A Non-Enzymatic Sensor Based on Trimetallic Nanoalloy with Poly (Diallyldimethylammonium Chloride)-Capped Reduced Graphene Oxide for Dynamic Monitoring Hydrogen Peroxide Production by Cancerous Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. One-Pot Synthesis of PDDA-AuPtAg/RGO Nanohybrids
2.3. Preparation of Modified Electrodes
2.4. Real-Time Detection of H2O2 Released From Living Cells
3. Results and Discussion
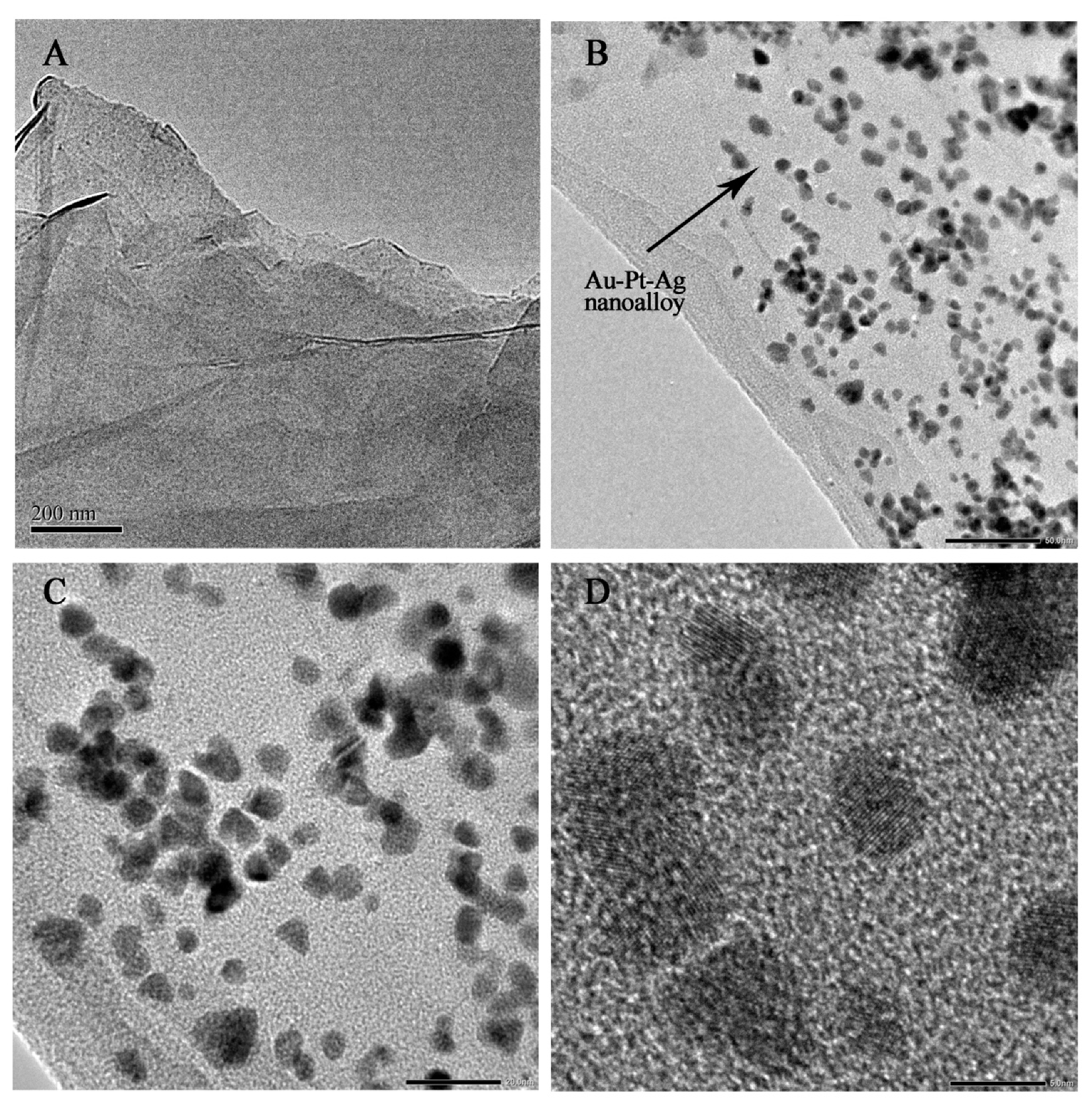
3.1. Characterization of PDDA-AuPtAg/RGO Nanocomposites
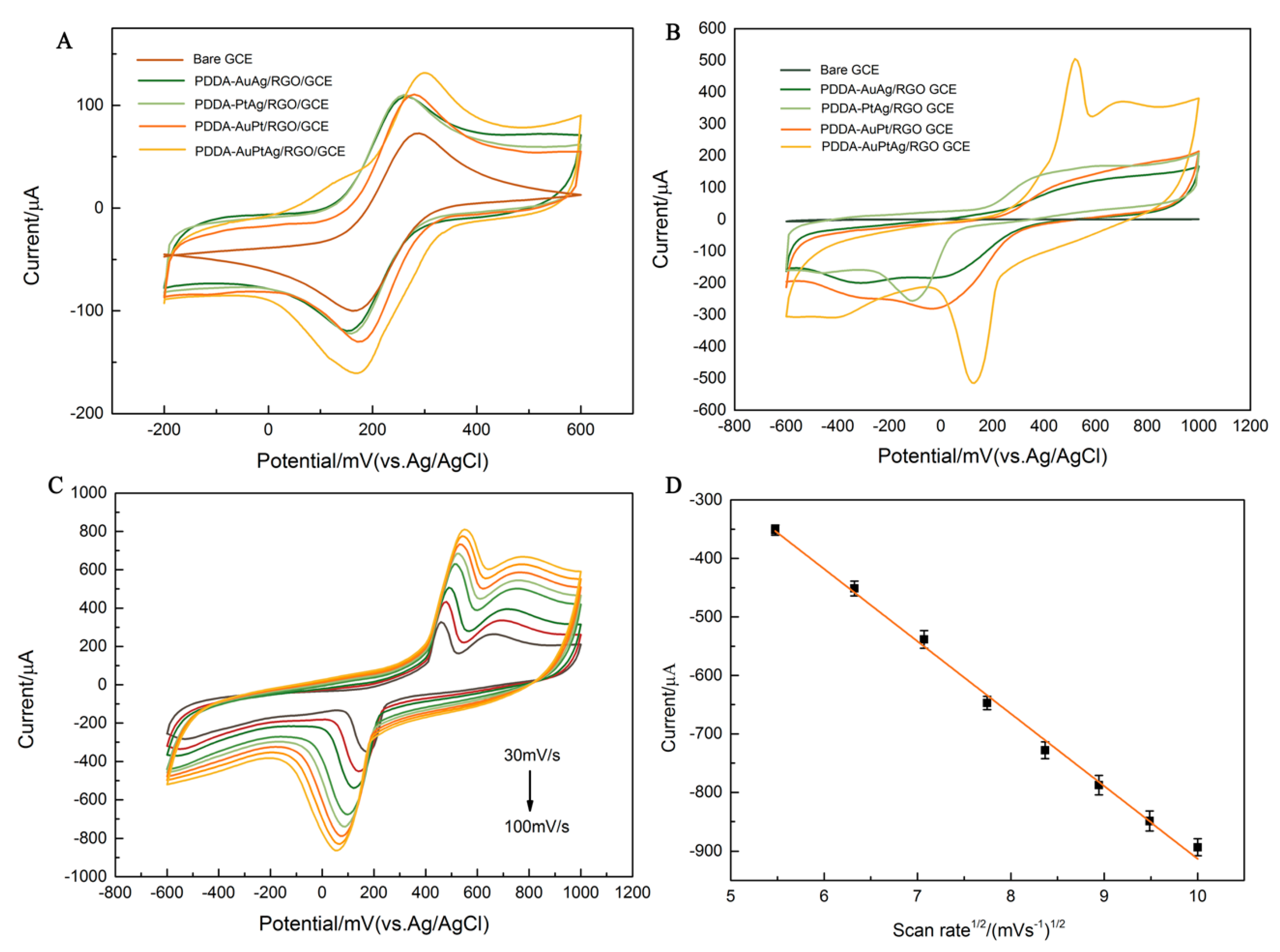
3.2. Electrochemical Behavior of Obtained Materials
3.3. Electrochemical Response to H2O2 by PDDA-AuPtAg/RGO/GCE
3.4. Performance of the Proposed Sensor
3.4.1. Optimization of the Experimental Variables
3.4.2. Amperometric Response towards H2O2
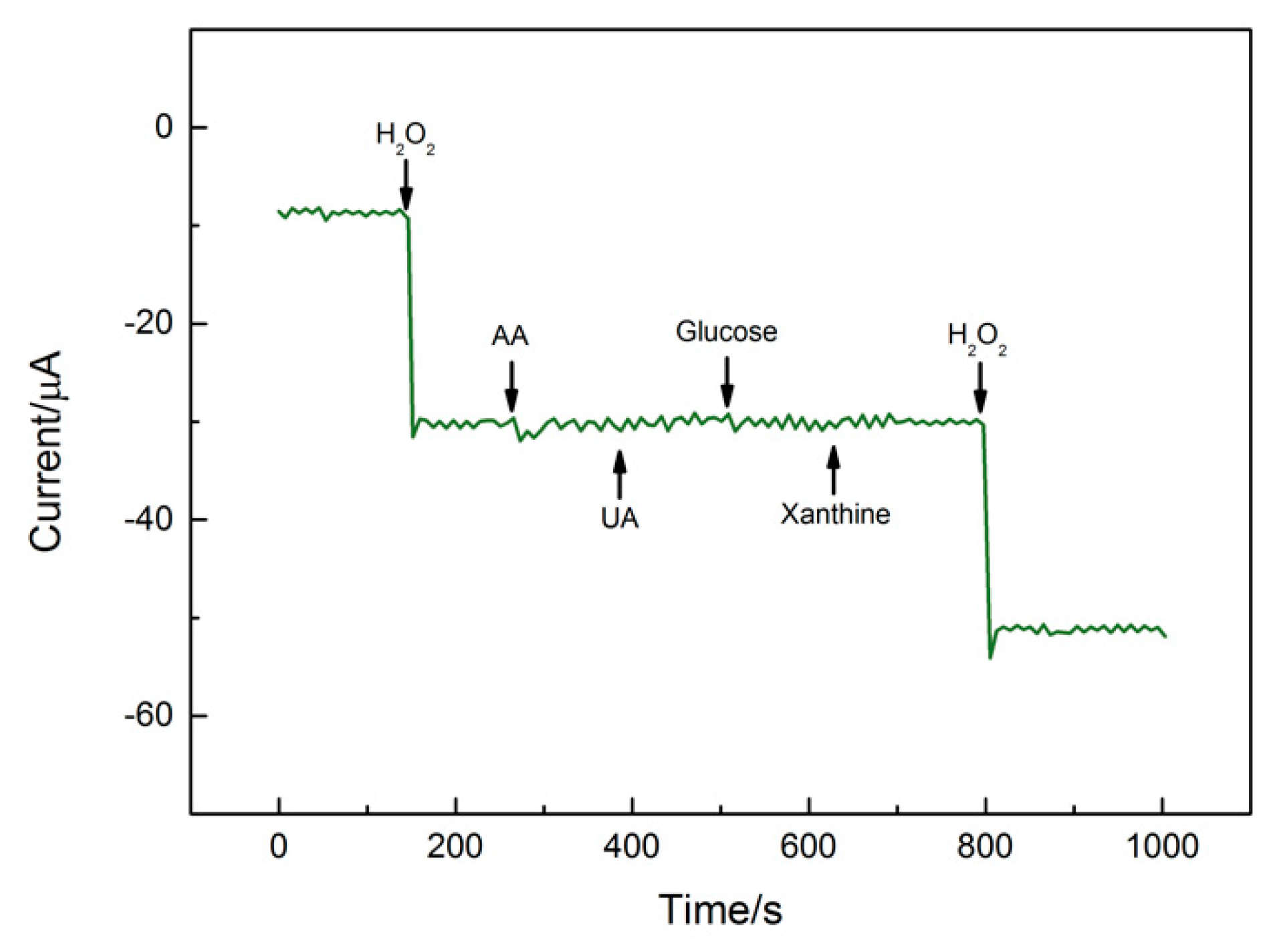
3.4.3. Interference Immunity, Repeatability, and Stability
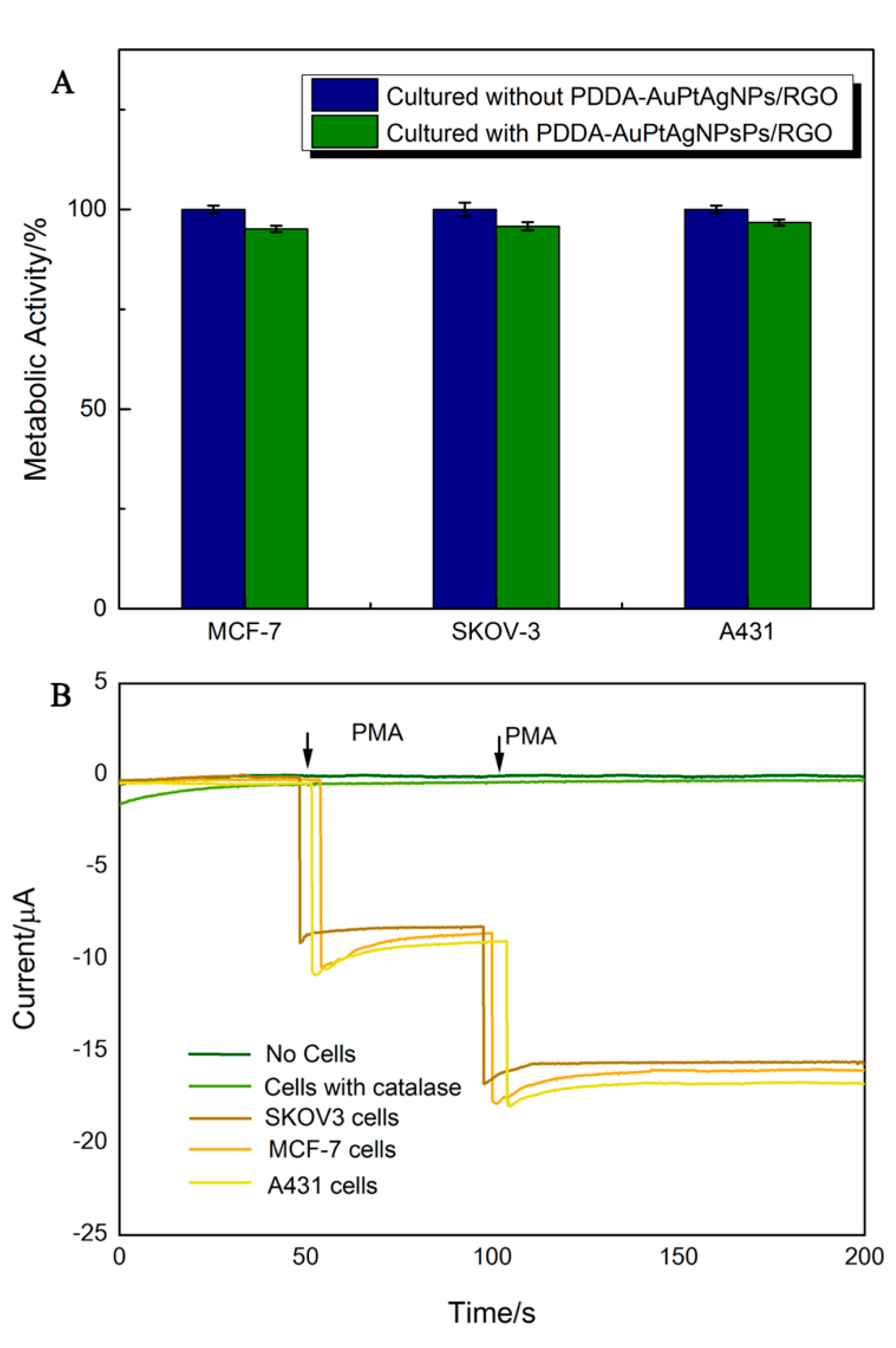
3.5. In Situ Monitoring of H2O2 Released From Living Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Espina, C.; Bauld, L.; Bonanni, B.; Brenner, H.; Brown, K.; Dillner, J.; Forman, D.; Kampman, E.; Nilbert, M. Cancer Prevention Europe. Mol. Oncol. 2019, 13, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Luo, Y.; Sun, Z.; Wang, C.; Zhang, M.; Fu, H.; Qiao, Y.; Su, M. X-ray enabled detection and eradication of circulating tumor cells with nanoparticles. Biosens. Bioelectron. 2012, 38, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Castellucci, P.; Graziani, T.; Farolfi, A.; Fonti, C.; Lodi, F.; Fanti, S. 68 Ga-PSMA-11 PET/CT in recurrent prostate cancer: efficacy in different clinical stages of PSA failure after radical therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Gao, J.; Wu, C.; Zhao, S.; Yu, J.; Zhu, R.; Zhang, Q.; Wu, G.; Xue, X.; Wu, J. CT characteristics and pathologic basis of solitary cystic lung cancer. Radiology 2019, 291, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Tada-Oikawa, S.; Hiraku, Y.; Kojima, M.; Kawanishi, S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005, 76, 1439–1453. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discovery 2013, 12, 931. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Miao, P.; Li, H.; Tu, Y. An Electrochemiluminescent Platform for Living Cell Oxygen Metabolism Monitoring. J. Anal. Test. 2018, 2, 184–189. [Google Scholar] [CrossRef]
- Benhar, M.; Engelberg, D.; Levitzki, A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002, 3, 420–425. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide–production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xu, J.; Zhang, J.; Hu, Y.; Pan, Y.; Miao, P. Facile Strategy for Electrochemical Analysis of Hydrogen Peroxide Based on Multifunctional Fe3O4@Ag Nanocomposites. ACS Appl. Bio Mater. 2018, 1, 367–373. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kishikawa, N.; Ohyama, K.; Ohba, Y.; Kohno, M.; Masuda, T.; Takadate, A.; Nakashima, K.; Kuroda, N. Evaluation of chemiluminescence reagents for selective detection of reactive oxygen species. Anal. Chim. Acta 2010, 665, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, J.; Wang, Q.; Gu, Y.; Wang, P. A reaction based one-and two-photon fluorescent probe for selective imaging H2O2 in living cells and tissues. Sens. Actuators B Chem. 2018, 254, 411–416. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, Q.; Gong, P.; Liu, B.; Wang, J.; Yang, S. A new enzymatic immobilization carrier based on graphene capsule for hydrogen peroxide biosensors. Electrochim. Acta 2015, 151, 186–194. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2014, 87, 230–249. [Google Scholar] [CrossRef]
- Dai, H.; Lü, W.; Zuo, X.; Zhu, Q.; Pan, C.; Niu, X.; Liu, J.; Chen, H.; Chen, X. A novel biosensor based on boronic acid functionalized metal-organic frameworks for the determination of hydrogen peroxide released from living cells. Biosens. Bioelectron. 2017, 95, 131–137. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, M.; Meng, X.; Xiang, J.; Wang, L.; Ren, Q.; Guo, S. Graphene/intermetallic PtPb nanoplates composites for boosting electrochemical detection of H2O2 released from cells. Anal. Chem. 2017, 89, 3761–3767. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Ju, J.; Chen, W. Electrochemical sensor based on graphene-supported tin oxide nanoclusters for nonenzymatic detection of hydrogen peroxide. Electrochim. Acta 2016, 210, 181–189. [Google Scholar] [CrossRef]
- Bai, Z.; Li, G.; Liang, J.; Su, J.; Zhang, Y.; Chen, H.; Huang, Y.; Sui, W.; Zhao, Y. Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2016, 82, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Lee, H.F.; Chen, S.-M.; Thirumalraj, B. An electrochemical facile fabrication of platinum nanoparticle decorated reduced graphene oxide; application for enhanced electrochemical sensing of H2O2. Rsc Adv. 2015, 5, 105567–105573. [Google Scholar] [CrossRef]
- Karuppiah, C.; Palanisamy, S.; Chen, S.-M. An ultrahigh selective and sensitive enzyme-free hydrogen peroxide sensor based on palladium nanoparticles and nafion-modified electrode. Electrocatalysis 2014, 5, 177–185. [Google Scholar] [CrossRef]
- Palanisamy, S.; Chen, S.-M.; Sarawathi, R. A novel nonenzymatic hydrogen peroxide sensor based on reduced graphene oxide/ZnO composite modified electrode. Sens. Actuators B Chem. 2012, 166, 372–377. [Google Scholar] [CrossRef]
- He, W.; Wamer, W.; Xia, Q.; Yin, J.-J.; Fu, P.P. Enzyme-like activity of nanomaterials. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 186–211. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Chen, T.-W.; Palanisamy, S.; Chen, S.-M. Non-enzymatic sensing of hydrogen peroxide using a glassy carbon electrode modified with a composite consisting of chitosan-encapsulated graphite and platinum nanoparticles. Microchim. Acta 2016, 183, 2861–2869. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Jiang, H.; Wang, X. Pt modified carbon fiber microelectrode for electrochemically catalytic reduction of hydrogen peroxide and its application in living cell H2O2 detection. J. Electroanal. Chem. 2016, 781, 233–237. [Google Scholar] [CrossRef]
- Guler, M.; Turkoglu, V.; Bulut, A.; Zahmakiran, M. Electrochemical sensing of hydrogen peroxide using Pd@ Ag bimetallic nanoparticles decorated functionalized reduced graphene oxide. Electrochim. Acta 2018, 263, 118–126. [Google Scholar] [CrossRef]
- Kang, J.-X.; Chen, T.-W.; Zhang, D.-F.; Guo, L. PtNiAu trimetallic nanoalloys enabled by a digestive-assisted process as highly efficient catalyst for hydrogen generation. Nano Energy 2016, 23, 145–152. [Google Scholar] [CrossRef]
- Han, S.H.; Liu, H.M.; Chen, P.; Jiang, J.X.; Chen, Y. Porous trimetallic PtRhCu cubic nanoboxes for ethanol electrooxidation. Adv. Energy Mater. 2018, 8, 1801326. [Google Scholar] [CrossRef]
- Singh, S.; Tuteja, S.K.; Sillu, D.; Deep, A.; Suri, C.R. Gold nanoparticles-reduced graphene oxide based electrochemical immunosensor for the cardiac biomarker myoglobin. Microchim. Acta 2016, 183, 1729–1738. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Tung, T.T.; Losic, D. Carbon nanomaterial based biosensors for non-invasive detection of cancer and disease biomarkers for clinical diagnosis. Sensors 2017, 17, 1919. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.; Losic, D. Recent advances in sensing applications of graphene assemblies and their composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Li, C.; Tang, L.; Zhang, Z.; Yang, M. A novel composite film derived from cysteic acid and PDDA-functionalized graphene: enhanced sensing material for electrochemical determination of metronidazole. Talanta 2013, 104, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, Y.; Liao, H.; Engelhard, M.H.; Yin, G.; Lin, Y. Polyelectrolyte-induced reduction of exfoliated graphite oxide: A facile route to synthesis of soluble graphene nanosheets. ACS Nano 2011, 5, 1785–1791. [Google Scholar] [CrossRef]
- Xiang, Y.; Banks, M.K.; Wu, R.; Xu, W.; Chen, S. Synthesis of thermo-sensitive PDDA-co-PNIPAM/graphene hybrid via electrostatic interactions and its thermal modulated phase transition. Mater. Chem. Phys. 2018, 220, 58–65. [Google Scholar] [CrossRef]
- Hu, C.; Zhai, X.; Liu, L.; Zhao, Y.; Jiang, L.; Qu, L. Spontaneous reduction and assembly of graphene oxide into three-dimensional graphene network on arbitrary conductive substrates. Sci. Rep. 2013, 3, 2065. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Jafari-Asl, M.; Rezaei, B. A new strategy for the synthesis of 3-D Pt nanoparticles on reduced graphene oxide through surface functionalization, Application for methanol oxidation and oxygen reduction. Electrochim. Acta 2014, 130, 397–405. [Google Scholar] [CrossRef]
- Guan, Y.; Dai, M.; Liu, T.; Liu, Y.; Liu, F.; Liang, X.; Suo, H.; Sun, P.; Lu, G. Effect of the dispersants on the performance of fuel cell type CO sensor with Pt–C/Nafion electrodes. Sens. Actuators B Chem. 2016, 230, 61–69. [Google Scholar] [CrossRef]
- Wang, D.; Markus, J.; Kim, Y.-J.; Wang, C.; Pérez, Z.E.J.; Ahn, S.; Aceituno, V.C.; Mathiyalagan, R.; Yang, D.C. Coalescence of functional gold and monodisperse silver nanoparticles mediated by black Panax ginseng Meyer root extract. Int. J. Nanomed. 2016, 11, 6621. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; Patil, M.; Epur, R.; Yun, Y.; Shanov, V.; Schulz, M.; Heineman, W.R.; Datta, M.K.; Kumta, P.N. Gold-coated carbon nanotube electrode arrays: Immunosensors for impedimetric detection of bone biomarkers. Biosens. Bioelectron. 2016, 77, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wan, G.; Wu, L.; Zeng, M.; Lin, S.; Wang, G. Peroxidase-like activity of Au@ TiO2 yolk-shell nanostructure and its application for colorimetric detection of H2O2 and glucose. Sens. Actuators B Chem. 2018, 257, 166–177. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, Y.; Song, Y.; Gu, Y.; Yan, X.; Lu, N.; Liu, H.; Xu, Z.; Xu, H.; Zhang, Z. AuPt/MOF–Graphene: A Synergistic Catalyst with Surprisingly High Peroxidase-Like Activity and Its Application for H2O2 Detection. Anal. Chem. 2019, 91, 10589–10595. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, Z.; Cao, H.; Huang, Y. Peroxidase-like activity of chitosan stabilized silver nanoparticles for visual and colorimetric detection of glucose. Analyst 2012, 137, 5560–5564. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Tuan, N.T.; Jeong, H.; Lee, S.H.; Kim, N.H.; Lee, J.H. Enhanced electrocatalytic performance of an ultrafine AuPt nanoalloy framework embedded in graphene towards epinephrine sensing. Biosens. Bioelectron. 2017, 89, 750–757. [Google Scholar] [CrossRef]
- Khan, M.U.; Qurashi, N.A.; Khan, M.S.; Jabeen, F.; Umar, A.; Yaqoob, J.; Wajid, M. Generation of reactive oxygen species and their impact on the health related parameters: A critical review. Int. J. Biosci. 2016, 9, 303–323. [Google Scholar]
- Griffith, A.W.; Cooper, J.M. Single-cell measurements of human neutrophil activation using electrorotation. Anal. Chem. 1998, 70, 2607–2612. [Google Scholar] [CrossRef]
- Wu, P.; Qian, Y.; Du, P.; Zhang, H.; Cai, C. Facile synthesis of nitrogen-doped graphene for measuring the releasing process of hydrogen peroxide from living cells. J. Mater. Chem. 2012, 22, 6402–6412. [Google Scholar] [CrossRef]
- Dou, B.; Yang, J.; Yuan, R.; Xiang, Y. Trimetallic hybrid nanoflower-decorated MoS2 nanosheet sensor for direct in situ monitoring of H2O2 secreted from live cancer cells. Anal. Chem. 2018, 90, 5945–5950. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, J.; Pan, M.; Liu, X.; Li, B.; Liu, J.; Chen, Q. A Non-Enzymatic Sensor Based on Trimetallic Nanoalloy with Poly (Diallyldimethylammonium Chloride)-Capped Reduced Graphene Oxide for Dynamic Monitoring Hydrogen Peroxide Production by Cancerous Cells. Sensors 2020, 20, 71. https://doi.org/10.3390/s20010071
Jiao J, Pan M, Liu X, Li B, Liu J, Chen Q. A Non-Enzymatic Sensor Based on Trimetallic Nanoalloy with Poly (Diallyldimethylammonium Chloride)-Capped Reduced Graphene Oxide for Dynamic Monitoring Hydrogen Peroxide Production by Cancerous Cells. Sensors. 2020; 20(1):71. https://doi.org/10.3390/s20010071
Chicago/Turabian StyleJiao, Jun, Meixin Pan, Xinran Liu, Binshuai Li, Jian Liu, and Qiang Chen. 2020. "A Non-Enzymatic Sensor Based on Trimetallic Nanoalloy with Poly (Diallyldimethylammonium Chloride)-Capped Reduced Graphene Oxide for Dynamic Monitoring Hydrogen Peroxide Production by Cancerous Cells" Sensors 20, no. 1: 71. https://doi.org/10.3390/s20010071
APA StyleJiao, J., Pan, M., Liu, X., Li, B., Liu, J., & Chen, Q. (2020). A Non-Enzymatic Sensor Based on Trimetallic Nanoalloy with Poly (Diallyldimethylammonium Chloride)-Capped Reduced Graphene Oxide for Dynamic Monitoring Hydrogen Peroxide Production by Cancerous Cells. Sensors, 20(1), 71. https://doi.org/10.3390/s20010071




