Electromicrofluidic Device for Interference-Free Rapid Antibiotic Susceptibility Testing of Escherichia coli from Real Samples
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
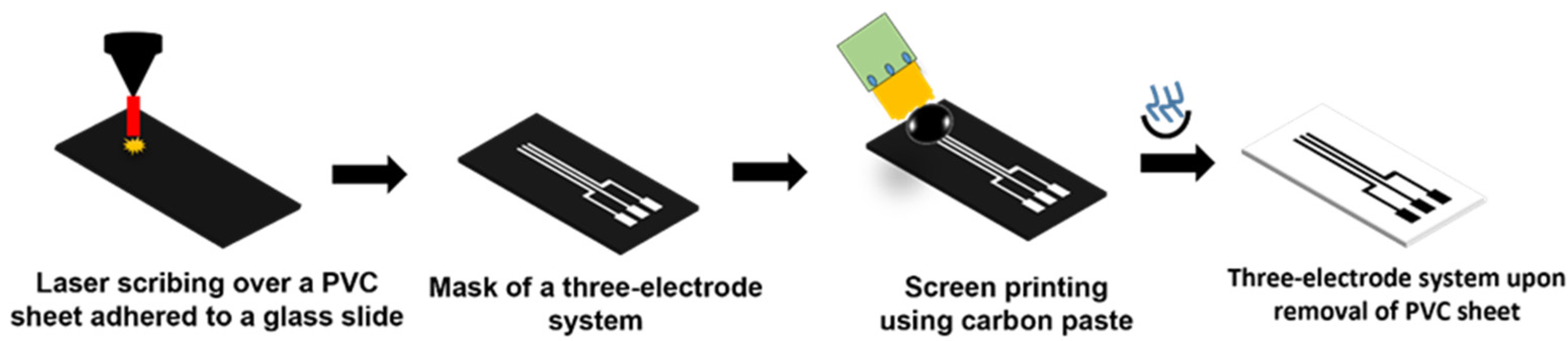
2.2. Development of Three-Electrode System and Microfluidic Device
2.3. Fabrication of LIG Heater
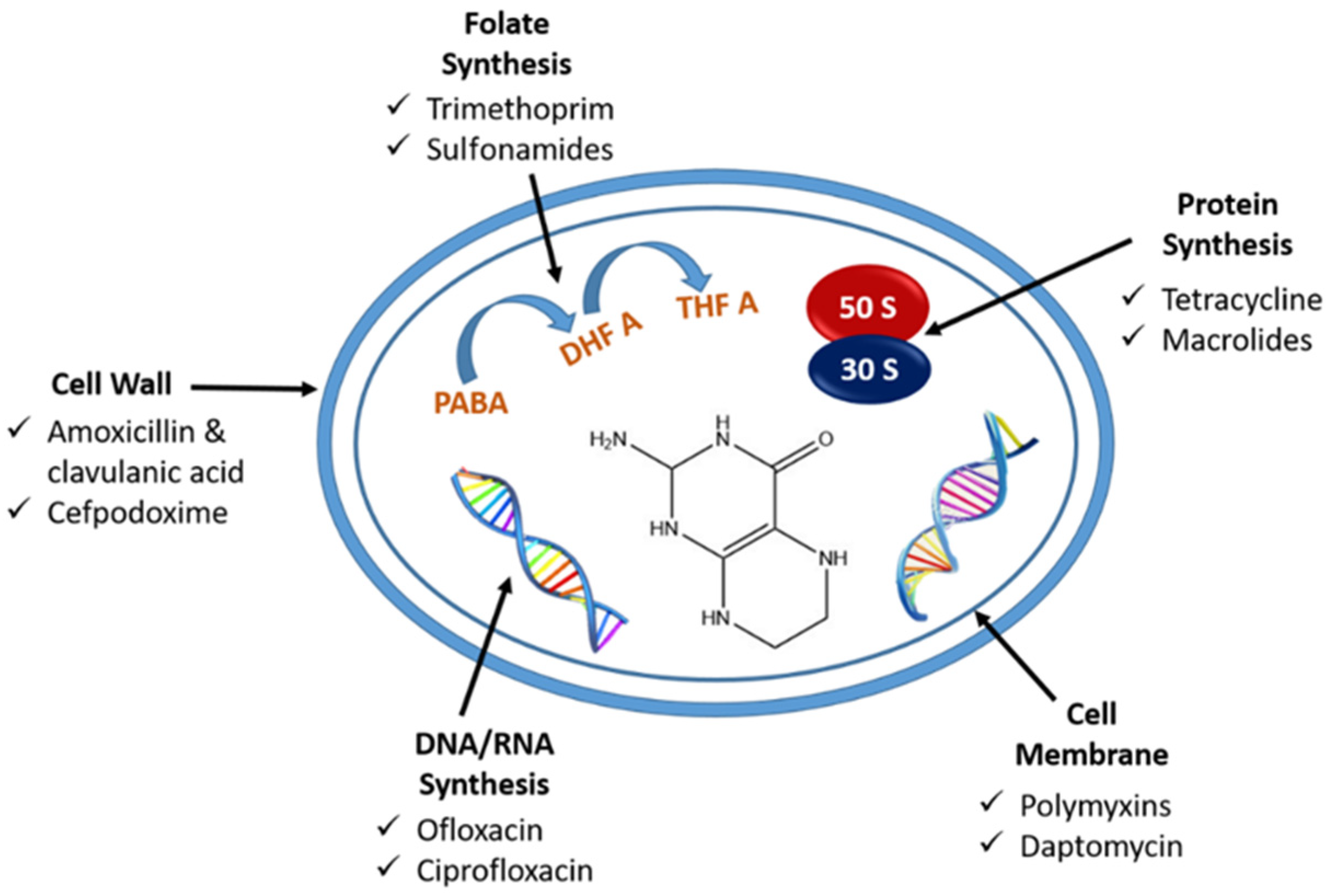
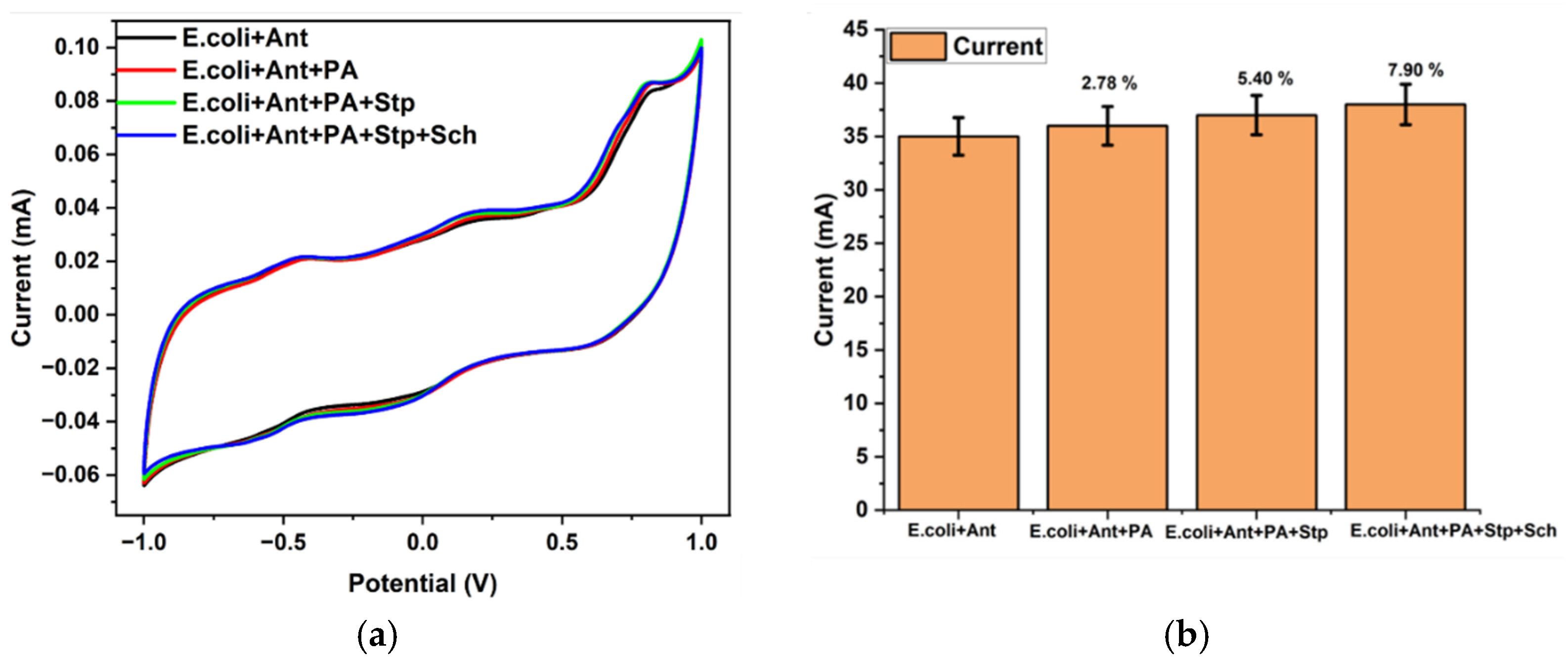
2.4. Effect of Antibiotic on E. coli
2.5. Evaluation of Real Samples
3. Results and Discussion
3.1. Off-Chip Minimum Inhibitory Concentration (MIC) Measurement
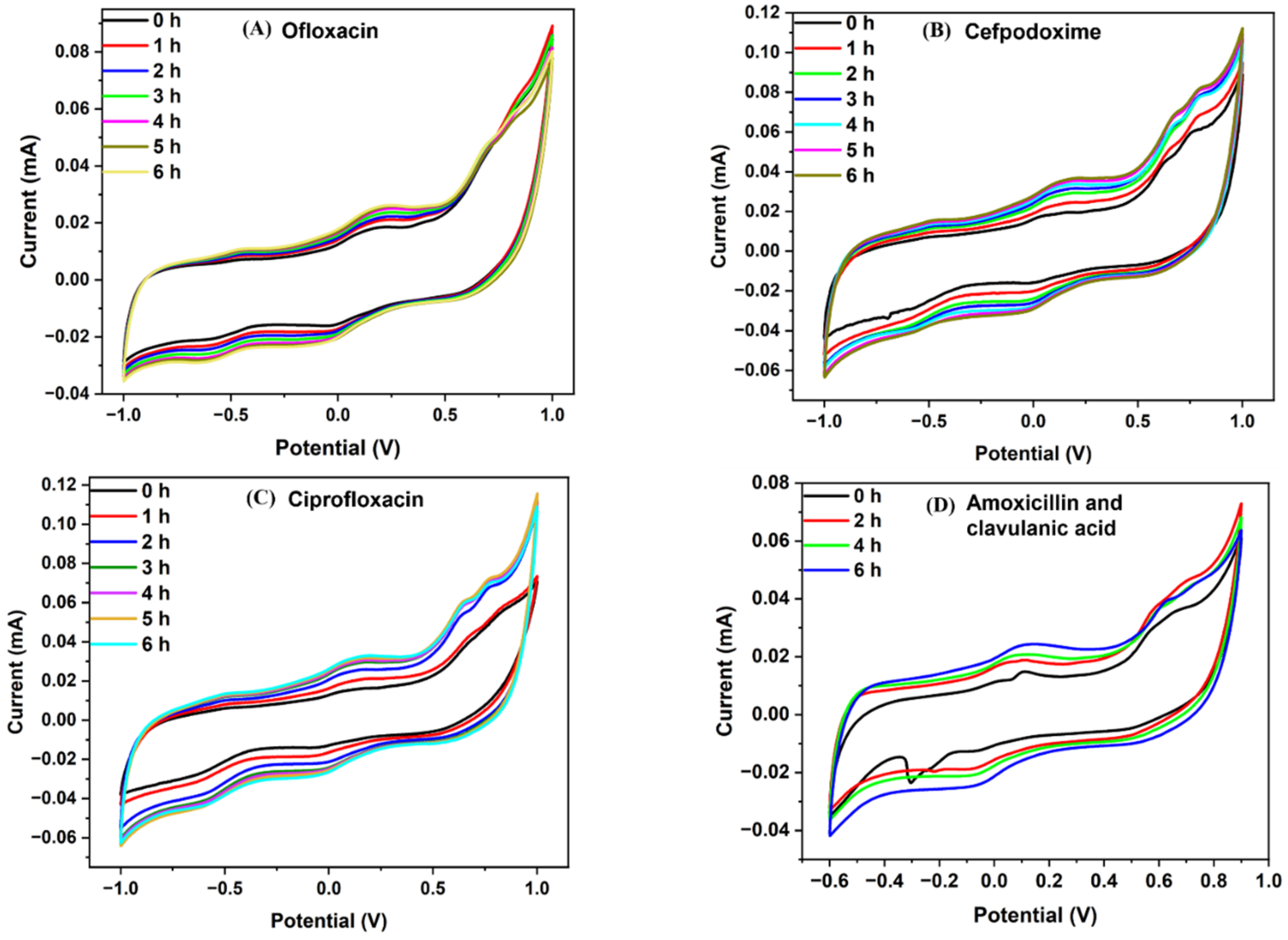
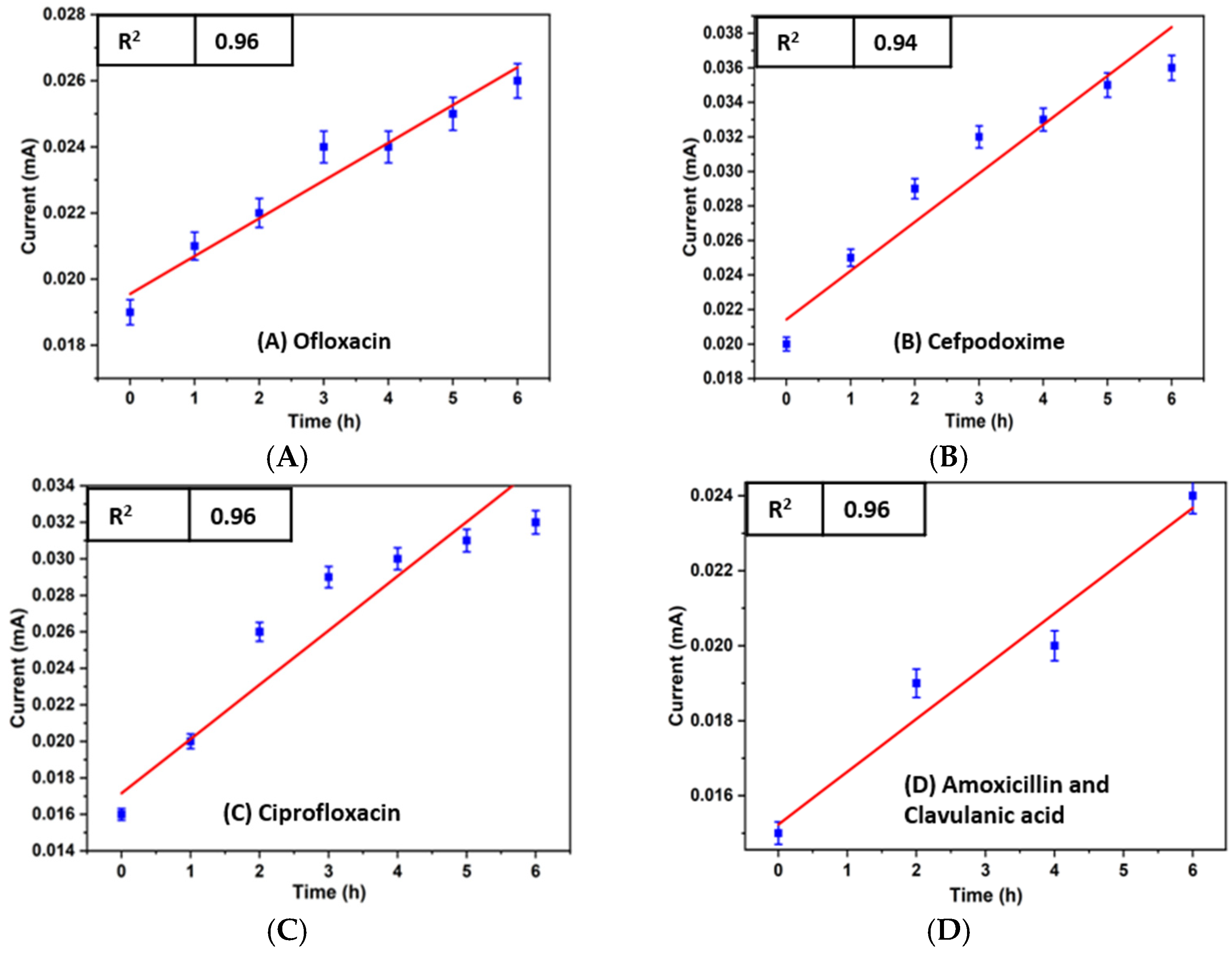
3.2. Electrochemical Detection of Antibiotic Effect over the Bacterial Growth
3.3. Interference Study
3.4. Antibiotic Susceptibility Testing Using Synthetic Urine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.T.; Wang, J.C.; Chuang, H.S. Developing Rapid Antimicrobial Susceptibility Testing for Motile/Non-Motile Bacteria Treated with Antibiotics Covering Five Bactericidal Mechanisms based on Bead-Based Optical Diffusometry. Biosensors 2020, 10, 181. [Google Scholar] [CrossRef]
- Farshidfar, N.; Assar, S.; Amiri, M.A.; Sahmeddini, S.; Hamedani, S.; Zarei, M.; Tayebi, L. The Feasible Application of Microfluidic Tissue/Organ-on-a-Chip as an Impersonator of Oral Tissues and Organs: A Direction for Future Research. Biodes. Manuf. 2023, 6, 478–506. [Google Scholar] [CrossRef]
- Verma, A.; Verma, M.; Singh, A. Animal tissue culture principles and applications. In Animal Biotechnology; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128117101. [Google Scholar]
- Tarditto, L.V.; Zon, M.A.; Ovando, H.G.; Vettorazzi, N.R.; Arévalo, F.J.; Fernández, H. Electrochemical Magneto Immunosensor Based on Endogenous β-Galactosidase Enzyme to Determine Enterotoxicogenic Escherichia coli F4 (K88) in Swine Feces Using Square Wave Voltammetry. Talanta 2017, 174, 507–513. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Veloo, A.C.M.; Seme, K.; Raangs, E.; Rurenga, P.; Singadji, Z.; Wekema-Mulder, G.; Van Winkelhoff, A.J. Antibiotic Susceptibility Profiles of Oral Pathogens. Int. J. Antimicrob. Agents 2012, 40, 450–454. [Google Scholar] [CrossRef]
- Karasinski, J.; White, L.; Zhang, Y.; Wang, E.; Andreescu, S.; Sadik, O.A.; Lavine, B.K.; Vora, M. Detection and Identification of Bacteria Using Antibiotic Susceptibility and a Multi-Array Electrochemical Sensor with Pattern Recognition. Biosens. Bioelectron. 2007, 22, 2643–2649. [Google Scholar] [CrossRef]
- Pandey, A.; Gurbuz, Y.; Ozguz, V.; Niazi, J.H.; Qureshi, A. Graphene-Interfaced Electrical Biosensor for Label-Free and Sensitive Detection of Foodborne Pathogenic E. Coli O157:H7. Biosens. Bioelectron. 2017, 91, 225–231. [Google Scholar] [CrossRef]
- Abu-Sini, M.K.; Maharmah, R.A.; Abulebdah, D.H.; Al-Sabi, M.N.S. Isolation and Identification of Coliform Bacteria and Multidrug-Resistant Escherichia coli from Water Intended for Drug Compounding in Community Pharmacies in Jordan. Healthcare 2023, 11, 299. [Google Scholar] [CrossRef]
- Paulose, A.K.; Hou, Y.; Huang, Y.; Dileep, N.C.; Chiu, C.; Pal, A.; Kalaimani, V.M.; Lin, Z.; Chang, C.; Chen, C.; et al. Rapid Escherichia coli Cloned DNA Detection in Serum Using an Electrical Double Layer-Gated Field-Effect Transistor-Based DNA Sensor. Anal. Chem. 2023, 95, 6871–6878. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, A.K.; Ali, M.R.; Chander, Y. Antimicrobial Susceptibility Profile of Extended Spectrum β-Lactamase (ESBL) Producing Escherichia coli from Various Clinical Samples. Infect. Dis. Res. Treat. 2014, 7, IDRT.S13820. [Google Scholar] [CrossRef]
- Behera, B.; Anil Vishnu, G.K.; Chatterjee, S.; Sitaramgupta, V.V.S.N.; Sreekumar, N.; Nagabhushan, A.; Rajendran, N.; Prathik, B.H.; Pandya, H.J. Emerging Technologies for Antibiotic Susceptibility Testing. Biosens. Bioelectron. 2019, 142, 111552. [Google Scholar] [CrossRef]
- Cunha, A.P.; Henriques, R.; Cardoso, S.; Freitas, P.P.; Carvalho, C.M. Rapid and Multiplex Detection of Nosocomial Pathogens on a Phage-Based Magnetoresistive Lab-on-Chip Platform. Biotechnol. Bioeng. 2021, 118, 3164–3174. [Google Scholar] [CrossRef]
- Jeon, H.; Khan, Z.A.; Barakat, E.; Park, S. Label-Free Electrochemical Microfluidic Chip for the Antimicrobial Susceptibility Testing. Antibiotics 2020, 9, 348. [Google Scholar] [CrossRef]
- Webster, T.A.; Sismaet, H.J.; Chan, I.P.J.; Goluch, E.D. Electrochemically Monitoring the Antibiotic Susceptibility of Pseudomonas aeruginosa Biofilms. Analyst 2015, 140, 7195–7201. [Google Scholar] [CrossRef]
- Ding, C.; Liu, Y.; Guo, Y.; Guo, X.; Kang, Q.; Yan, X.; He, Z. Precise Digital Bacteria Enumeration and Antibiotic Susceptibility Testing via a Portable Vibrating Capillary-Based Droplet Platform. Sens. Actuators B Chem. 2023, 380, 133254. [Google Scholar] [CrossRef]
- Rao, R.P.; Sharma, S.; Mehrotra, T.; Das, R.; Kumar, R.; Singh, R.; Roy, I.; Basu, T. Rapid Electrochemical Monitoring of Bacterial Respiration for Gram-Positive and Gram-Negative Microbes: Potential Application in Antimicrobial Susceptibility Testing. Anal. Chem. 2020, 92, 4266–4274. [Google Scholar] [CrossRef]
- Abbas, N.; Song, S.; Chang, M.S.; Chun, M.S. Point-of-Care Diagnostic Devices for Detection of Escherichia coli O157:H7 Using Microfluidic Systems: A Focused Review. Biosensors 2023, 13, 741. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, H.; Park, J.; Abbas, N.; Kang, S.; Hyun, H.; Seong, H.; Yoon, J.G.; Noh, J.Y.; Kim, W.J.; et al. Collection and Detection of SARS-CoV-2 in Exhaled Breath Using Face Mask. PLoS ONE 2022, 17, e0270765. [Google Scholar] [CrossRef]
- Mairhofer, J.; Roppert, K.; Ertl, P. Microfluidic Systems for Pathogen Sensing: A Review. Sensors 2009, 9, 4804–4823. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Xue, Z.; Xia, H.; Yang, N.; Zhang, R. Numerical Analysis of Capture and Isolation of Magnetic Nanoparticles in Microfluidic System. Mod. Phys. Lett. B 2018, 32, 1840075. [Google Scholar] [CrossRef]
- Hewlin, R.; Edwards, M.; Smith, M. A 2D Transient Computational Multi-Physics Model for Analyzing Magnetic and Non-Magnetic Particle (Red Blood Cells and E. Coli Bacteria) Dynamics in a Travelling Wave Ferro-Magnetic Microfluidic Device for Potential Cell Separation and Sorting. J. Eng. Sci. Med. Diagn. Ther. 2023, 7, 021004. [Google Scholar] [CrossRef]
- Besant, J.D.; Sargent, E.H.; Kelley, S.O. Rapid Electrochemical Phenotypic Profiling of Antibiotic-Resistant Bacteria. Lab Chip 2015, 15, 2799–2807. [Google Scholar] [CrossRef]
- Grigorov, E.; Peykov, S.; Kirov, B. Novel Microfluidics Device for Rapid Antibiotics Susceptibility Screening. Appl. Sci. 2022, 12, 2198. [Google Scholar] [CrossRef]
- Fande, S.; Amreen, K.; Sriram, D.; Goel, S. Microfluidic Electrochemical Device for Real-Time Culturing and Interference-Free Detection of Escherichia coli. Anal. Chim. Acta 2023, 1237, 340591. [Google Scholar] [CrossRef]
- Tang, P.C.; Eriksson, O.; Sjögren, J.; Fatsis-Kavalopoulos, N.; Kreuger, J.; Andersson, D.I. A Microfluidic Chip for Studies of the Dynamics of Antibiotic Resistance Selection in Bacterial Biofilms. Front. Cell. Infect. Microbiol. 2022, 12, 896149. [Google Scholar] [CrossRef]
- Zhang, Y.; Gholizadeh, H.; Young, P.; Traini, D.; Li, M.; Ong, H.X.; Cheng, S. Real-Time in-Situ Electrochemical Monitoring of Pseudomonas aeruginosa Biofilms Grown on Air–Liquid Interface and Its Antibiotic Susceptibility Using a Novel Dual-Chamber Microfluidic Device. Biotechnol. Bioeng. 2023, 120, 702–714. [Google Scholar] [CrossRef]
- Chikezie, I.O. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Using a Novel Dilution Tube Method. Afr. J. Microbiol. Res. 2017, 11, 977–980. [Google Scholar] [CrossRef]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A Fully Automated Microfluidic-Based Electrochemical Sensor for Real-Time Bacteria Detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef]
- Song, K.; Yu, Z.; Zu, X.; Huang, L.; Fu, D.; Yao, J.; Hu, Z.; Xue, Y. Microfluidic Chip for Detection of Drug Resistance at the Single-Cell Level. Micromachines 2023, 14, 46. [Google Scholar] [CrossRef]
- Srikanth, S.; Jayapiriya, U.S.; Dubey, S.K.; Javed, A.; Goel, S. A Protocol to Execute a Lab-on-Chip Platform for Simultaneous Culture and Electrochemical Detection of Bacteria. STAR Protoc. 2023, 4, 102327. [Google Scholar] [CrossRef]
- Srikanth, S.; Jayapiriya, U.S.; Dubey, S.K.; Javed, A.; Goel, S. Lab-On-Chip Integrated Platform with Screen Printed Electrodes and Laser Induced Graphene Heater for Simultaneous Culture and Electrochemical Detection of Bacteria. Available online: https://ssrn.com/abstract=4024173 (accessed on 1 September 2023).
- Haddad, L. Synthetic Urine and Method of Making Same. US Patent 7,109,035 B2, 19 September 2006. [Google Scholar]
- Some, S.; Mondal, R.; Mitra, D.; Jain, D.; Verma, D.; Das, S. Microbial Pollution of Water with Special Reference to Coliform Bacteria and Their Nexus with Environment. Energy Nexus 2021, 1, 100008. [Google Scholar] [CrossRef]
- Luo, J.; Fang, X.; Ye, D.; Li, H.; Chen, H.; Zhang, S.; Kong, J. A Real-Time Microfluidic Multiplex Electrochemical Loop-Mediated Isothermal Amplification Chip for Differentiating Bacteria. Biosens. Bioelectron. 2014, 60, 84–91. [Google Scholar] [CrossRef]




| Components | Quantity (mg/L) |
|---|---|
| Potassium Chloride (KCl) | 2000 |
| Sodium Sulfate (Na2SO4) | 2000 |
| Ammonium Phosphate ((NH4)3PO4) | 850 |
| Ammonium Diphosphate ((NH4)3PO4) | 850 |
| Calcium Chloride (CaCl2) | 250 |
| Magnesium Chloride (MgCl2) | 500 |
| Urea (CH4N2O) | 600 |
| Creatinine (C4H7N3O) | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fande, S.; Amreen, K.; Sriram, D.; Mateev, V.; Goel, S. Electromicrofluidic Device for Interference-Free Rapid Antibiotic Susceptibility Testing of Escherichia coli from Real Samples. Sensors 2023, 23, 9314. https://doi.org/10.3390/s23239314
Fande S, Amreen K, Sriram D, Mateev V, Goel S. Electromicrofluidic Device for Interference-Free Rapid Antibiotic Susceptibility Testing of Escherichia coli from Real Samples. Sensors. 2023; 23(23):9314. https://doi.org/10.3390/s23239314
Chicago/Turabian StyleFande, Sonal, Khairunnisa Amreen, D. Sriram, Valentin Mateev, and Sanket Goel. 2023. "Electromicrofluidic Device for Interference-Free Rapid Antibiotic Susceptibility Testing of Escherichia coli from Real Samples" Sensors 23, no. 23: 9314. https://doi.org/10.3390/s23239314





