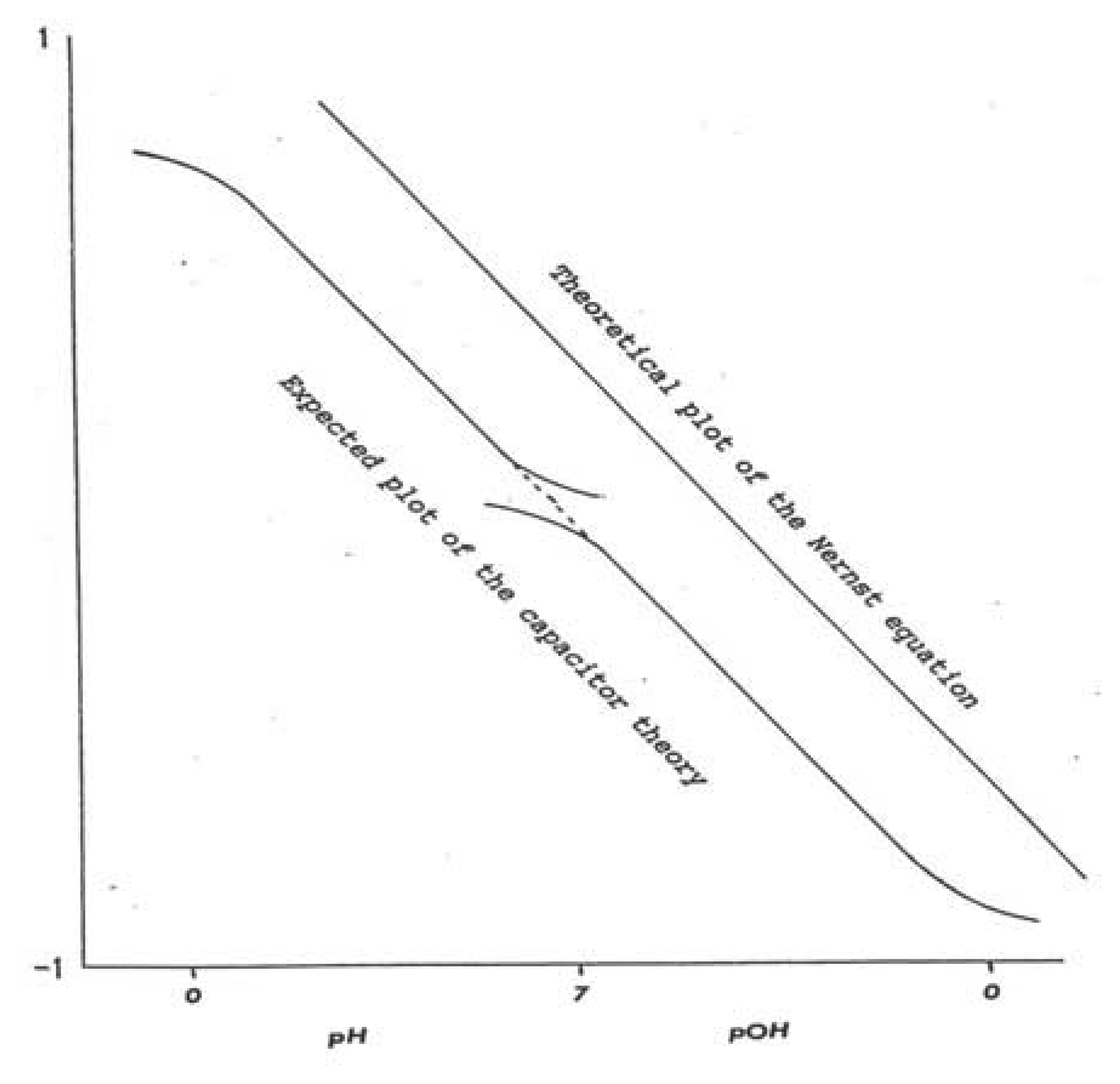
Theory: Nernst Equation, Slope, and Modified Boltzmann Equation
Experimental
pH Meter
Temperature
Stirring
Reference Electrode
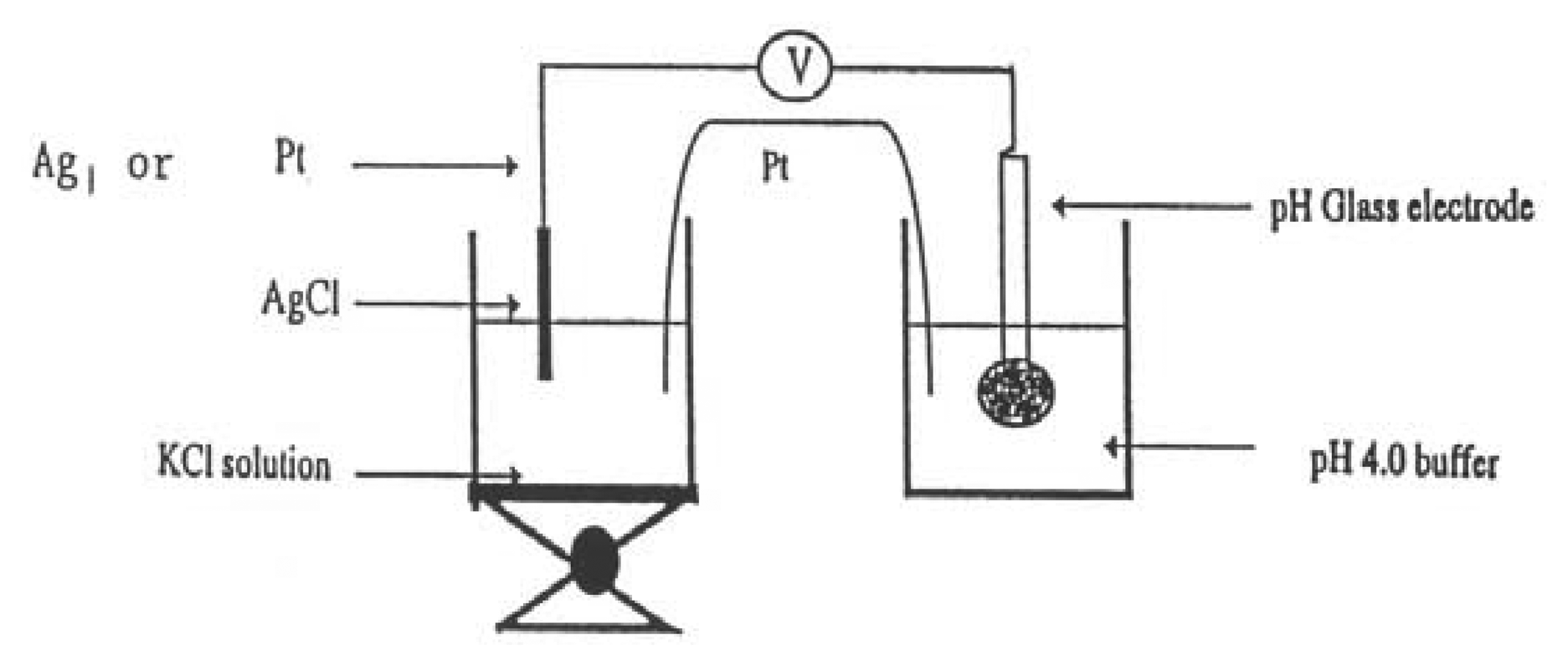
Junction Potential
Discussions
Standard Buffer
Sensitivity
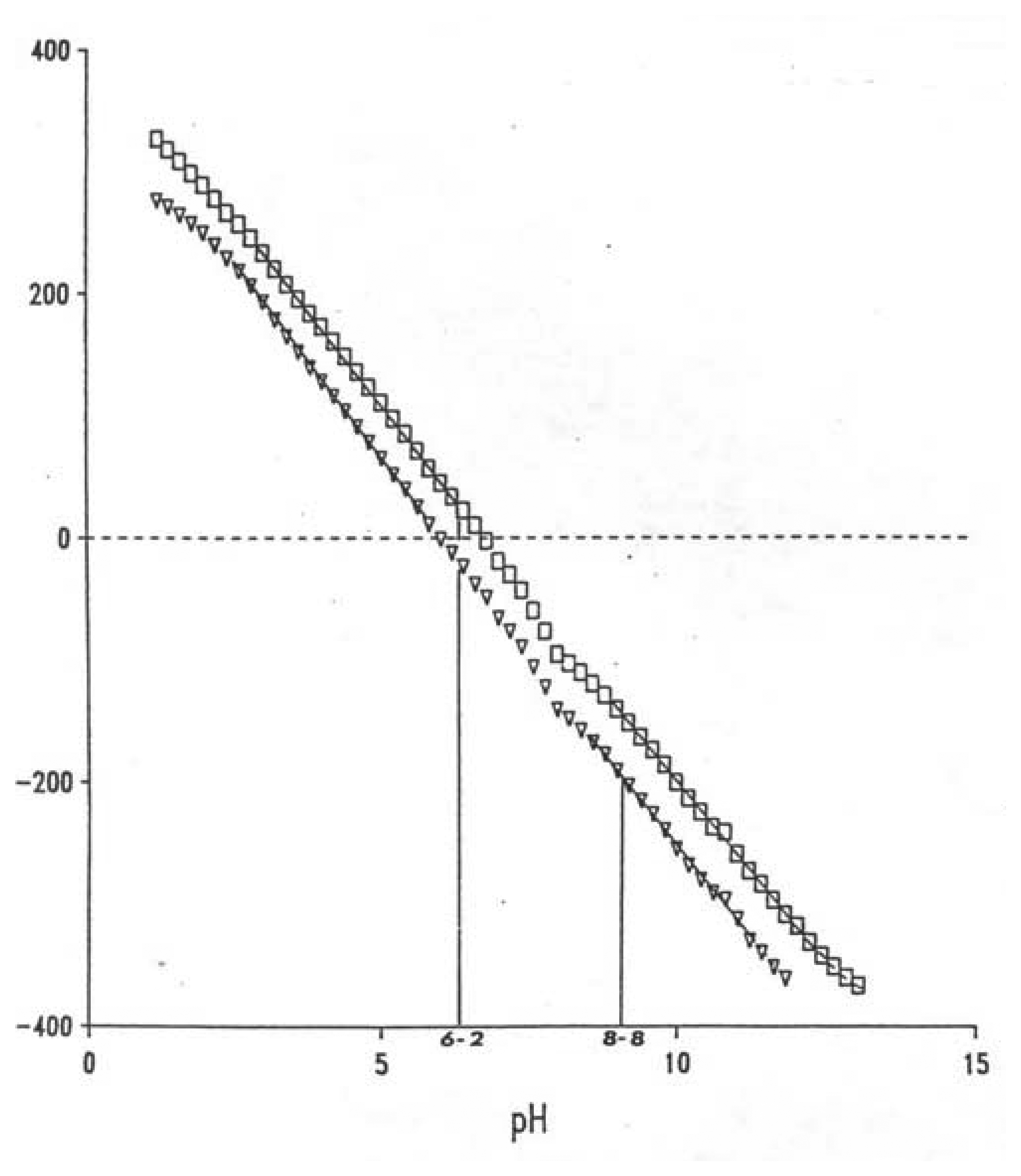
Suspension Effect
Glass Electrode
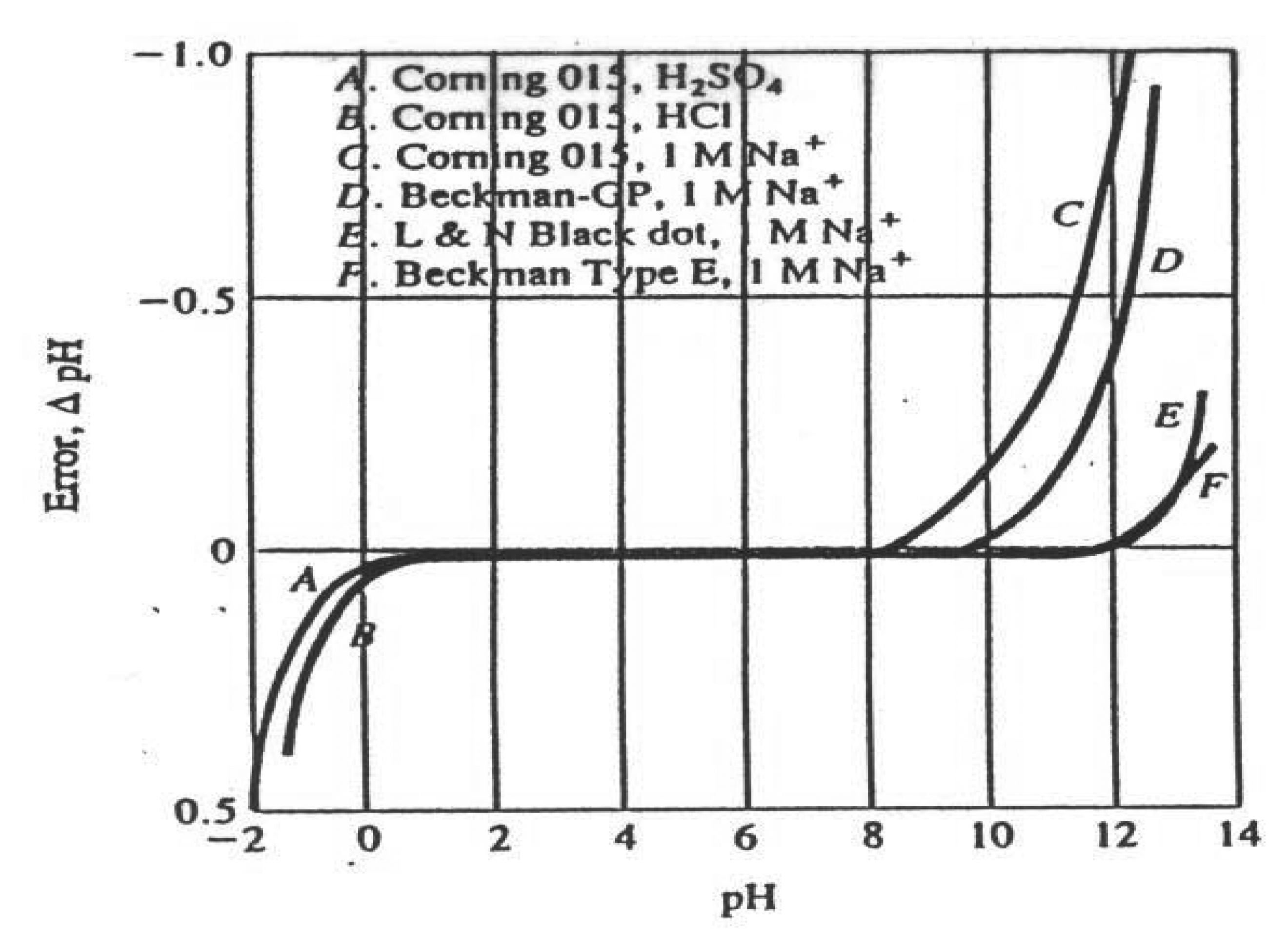
Limitations of the pH Glass Electrode
Frequency of Calibration
Summary
References
- Skoog, D.A. Principles of Instrumental Analysis, 3rd ed.; Saunders: Philadephia, PA, 1985. [Google Scholar]
- Galster, H. pH Measurement; VCH: Weinheim, Germany, 1991. [Google Scholar]
- Skoog, D. A.; West, D. M.; Holler, F. J. Fundamentals of Analytical Chemistry, 6th ed.; Saunders: Philadelphia, PA, 1992. [Google Scholar]
- Harris, D. C. Quantitative Chemical Analysis, 4th ed.; Freeman: NY, 1998. [Google Scholar]
- The Beckman Handbook of Applied Electrochemistry, 1st ed.; Beckman Instrument Industries: Fullerton, CA, 1980.
- Handbook of Electronic Technology, 3rd. ed.; Orion Research: Cambridge, U.K., 1982.
- Practice and Theory of pH Measurement; Ingold: Urdorf, Switzerland, 1989.
- Cheng, K. L. pH Glass Electrode and Its Mechanism. In Electrochemistry, Past and Present; Stock, J. T., Orno, M. V., Eds.; ACS Symposium Series: Washington, D.C, 1989; No. 390, pp. 286–302. [Google Scholar]
- Cheng, K. L. Misleading Ion Activity Concept in Nonfaradaic Potentiometry, Presented at ACS National Meeting, Anaheim, April 1995.
- Cheng, K. L. Microchem. J. 1990, 42, 5–24.
- Huang, C. M.; Jean, Y. C.; Cheng, K. L. J. Electrochem. Soc. 1995, 142, L175.
- Cheng, K. L.; Ashraf, N. Talanta 1990, 37, 659.
- Ashraf, N.; Hamdani, K.; Cheng, K.L. Advances in the Applications of Membrane-Mimetic Chemistry; Yen, T. F., et al., Eds.; Plenum: New York, 1994; pp. 209–225. [Google Scholar]
- Cheng, K. L. Microchem. J. 1998, 59, 437.
- Yang, S. X. New Developments of Nonfaradaic Potentiometry. Dissertation, University of Missouri-Kansas City, 1988. [Google Scholar]
- Beck, W. H.; Bottom, A. E.; Covington, A. K. Anal. Chem. 1968, 501–5.
- Huang, C. I.; Huang, H. J.; Cheng, K. L. Advances in Applications of Membrane-Mimetic Chemistry; Yen, T. F., Ed.; Plenum: NY, 1994. [Google Scholar]
- Temsamani, K. L.; Cheng, K. L. Sensors and Actuators B 2001, 76, 551–555.
- Crow, D. R. Principles and Applications of Electrochemistry, 3rd ed.; Chapman and Hall: New York, 1988. [Google Scholar]
- Bard, A. J.; Parsons, R.; Jordon, J. Standard Potentials in Aqueous Solutions; Dekker: New York, 1985. [Google Scholar]
- Milazzo, G.; Sagloo, C. Tables of Standard Electrode Potentials; Wiley: New York, 1978. [Google Scholar]
- Baucke, F. G. K. Electroanal. Chem. 1994, 367, 131.
- Yang, S. X.; Cheng, K.L.; Kurtz, L.T.; Peck, T. R. Particul. Sci. Tech. 1989, 7, 139.
- Genz, A.-K.; Busch, R.; Von Engelhaidt, W. Comp. Biochem. Physiol. A. Physiol. 1997, 118(A(2)), 407–408.
- Cheng, K. L. J. Chem. Educ. 1999, 76, 1029.
- Hamdani, K.; Cheng, K. L. Colloid Surface 1992, 63, 239–31.
- Su, Y. S.; Cheng, K. L.; Jean, Y. C. Talanta 1997, 44, 1757–1763.
- Cheng, K. L.; Song, H. Z.; Yang, S. J. Chem. Soc., Chem. Commun. 1988, 1333.
- Cheng, K. L. J. Coll. Interface. Sci. 2001, 239, 385–390.
- Hamdani, K.; Cheng, K. L. Microchem. J. 1999, 61, 195.
- Cheng, K. L. A Critical Review of Misleading Ion Activity and Ion Activity Coefficient Concepts. Paper presented at the Pittcon Conference, Atlanta, GA, March 1999.
- Bates, R. Determination of pH, 2nd ed.; Wiley: New York, 1974. [Google Scholar]
- Wu, Y. C.; Koch, W. F.; Marinenko, G. J. Res. NBC 1989, 395.
- Su, S. Y.; Cheng, K. L. Interference of pH Glass Electrode Containing La203 or Nd203caused by Phosphate and Fluoride Adsorption. Paper presented at the Eastern Analytical Symposium, Summit, NJ; 1997. [Google Scholar]
- Bockris, J. O'M.; Reddy, A.K.N. Modern Electrochemistry; Plenum: New York, 1970; p. p. 18. [Google Scholar]
- Cheng, K. L. Microchem. J. 2002, 72, 269–276.
© 2005 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cheng, K.L.; Zhu, D.-M. On Calibration of pH Meters. Sensors 2005, 5, 209-219. https://doi.org/10.3390/s5040209
Cheng KL, Zhu D-M. On Calibration of pH Meters. Sensors. 2005; 5(4):209-219. https://doi.org/10.3390/s5040209
Chicago/Turabian StyleCheng, K. L., and Da-Ming Zhu. 2005. "On Calibration of pH Meters" Sensors 5, no. 4: 209-219. https://doi.org/10.3390/s5040209
APA StyleCheng, K. L., & Zhu, D.-M. (2005). On Calibration of pH Meters. Sensors, 5(4), 209-219. https://doi.org/10.3390/s5040209



