A Highly Sensitive and Selective ppb-Level Acetone Sensor Based on a Pt-Doped 3D Porous SnO2 Hierarchical Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Pure SnO2 Microflowers
2.3. Synthesis of the Pt-Doped 3DS and Pt-Doped 3DPS Microflowers
2.4. Characterization of the Sensing Materials
2.5. Fabrication and Characterization of the Gas Sensor
3. Results
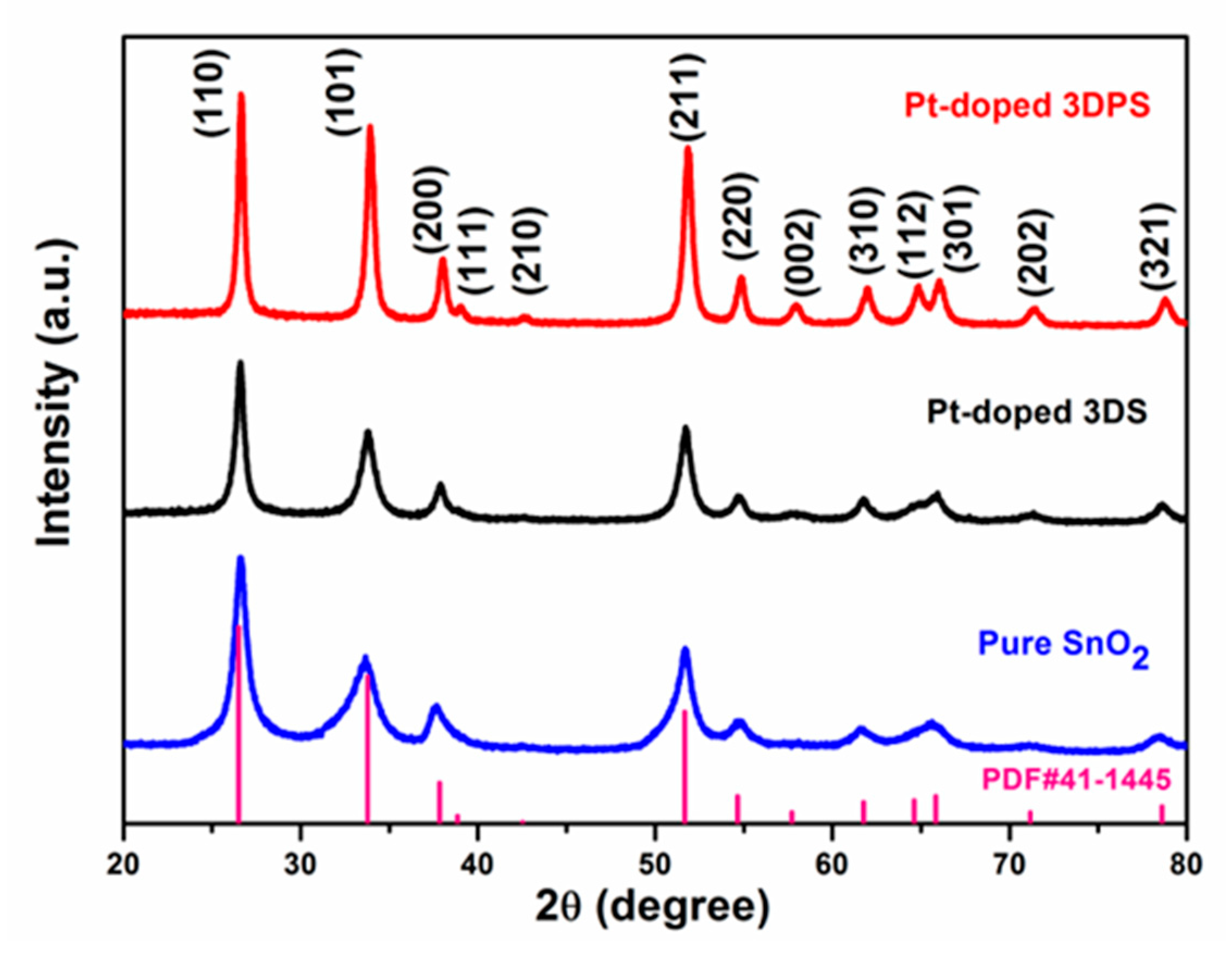
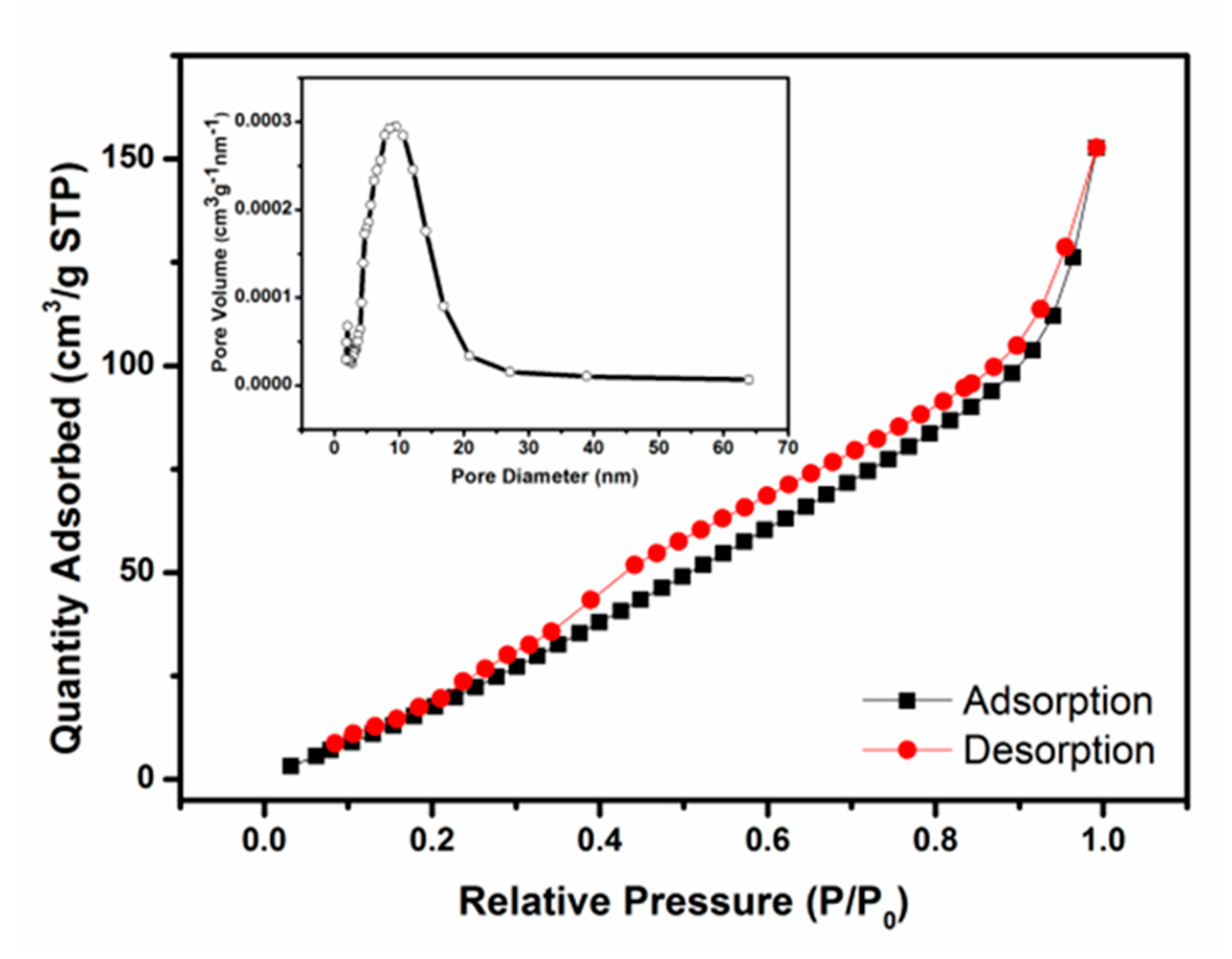
3.1. Structure, Morphology and Composition of the Sensing Materials
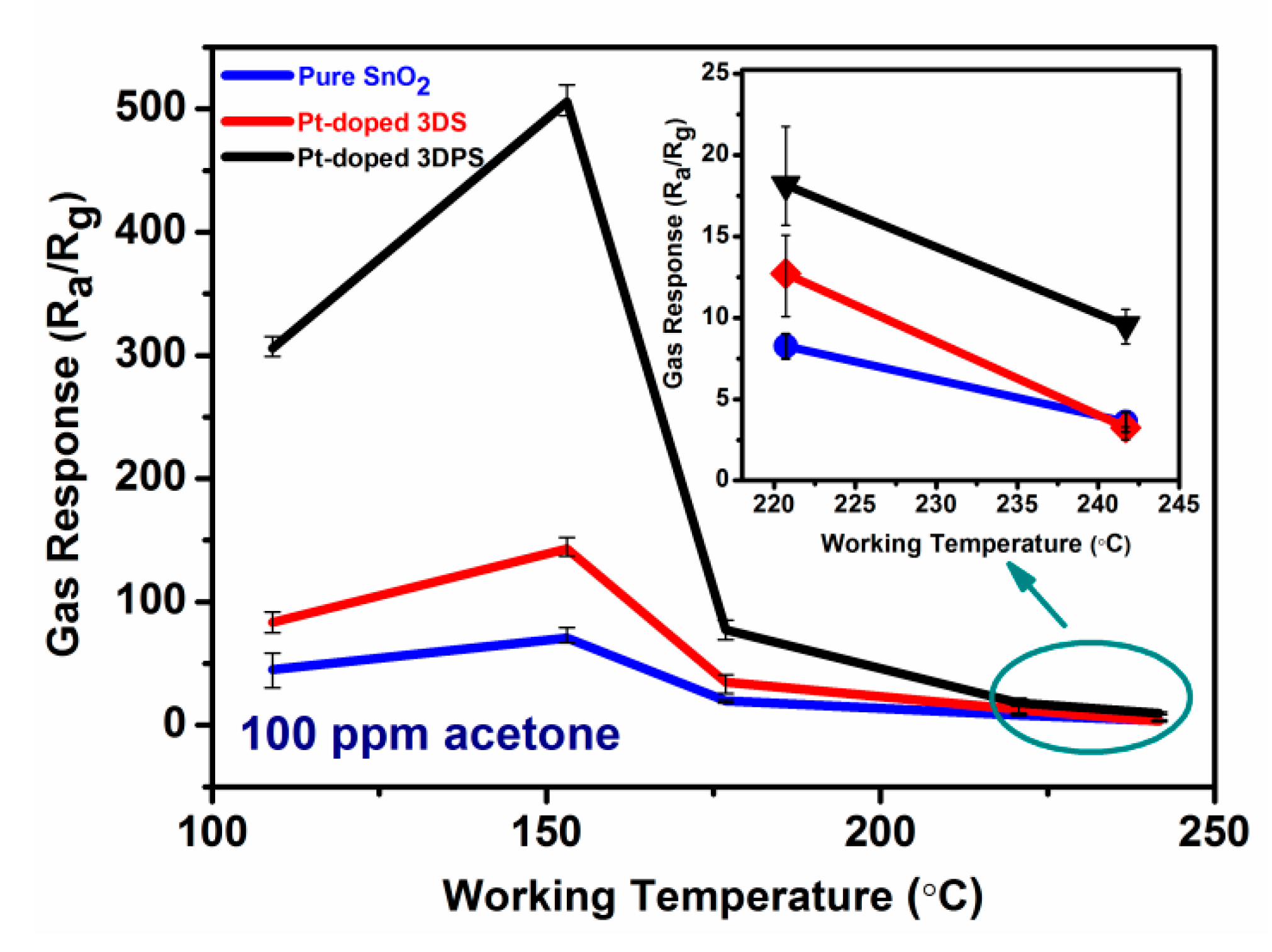
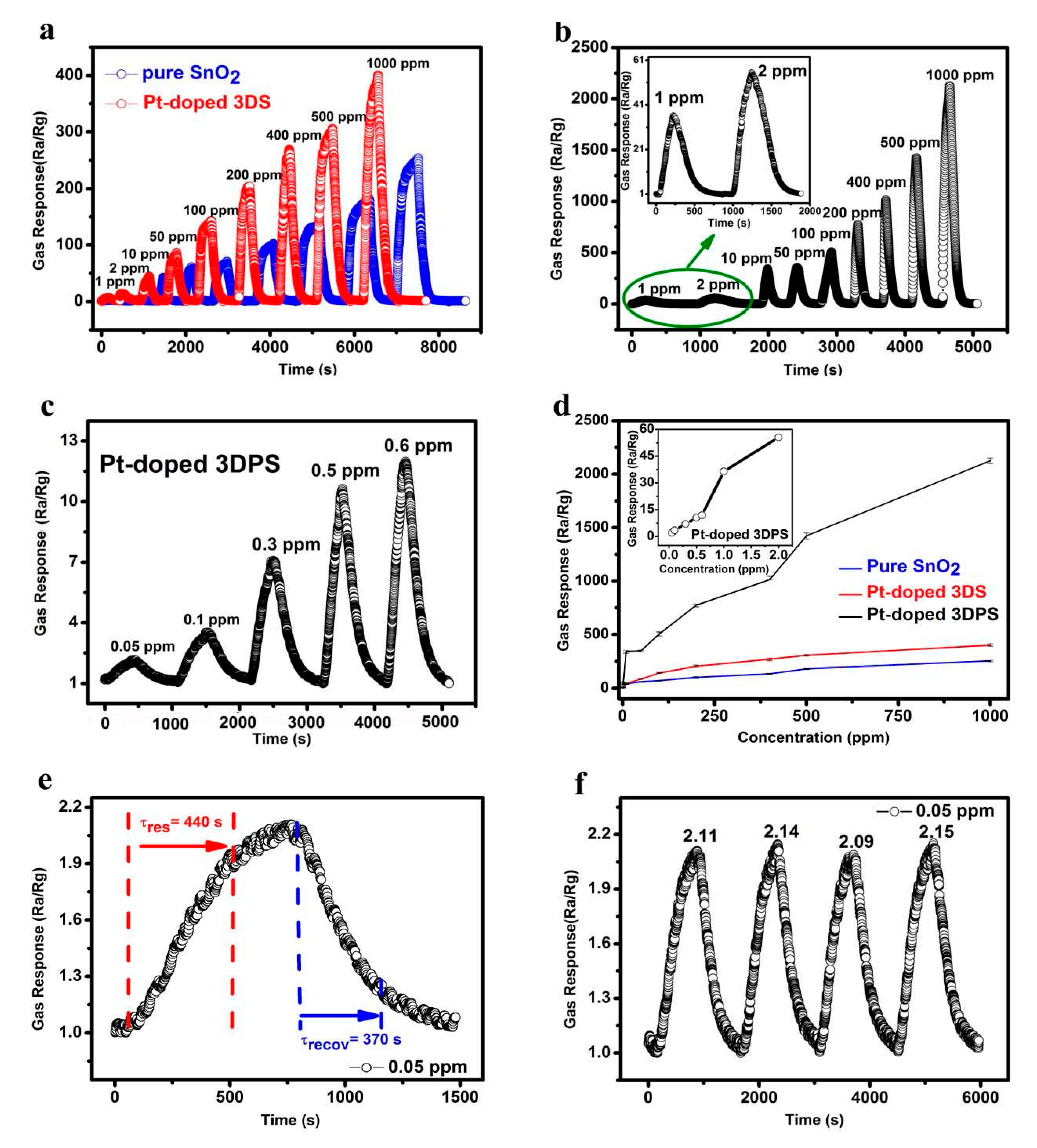
3.2. The Characterization of Gas Sensor Performance
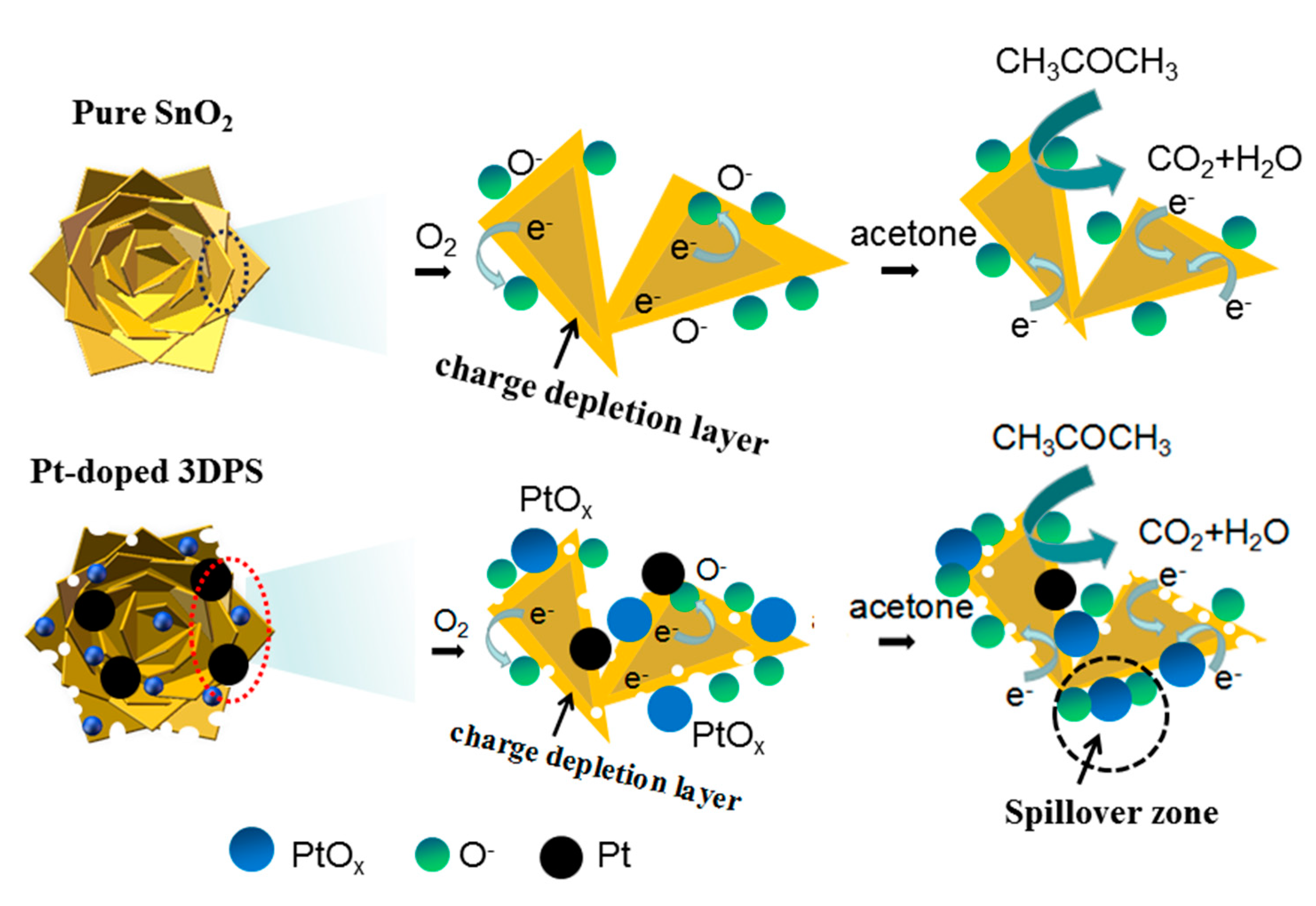
3.3. Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Bamsaoud, S.F.; Rane, S.B.; Karekar, R.N.; Aiyer, R.C. Nano particulate SnO2 based resistive films as a hydrogen and acetone vapour sensor. Sens. Actuators B 2011, 153, 382–391. [Google Scholar] [CrossRef]
- Likhodii, S.S.; Musa, K.; Cunnane, S.C. Breath acetone as a measure of systemic ketosis assessed in a rat model of the ketogenic diet. Clin. Chem. 2002, 48, 115–120. [Google Scholar] [PubMed]
- Liu, X.; Hu, J.F.; Cheng, B.; Qin, H.; Jiang, M. Acetone gas sensing properties of SmFe1-xMgxO3 perovskite oxides. Sens. Actuators B 2008, 134, 483–487. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Sens. Actuators B 2014, 47, 66–76. [Google Scholar] [CrossRef]
- Kim, S.-J.; Choi, S.-J.; Jang, J.-S.; Cho, H.-J.; Kim, I.-D. Innovative Nanosensor for Disease Diagnosis. ACC Chem. Res. 2017, 50, 1587–1596. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Choi, J.-K.; Woo, H.-S.; Kim, S.-J.; Jung, S.-Y.; Seong, T.-Y.; Kim, I.-D. Facile Control of C2H5OH Sensing Characteristics by Decorating Discrete Ag Nanoclusters on SnO2 Nanowire Networks. ACS Appl. Mater. Interfaces 2011, 3, 3140–3145. [Google Scholar] [CrossRef]
- Suematsu, K.; Sasaki, M.; Ma, N.; Yuasa, M.; Shimanoe, K. Antimony-Doped Tin Dioxide Gas Sensors Exhibiting High Stability in the Sensitivity to Humidity Changes. ACS Sens. 2016, 1, 913–920. [Google Scholar] [CrossRef]
- Liu, J.Y.; Dai, M.J.; Wang, T.S.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, Y.; Zhang, Z.L.; Qu, F.Y.; Umar, A.; Wu, X. Hierarchical SnO2 Nanostructures Made of Intermingled Ultrathin Nanosheets for Environmental Remediation, Smart Gas Sensor, and Supercapacitor Applications. ACS Appl. Mater. Interfaces 2014, 6, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Mei, X.D.; Cai, Y.X.; Ma, J.; Sun, Y.F.; Liang, X.H.; Liu, F.M.; Lu, G.Y. Synthesis and gas sensing properties of hierarchical SnO2 nanostructures. Sens. Actuators B 2013, 187, 301–307. [Google Scholar] [CrossRef]
- Wei, F.J.; Zhang, H.J.; Nguyen, M.; Ying, M.X.; Gao, R.; Jiao, Z. Template-free synthesis of flower-like SnO2 hierarchical nanostructures with improved gas sensing performance. Sens. Actuators B 2015, 215, 15–23. [Google Scholar] [CrossRef]
- Jing, Z.H.; Zhan, J.H. Fabrication and Gas-Sensing Properties of Porous ZnO Nanoplates. Adv. Mater. 2008, 20, 4547–4551. [Google Scholar] [CrossRef]
- Kou, X.Y.; Wang, C.; Ding, M.D.; Feng, C.H.; Li, X.; Ma, J.; Zhang, H.; Sun, Y.F.; Lu, G.Y. Synthesis of Co-doped SnO2 nanofibers and their enhanced gas-sensing properties. Sens. Actuators B 2016, 236, 425–432. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Gong, H.B.; Ju, D.X.; Cao, B.Q. Au nanoparticle-functionalized 3D SnO2 microstructures for high performance gas sensor. Sens. Actuators B 2016, 226, 266–272. [Google Scholar] [CrossRef]
- Liu, L.Y.; Liu, S.T. Oxygen Vacancies as an Efficient Strategy for Promotion of Low Concentration SO2 Gas Sensing: The Case of Au-Modified SnO2. ACS Sustain. Chem. Eng. 2018, 6, 13427–13434. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.N.; Umar, A.; Chen, W.G.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens. Actuators B 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.J.; Lee, I.; Youn, D.Y.; Park, C.O.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Thin-Wall Assembled SnO2 Fibers Functionalized by Catalytic Pt Nanoparticles and their Superior Exhaled-Breath-Sensing Properties for the Diagnosis of Diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Wang, S.M.; Xiao, B.X.; Yang, T.Y.; Wang, P.; Xiao, C.H.; Li, Z.H.; Zhao, R.; Zhang, M.Z. Enhanced HCHO gas sensing properties by Ag-loaded sunflower-like In2O3 hierarchical nanostructures. J. Mater. Chem. A 2014, 2, 6598–6604. [Google Scholar] [CrossRef]
- Liu, X.J.; Tian, X.Y.; Jiang, X.M.; Jiang, L.; Hou, P.Y.; Zhang, S.W.; Sun, X.; Yang, H.C.; Cao, R.Y.; Xu, X.J. Facile preparation of hierarchical Sb-doped In2O3 microstructures for acetone detection. Sens. Actuators B 2018, 270, 304–311. [Google Scholar] [CrossRef]
- Kim, D.H.; Shim, Y.S.; Jeon, J.M.; Jeon, H.Y.; Park, S.S.; Kim, Y.W.; Kim, J.S.; Lee, J.H.; Jang, H.W. Vertically Ordered Hematite Nanotube Array as an Ultrasensitive and Rapid Response Acetone Sensor. ACS Appl. Mater. Interfaces 2014, 6, 14779–14784. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Zhou, X.; Guo, H.; Wang, C.; Liu, J.Y.; Sun, P.; Liu, F.M.; Lu, G.Y. Design of Au@ZnO Yolk-Shell Nanospheres with Enhanced Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 18661–18667. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N.; Shimanoe, K. New perspectives of gas sensor technology. Sens. Actuators B 2009, 138, 100–107. [Google Scholar] [CrossRef]
- Kolmakov, A.; Zhang, Y.; Cheng, G.; Moskovits, M. Detection of CO and O2 using tin oxide nanowire sensors. Adv. Mater. 2003, 15, 997–1000. [Google Scholar] [CrossRef]
- Wang, Y.L.; Jiang, X.C.; Xia, Y.N. A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J. Am. Chem. Soc. 2003, 125, 16176–16177. [Google Scholar] [CrossRef]
- Hernández-Fernández, P.; Nuño, R.; Fatás, E.; Fierro, J.L.G.; Ocón, P. MWCNT-supported PtRu catalysts for the electrooxidation of methanol: Effect of the functionalized support. Int. J. Hydrogen Energy 2011, 36, 8267–8278. [Google Scholar] [CrossRef]
- Yasuda, K.; Nobu, M.; Masui, T.; Imanaka, N. Complete oxidation of acetaldehyde on Pt/CeO2–ZrO2–Bi2O3 catalysts. Mater. Res. Bull. 2010, 45, 1278–1282. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Qiao, J.H.; Yuan, J.H.; Shen, J.F.; Wang, A.J.; Huang, S.T. Controllable synthesis of PtPd nanocubes on graphene as advanced catalysts for ethanol oxidation. Int. J. Hydrogen Energy 2018, 43, 4902–4911. [Google Scholar] [CrossRef]
- Romanovskaya, V.; Ivanovskaya, M.; Bogdanov, P. A study of sensing properties of Pt-and Au-loaded In2O3 ceramics. Sens. Actuators B 1999, 56, 31–36. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.C.; Zhuang, J.; Wang, L.Y.; Zhang, J.B.; Li, H.Y.; Liu, Z.; Han, Y.; Jiang, X.X.; Zhang, P. Improved selective acetone sensing properties of Co-doped ZnO nanofibers by electrospinning. Sens. Actuators B 2011, 155, 782–788. [Google Scholar] [CrossRef]
- Ding, D.; Xue, Q.; Lu, W.; Xiong, Y.; Zhang, J.Q.; Pan, X.L.; Tao, B.H. Chemically functionalized 3D reticular graphene oxide frameworks decorated with MOF-derived Co3O4: Towards highly sensitive and selective detection to acetone. Sens. Actuators B 2018, 259, 289–298. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Han, Y.T.; Chen, X.W. Glucose-assisted synthesis of hierarchical flower-like Co3O4 nanostructures assembled by porous nanosheets for enhanced acetone sensing. Sens. Actuators B 2019, 288, 699–706. [Google Scholar] [CrossRef]
- Zhang, S.F.; Jiang, W.H.; Li, Y.W.; Yang, X.L.; Sun, P.; Liu, F.M.; Yan, X.; Gao, Y.; Liang, X.; Ma, J.; et al. Highly-sensitivity acetone sensors based on spinel-type oxide (NiFe2O4) through optimization of porous structure. Sens. Actuators B 2019, 291, 266–274. [Google Scholar] [CrossRef]
- Kou, X.Y.; Xie, N.; Chen, F.; Wang, T.S.; Guo, L.L.; Wang, C.; Wang, Q.J.; Ma, J.; Sun, Y.F.; Zhang, H.; et al. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Li, F.; Zang, T.; Gao, X.; Wang, R. Coaxial electrospinning heterojunction SnO2/Au-doped In2O3 core-shell nanofibers for acetone gas sensor. Sens. Actuators B 2017, 252, 822–830. [Google Scholar] [CrossRef]
- Jang, J.S.; Choi, S.J.; Kim, S.J.; Hakim, M.; Kim, I.D. Rational Design of highly porous SnO2 nanotubes functionalized with biomimetic nanocatalysts for direct observation of simulated diabetes. Adv. Funct. Mater. 2016, 26, 4740–4748. [Google Scholar] [CrossRef]
- Li, G.J.; Cheng, Z.X.; Xiang, Q.; Yan, L.M.; Wang, X.H.; Xu, J.Q. Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone. Sens. Actuators B 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Zhang, S.D.; Yan, M.J.; Liang, K.Y.; Turak, A.; Zhang, B.X.; Meng, D.; Wang, C.X.; Qu, F.D.; Cheng, W.; Yang, M.H. An acetone gas sensor based on nanosized Pt-loaded Fe2O3 nanocubes. Sens. Actuators B 2019, 290, 59–67. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Wang, W.D.; Xue, Y.; Sun, Y.J.; Li, P.W.; Lian, K.; Zhang, W.D.; Chen, L.; Shi, J.; et al. Synthesis and gas sensing properties of NiO/SnO2 hierarchical structures toward ppb-level acetone detection. Mater. Res. Bull. 2018, 102, 294–303. [Google Scholar] [CrossRef]
- Xu, J.Q.; Chen, Y.P.; Chen, D.Y.; Shen, J.N. Hydrothermal synthesis and gas sensing characters of ZnO nanorods. Sens. Actuators B 2006, 113, 526–531. [Google Scholar]
- Chang, C.S. Oxygen chemisorption on tin oxide: Correlation between electrical conductivity and EPR measurements. J. Vac. Sci. Technol. 1980, 17, 366–369. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K.; Gulina, L.B.; Tolstoy, V.P. Gas sensor application of Ag nanoclusters synthesized by SILD method. Sens. Actuators B 2012, 166, 402–410. [Google Scholar] [CrossRef]
- Matsushima, S.; Teraoka, Y.; Miura, N.; Yamazoe, N. Electronic Interaction Between Metal Additives and Tin Dioxide in Tin Dioxide-Based Gas Sensors. Jpn. J. Appl. Phys. 1988, 27, 1798–1802. [Google Scholar] [CrossRef]
- Jow, N. The Role of Precious Metal Catalysts. Solid State Gas Sens. 1987, 124–138. [Google Scholar]
- Yamazoe, N. New Approaches for Improving Semiconductor Gas Sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Gentry, S.J.; Jones, T.A. The Role of Catalysis in Solid-State Gas Sensors. Sens. Actuators 1986, 10, 141–163. [Google Scholar] [CrossRef]
- Kohl, D. Surface Processes in the Detection of Reducing Gases with SnO2-Based Devices. Sens. Actuators 1989, 18, 71–113. [Google Scholar] [CrossRef]
- Tsu, K.; Boudart, M. Recombination of atoms at the surface of thermocouple probes. Can. J. Chem. 1961, 39, 1239–1246. [Google Scholar] [CrossRef]
- Yuasa, M.; Kida, T.; Shimanoe, K. Preparation of a Stable Sol Suspension of Pd-Loaded SnO2 Nanocrystals by a Photochemical Deposition Method for Highly Sensitive Semiconductor Gas Sensors. ACS Appl. Mater. Interfaces 2012, 4, 4231–4236. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, A.A.; Malyshev, V.V. Sensors for the ultra-fast monitoring of explosive gas concentrations. Sens. Actuators B 2013, 189, 260–267. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Hanaor, D.A.H.; Ghadiri, M.; Chrzanowski, W.; Gan, Y.X. Scalable Surface Area Characterization by Electrokinetic Analysis of Complex Anion Adsorption. Langmuir 2014, 30, 15143–15152. [Google Scholar] [CrossRef]
- Williams, D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuators B 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Kulkarni, G.U.; Vinod, C.P.; Rao, C.N.R. Nanoscale catalysis by gold. Surf. Chem. Catal. 2002, 191–206. [Google Scholar]





| S.N. | Materials | Temp. (°C) | Response (Concentration) | tres (s) | trecov (s) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Co3O4/FGH composites | 250 | 4.06 (1 ppm) | 20 | --- | [34] |
| 2 | Hierarchical flower-like Co3O4 nanostructures | 130 | 48.1 (100 ppm) | 18 | 13 | [35] |
| 3 | Porous NiFe2O4 microspheres | 250 | 27.4 (100 ppm) | 2 | 406 | [36] |
| 4 | Rh-doped SnO2 nano-fibers | 200 | 60.6 (50 ppm) | 2 | 64 | [37] |
| 5 | SnO2/Au-doped In2O3 core-shell nano-fibers | 280 | 14 (100 ppm) | 2 | 9 | [38] |
| 6 | Pt-PS_SnO2 NTs | 350 | 34.8 (1 ppm) | --- | --- | [39] |
| 7 | PdAu/SnO2 3D nanosheets | 250 | 6.5 (2 ppm) | 5 | 4 | [40] |
| 8 | Pt-loaded Fe2O3 nanocubes | 139 | 25.7 (100 ppm) and 1.1 (1 ppm) | 3 | 22 | [41] |
| 9 | Ni1Sn3 | 300 | 2.44 (0.5 ppm) | --- | --- | [42] |
| 10 | Pt-doped 3D porous SnO2 | 153 | 505.7 (100 ppm) 2.1 (0.05 ppm) | 130 440 | 140 370 | In this work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, W.; Hu, X.; Min, X.; Qiu, J.; Tian, R.; Ji, P.; Qin, W.; Wang, H.; Pan, T.; Cheng, S.; et al. A Highly Sensitive and Selective ppb-Level Acetone Sensor Based on a Pt-Doped 3D Porous SnO2 Hierarchical Structure. Sensors 2020, 20, 1150. https://doi.org/10.3390/s20041150
Quan W, Hu X, Min X, Qiu J, Tian R, Ji P, Qin W, Wang H, Pan T, Cheng S, et al. A Highly Sensitive and Selective ppb-Level Acetone Sensor Based on a Pt-Doped 3D Porous SnO2 Hierarchical Structure. Sensors. 2020; 20(4):1150. https://doi.org/10.3390/s20041150
Chicago/Turabian StyleQuan, Wenjing, Xuefeng Hu, Xinjie Min, Junwen Qiu, Renbing Tian, Peng Ji, Weiwei Qin, Haixin Wang, Ting Pan, Suishi Cheng, and et al. 2020. "A Highly Sensitive and Selective ppb-Level Acetone Sensor Based on a Pt-Doped 3D Porous SnO2 Hierarchical Structure" Sensors 20, no. 4: 1150. https://doi.org/10.3390/s20041150





