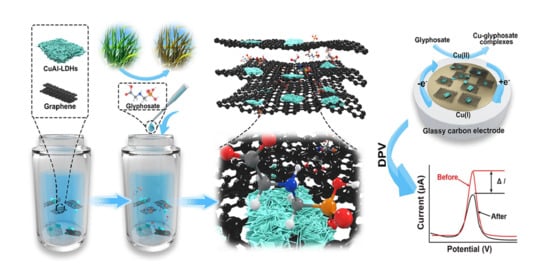
Performance of CuAl-LDH/Gr Nanocomposite-Based Electrochemical Sensor with Regard to Trace Glyphosate Detection in Water
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Apparatus
2.2. Synthesis of CuAl-LDH Composites
2.3. Fabrication of CuAl-LDH/Gr-Modified GCE
2.4. Electrochemical Behavior Tests
3. Results and Discussion
3.1. Characterization of CuAl-LDH/Gr Nanocomposites
3.2. Electrochemical Behaviors on CV
3.3. Electrochemical Impedance Spectroscopy Tests
3.4. Glyphosate Sensing Mechanism
3.5. Optimization Experiments
3.5.1. Buffer Electrolyte Solution and pH
3.5.2. The CuAl-LDH/Gr Ratio and the Volume
3.5.3. The Deposition Voltage and the Accumulation Time
3.6. Differential Pulse Voltammetry Detection of Glyphosate
3.7. Repeatability, Stability and Anti-Interference
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, M.; Li, H.; Jaisi, D.P. Degradation of glyphosate and bioavailability of phosphorus derived from glyphosate in a soil-water system. Water Res. 2019, 163, 114840–114850. [Google Scholar] [PubMed]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Caroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health A Glob. Access Sci. Source 2016, 15, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, V.; Montanarella, L.; Jones, A.; Fernandez-Ugalde, O.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Letter to the editor regarding: Uncontrolled GMOs and their associated pesticides make the conclusions unreliable. Food. Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Noori, J.S.; Dimaki, M.; Mortensen, J.; Svendsen, W.E. Detection of ghyphosate in drinking water: A fast and direct detection method without sample pretreatement. Sensors 2018, 9, 2961. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Smith, S.; Smith, G.; Wang, W.; Li, Y. Glyphosate contamination in grains and food. An overview. Food Control 2019, 106, 106710–106717. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616, 255–268. [Google Scholar] [CrossRef]
- Nedelkoska, T.V.; Low, G.K.C. High-performance liquid chromatographic determination of glyphosate in water and plant material after pre-column derivatisation with 9-fluorenylmethyl chloroformate. Anal. Chim. Acta 2004, 511, 145–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Qu, Q.; Wang, G.; Wang, C. Determination of glyphosate and aminomethylphosphonic acid in soybean samples by high performance liquid chromatography using a novel fluorescent labeling reagent. Anal. Method. 2013, 5, 6465–6472. [Google Scholar] [CrossRef] [Green Version]
- Moreno-González, D.; Rodríguez-Ramírez, R.; del Olmo-Iruela, M.; García-Campaña, A.M. Validation of a new method based on salting-out assisted liquid-liquid extraction and UHPLC-MS/MS for the determination of betalactam antibiotics in infant dairy products. Talanta 2017, 167, 493–498. [Google Scholar] [CrossRef]
- De Góes, R.E.; Muller, M.; Fabris, J.L. Spectroscopic detection of glyphosate in water assisted by laser-ablated silver nanoparticles. Sensors 2017, 17, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Majdinasab, M.; Hayat, A. A perspective on non-enzymatic electrochemical nanosensors for direct detection of pesticides. Curr. Opin. Electrochem. 2018, 11, 12–18. [Google Scholar] [CrossRef]
- Noori, J.S.; Mortensen, J.; Geto, A. Recent development on the electrochemical detection of selected pesticides: A focused review. Sensors 2020, 20, 2221. [Google Scholar] [CrossRef] [Green Version]
- Pintado, S.; Montoya, M.R.; Rodríguez-Amaro, R.; Mayén, M.; Mellado, J.M.R. Electrochemical determination of glyphosate in waters using electrogenerated copper ions. Int. J. Electrochem. Sci. 2012, 7, 2523–2530. [Google Scholar]
- Vaghela, C.; Kulkarni, M.; Haram, S.; Aiyer, R.; Karve, M. A novel inhibition based biosensor using urease nanoconjugate entrapped biocomposite membrane for potentiometric glyphosate detection. Int. J. Biol. Macromol. 2018, 108, 32–40. [Google Scholar] [CrossRef]
- Cahuantzi-Muñoz, S.L.; González-Fuentes, M.A.; Ortiz-Frade, L.A.; Torres, E.; Trejo, G.; Méndez-Albores, A. Electrochemical biosensor for sensitive quantification of glyphosate in maize kernels. Electroanalysis 2019, 31, 927–935.15. [Google Scholar] [CrossRef] [Green Version]
- Sok, V.; Fragoso, A. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Microchim. Acta 2019, 186, 569–576. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Yin, R.; Jensen, J.L. Molecularly imprinted polymer sensors for pesticide and insecticide detection in water. Analyst 2001, 126, 798–802. [Google Scholar] [CrossRef]
- Do, M.H.; Florea, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Tran-Thi, N.T.; Jaffrezic-Renault, N. Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int. J. Environ. Anal. Chem. 2015, 95, 1489–1501. [Google Scholar] [CrossRef]
- Khenifi, A.; Derriche, Z.; Forano, C.; Prevot, V.; Moustry, C.; Scavetta, E.; Ballarin, B.; Guadagnini, L.; Tonelli, D. Tonelli. Glyphosate and glufosinate detection at electrogenerated NiAl-LDH thin films. Anal. Chim. Acta 2009, 654, 97–102. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Zhang, Q.; Xu, G.; Dai, H.; Lin, Y. Binding-induced internal-displacement of signal-on photoelectrochemical response: A glyphosate detection platform based on graphitic carbon nitride. Sens. Actuators B Chem. 2016, 224, 798–804. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, C.; Zeng, G.M.; Tan, X.F.; Wang, H.; Huang, D.L.; Yang, K.H.; Wei, J.J.; Ma, C.; Nie, K. Boosting CO2 electrolysis performance via calcium-oxide-looping combined with in situ exsolved Ni–Fe nanoparticles in a symmetrical solid oxide electrolysis cell. J. Mater. Chem. A 2020, 8, 4141–4193. [Google Scholar] [CrossRef]
- Saha, S.; Ray, S.; Acharya, R.; Chatterjee, T.K.; Chakraborty, J. Magnesium, zinc and calcium aluminium layered double hydroxide-drug nanohybrids: A comprehensive study. J. Appl. Clay Sci. 2017, 135, 493–509. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.; Gao, J.; Wu, F.; Zhang, X. High performance NiFe layered double hydroxide for methyl orange dye and Cr (VI) adsorption. Chemosphere 2016, 152, 415–422. [Google Scholar] [CrossRef]
- Wimalasiri, Y.; Fan, R.; Zhao, X.S.; Zou, L. Assembly of Ni-Al layered double hydroxide and graphene electrodes for supercapacitors. Electrochim. Acta 2014, 134, 127–135. [Google Scholar] [CrossRef]
- Xu, R.X.; Yu, X.Y.; Gao, C.; Liu, J.H.; Compton, R.G.; Huang, X.J. Enhancing selectivity in stripping voltammetry by different adsorption behaviors: The use of nanostructured Mg–Al-layered double hydroxides to detect Cd (II). Analyst 2013, 138, 1812–1818. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Ternary layered double hydroxides (LDH) based on Cu-substituted ZnAl for the design of efficient antibacterial ceramics. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Zhou, J.; Min, M.; Liu, Y.; Tang, J.; Tang, W. Layered assembly of NiMn-layered double hydroxide on graphene oxide for enhanced non-enzymatic sugars and hydrogen peroxide detection. Sens. Actuators B Chem. 2018, 260, 408–417. [Google Scholar] [CrossRef]
- Kang, G.; Zhu, Z.; Tang, B.H.; Wu, C.H.; Wu, R.J. Rapid detection of ozone in the parts per billion range using a novel Ni–Al layered double hydroxide. Sens. Actuators B Chem. 2017, 241, 1203–1209. [Google Scholar] [CrossRef]
- Zhan, T.; Wang, X.; Li, X.; Song, Y.; Hou, W. Hemoglobin immobilized in exfoliated Co2Al LDH-graphene nanocomposite film: Direct electrochemistry and electrocatalysis toward trichloroacetic acid. Sens. Actuators B Chem. 2016, 228, 101–108. [Google Scholar] [CrossRef]
- Hansen, L.R.; Roslev, P. Developmental and lethal effects of glyphosate and a glyphosate-based product on Xenopus laevis embryos and tadpoles. Aquat. Toxicol. 2016, 179, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Liang, W.; Chen, L.; Li, Y.; He, X. Non-enzymatic glucose sensor with high sensitivity based on Cu-Al layered double hydroxides. Sens. Actuators B Chem. 2018, 273, 41–47. [Google Scholar] [CrossRef]
- Liang, H.; Miao, X.; Gong, J. One-step fabrication of layered double hydroxides/graphene hybrid as solid-phase extraction for stripping voltammetric detection of methyl parathion. Electrochem. Commun. 2012, 20, 149–152. [Google Scholar] [CrossRef]
- Shabnam, L.; Faisal, S.N.; Roy, A.K.; Haque, E.; Minett, A.I.; Gomes, V.G. Doped graphene/Cu nanocomposite: A high sensitivity non-enzymatic glucose sensor for food. Food Chem. 2017, 221, 751–759. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, B.; Cai, Y.; Cao, Q.; Tu, T.; Huang, B.; Fang, L.; Ye, X. Layer-by-layer chitosan-decorated pristine graphene on screen-printed electrodes by one-step electrodeposition for non-enzymatic hydrogen peroxide sensor. Talanta 2018, 190, 70–77. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Xu, J.; Zhao, Z.; Tian, G. Synthesis of CuZrO3 nanocomposites/graphene and their application in modified electrodes for the co-detection of trace Pb(II) and Cd(II). Sens. Actuators B Chem. 2018, 273, 1146–1155. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. FT-Raman and FT-IR spectroscopic study of synthetic Mg/Zn/Al-hydrotalcites. J. Raman Spectrosc. 2004, 35, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wang, L.; Shen, C.; Wang, C.; Hu, X.; Wang, G. An electrochemical sensor on the hierarchically porous Cu-BTC MOF platform for glyphosate determination. Sens. Actuators B Chem. 2019, 283, 487–494. [Google Scholar] [CrossRef]
- Le, W.Z.; Liu, Y.Q. Preparation of nano-copper oxide modified glassy carbon electrode by a novel film plating/potential cycling method and its characterization. Sens. Actuators B Chem. 2009, 141, 147–153. [Google Scholar] [CrossRef]
- Mouanga, M.; Puiggali, M.; Devos, O. EIS and LEIS investigation of aging low carbon steel with Zn–Ni coating. Electrochim. Acta 2013, 106, 82–90. [Google Scholar] [CrossRef]
- Zhou, C.; Tao, M.; Liu, J.; Liu, T.; Lu, X.; Xin, Z. ACS Effects of Interfacial Interaction on Corrosion Resistance of Polybenzoxazine/SiO2 Nanocomposite Coatings. Appl. Polym. Mater. 2019, 1, 381–391. [Google Scholar] [CrossRef]
- Huo, D.; Li, Q.; Zhang, Y.; Hou, C.; Lei, Y. A novel acetylcholinesterase biosensor based on ionic liquids-AuNPs-porous carbon composite matrix for detection of organophosphate pesticides. Sens. Actuators B Chem. 2014, 199, 410–417. [Google Scholar] [CrossRef]
- Sheals, J.; Persson, P.; Hedman, B. IR and EXAFS spectroscopic studies of glyphosate protonation and copper (II) complexes of glyphosate in aqueous solution. Inorg. Chem. 2001, 40, 4302–4309. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, L.; Huang, D.; Xian, Y.; Jin, L. A Nafion-coated bismuth film electrode for the determination of heavy metals in vegetable using differential pulse anodic stripping voltammetry: An alternative to mercury-based electrodes. Food Chem. 2008, 109, 834–839. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Yang, Z.; Xu, Q.; Hou, C.; Peng, J.; Hu, X. Simultaneous determination of ultratrace lead and cadmium by square wave stripping voltammetry with in situ depositing bismuth at Nafion-medical stone doped disposable electrode. J. Hazard Mater. 2011, 191, 26–31. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Yang, Q.; Dang, Z.; Zhang, L. Highly efficient removal of thallium in wastewater by MnFe2O4-biochar composite. ACS Appl. Mater. Interfaces 2019, 11, 15478–15488. [Google Scholar] [CrossRef]
- De Almeida, L.K.S.; Chigome, S.; Torto, N.; Frost, C.L.; Pletschke, B.I. A novel colorimetric sensor strip for the detection of glyphosate in water. Sens. Actuators B Chem. 2015, 206, 357–363. [Google Scholar] [CrossRef]
- Prasad, B.B.; Jauhari, D.; Tiwari, M.P. Doubly imprinted polymer nanofilm-modified electrochemical sensor for ultra-trace simultaneous analysis of glyphosate and glufosinate. Biosens. Bioelectron. 2014, 59, 81–88. [Google Scholar] [CrossRef]
- Lee, H.O.; Jung, D.U.; Lee, J.H.; Song, Y.S.; Park, C.; Kim, S.W. Detection of glyphosate by quantitative analysis of fluorescence and single DNA using DNA-labeled fluorescent magnetic core-shell nanoparticles. Sens. Actuators B. Chem. 2013, 177, 879–886. [Google Scholar] [CrossRef]







| Sample | Detected (ppb) | Spike (ppb) | Found (ppb) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 0 | 40 | 41.66 | 104.15 | 4.15 |
| 2 | 0 | 39.78 | 99.45 | ||
| 3 | 0 | 43.23 | 108.08 | ||
| 4 | 0 | 80 | 78.62 | 98.28 | 3.21 |
| 5 | 0 | 79.26 | 99.08 | ||
| 6 | 0 | 81.37 | 104.21 | ||
| 7 | 0 | 120 | 125.30 | 104.42 | 4.28 |
| 8 | 0 | 117.17 | 97.64 | ||
| 9 | 0 | 127.04 | 105.87 |
| Method | Materials | LOD (mol L−1) | Linear Range (mol L−1) | Ref |
|---|---|---|---|---|
| Fluorescence | Co-B/SiO2/dye NPs | 2.5 × 10−10 | 1.0 × 10−9–1.0 × 10−2 | [51] |
| Colorimetric | PVNA 1 | 5.9 × 10−7 | 5.9 × 10−6–2.9 × 10−3 | [49] |
| Potential biosensor | A-G@urease/AuNPs 2 | 5 × 10−7 | 5 × 10−7–5 × 10−5 | [16] |
| Amperometry biosensor | CNO/Tyr 3 | 6.5 × 10−9 | 1.5 × 10−9–1.0 × 10−5 | [18] |
| Electrochemical voltammetry | AuNPs/MIP 4 | 2.1 × 10−9 | 2.4 × 10−8–1.0 × 10−5 | [50] |
| Electrochemical voltammetry | NiAl-LDH 5 | 1.0 × 10−6 | 1.0 × 10−5–9.0 × 10−2 | [21] |
| Electrochemical voltammetry | CuAl-LDH/Gr NCs | 1.0 × 10−9 | 2.96 × 10−9–1.18 × 10−6 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liang, X.; Lu, Y.; Li, H.; Xu, X. Performance of CuAl-LDH/Gr Nanocomposite-Based Electrochemical Sensor with Regard to Trace Glyphosate Detection in Water. Sensors 2020, 20, 4146. https://doi.org/10.3390/s20154146
Zhang C, Liang X, Lu Y, Li H, Xu X. Performance of CuAl-LDH/Gr Nanocomposite-Based Electrochemical Sensor with Regard to Trace Glyphosate Detection in Water. Sensors. 2020; 20(15):4146. https://doi.org/10.3390/s20154146
Chicago/Turabian StyleZhang, Chuxuan, Xinqiang Liang, Yuanyuan Lu, Hua Li, and Xiangyang Xu. 2020. "Performance of CuAl-LDH/Gr Nanocomposite-Based Electrochemical Sensor with Regard to Trace Glyphosate Detection in Water" Sensors 20, no. 15: 4146. https://doi.org/10.3390/s20154146