Improvement of Sensing Performance of Impedancemetric C2H2 Sensor Using SmFeO3 Thin-Films Prepared by a Polymer Precursor Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Analysis of Precursor and Oxide Thin-Film
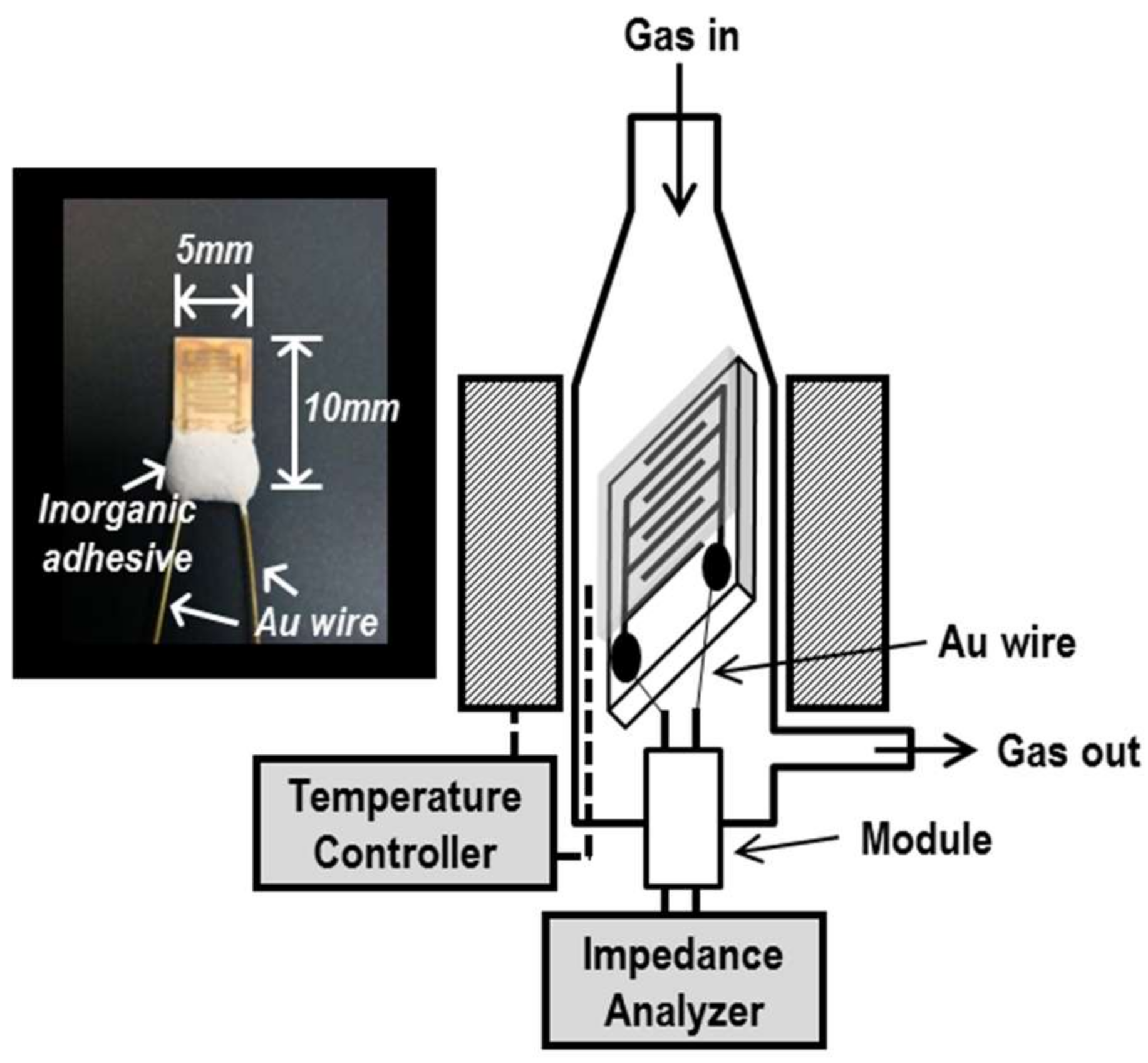
2.2. Measurement of Sensing Properties
3. Results and Discussion
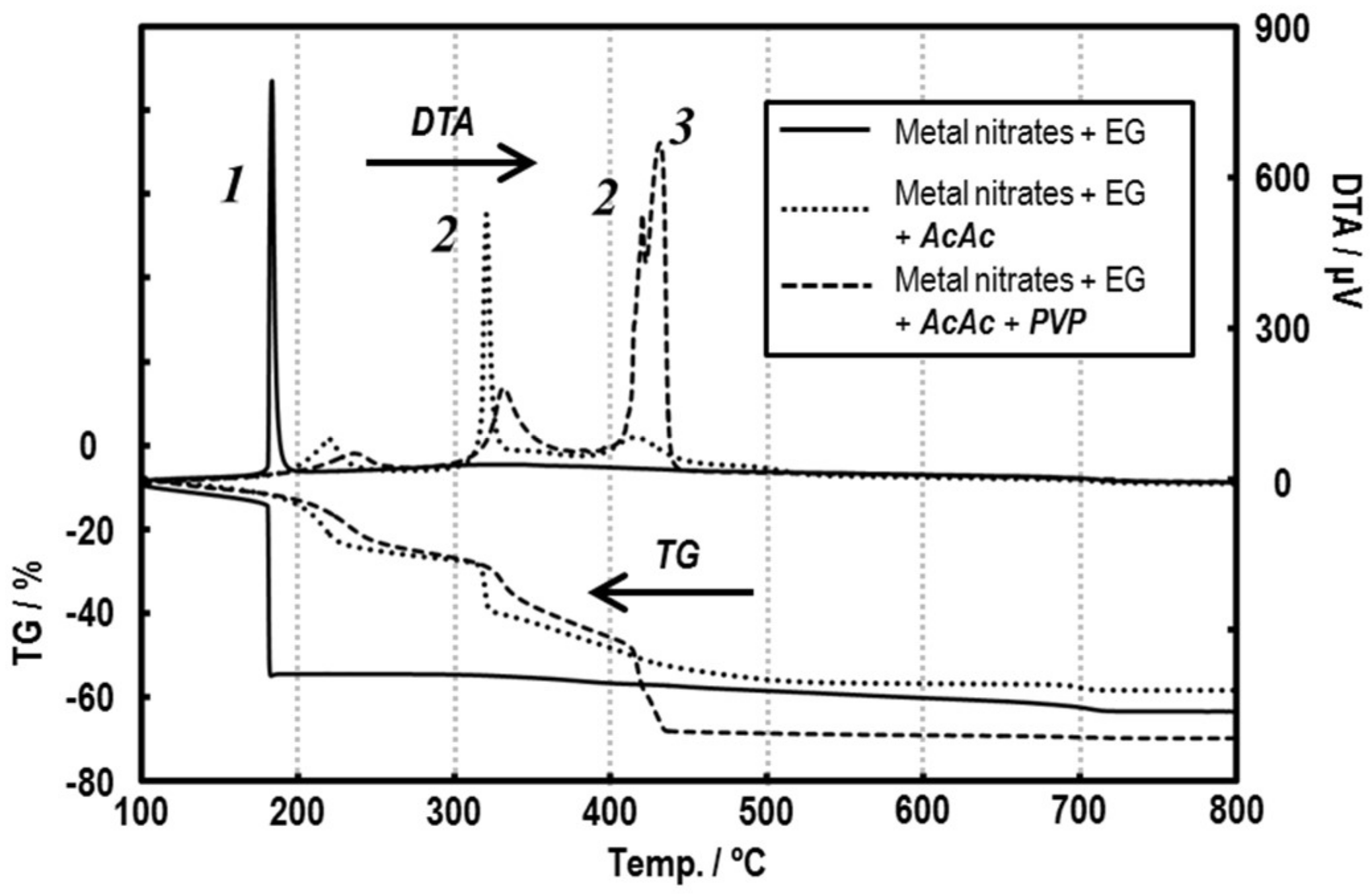
3.1. Preparation of the Precursor Solutions
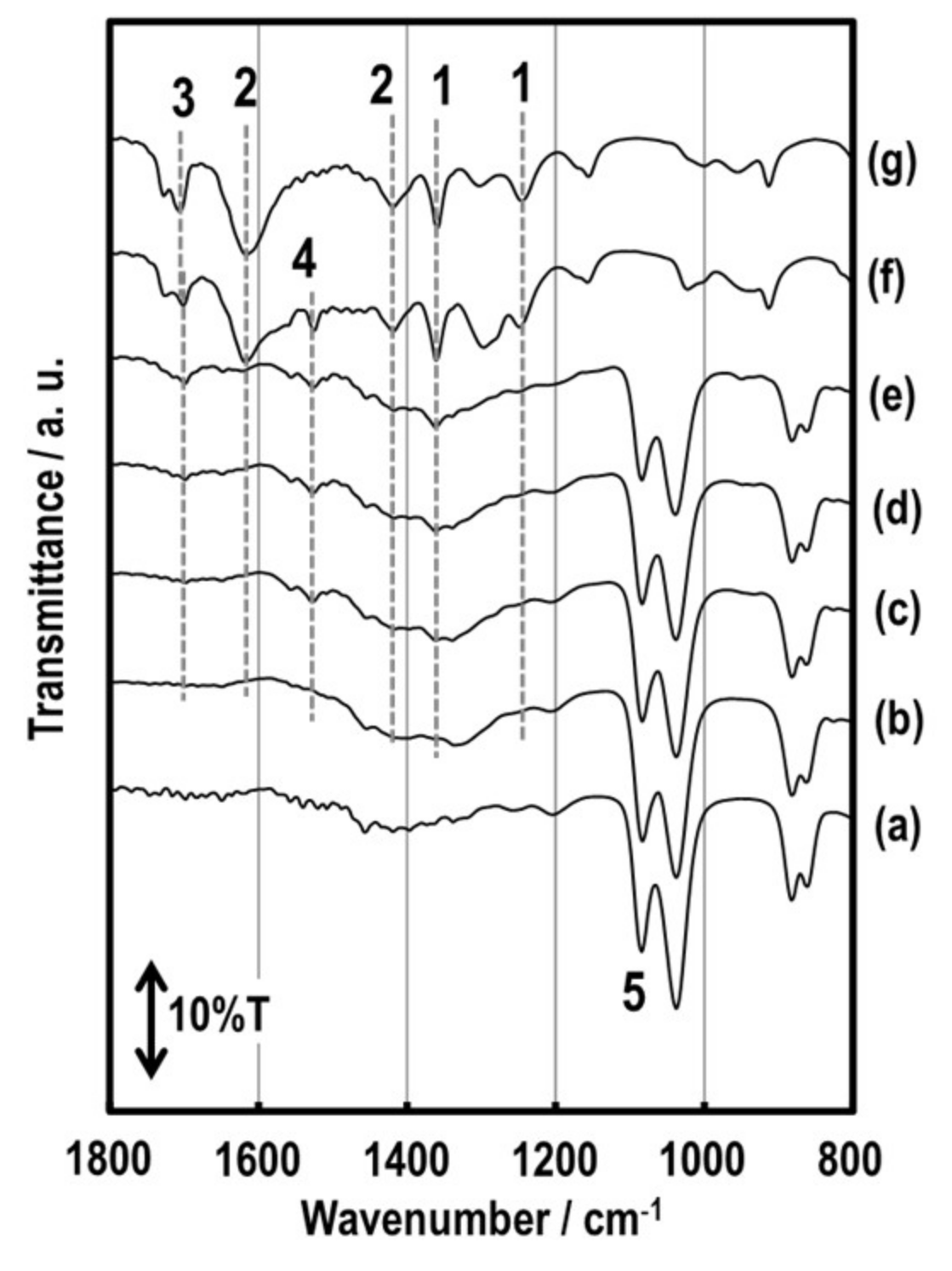
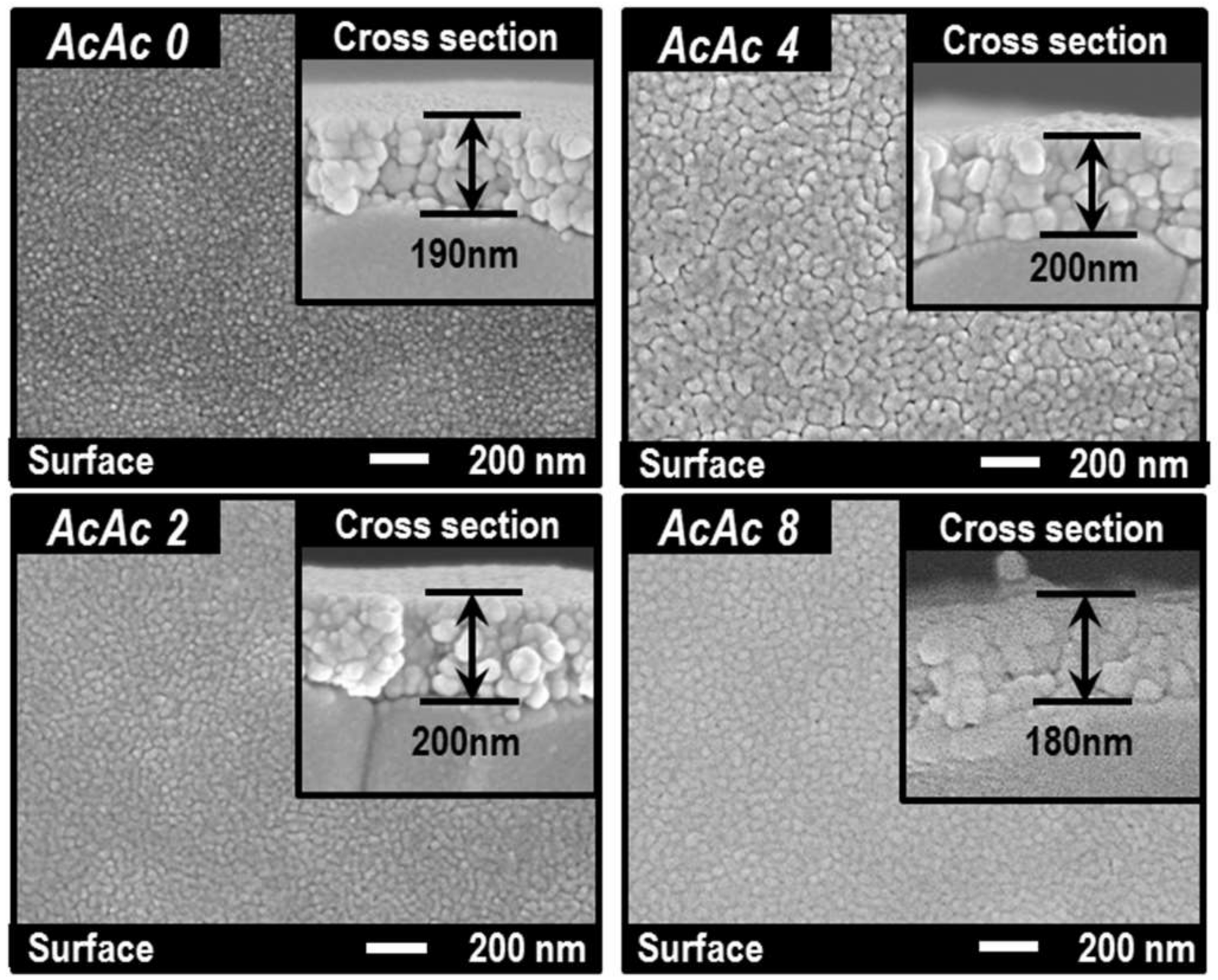
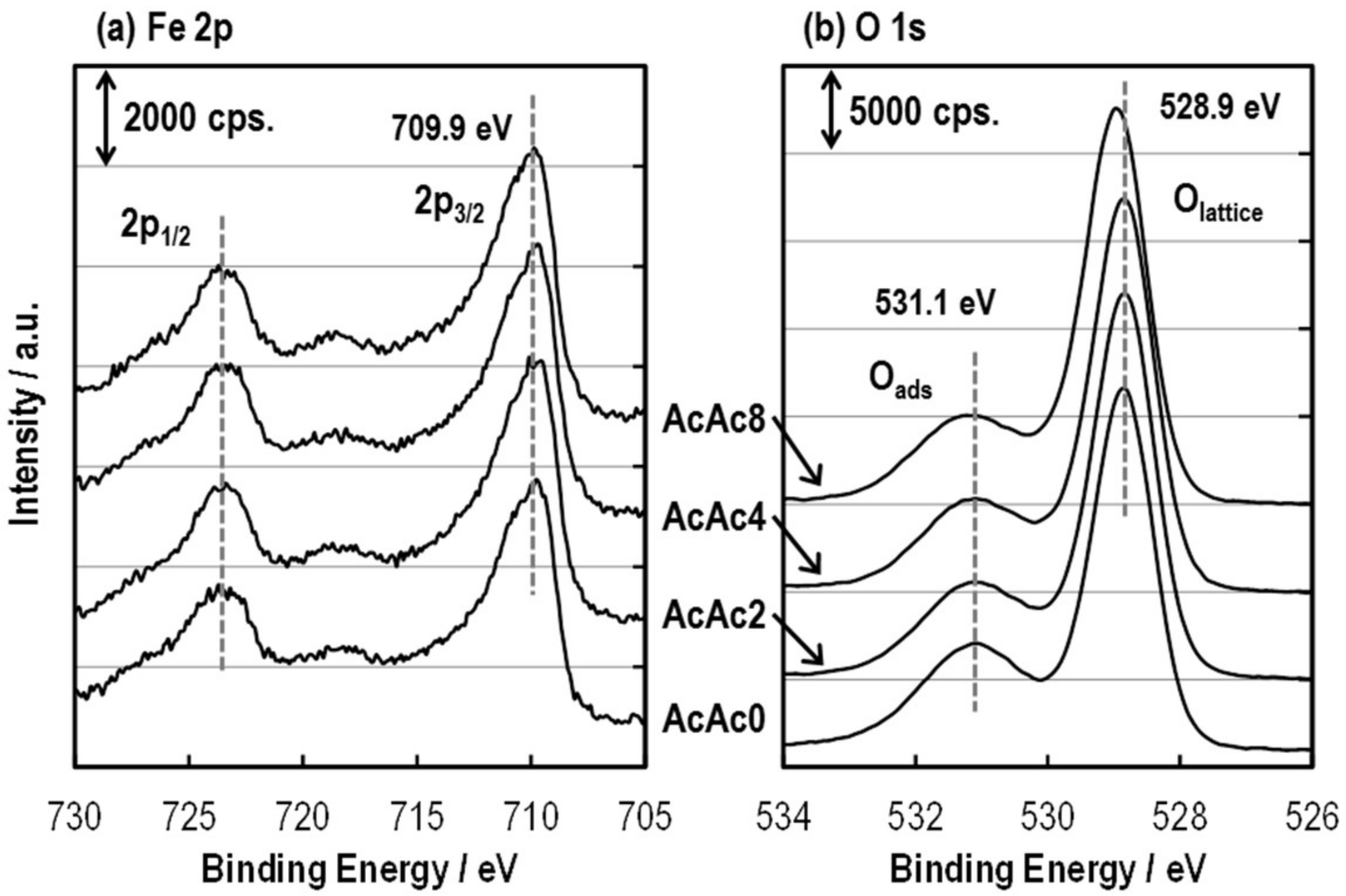
3.2. Structure of the SmFeO3 Thin-Films
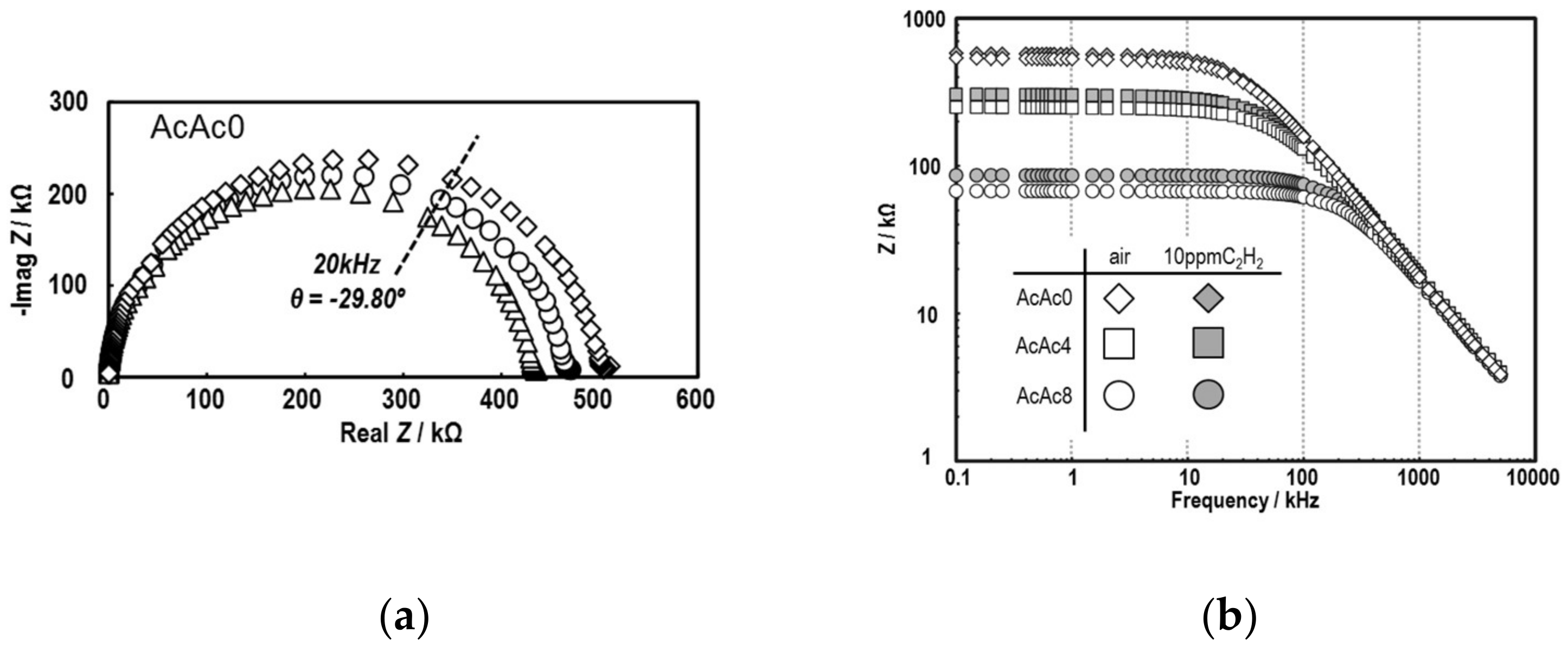
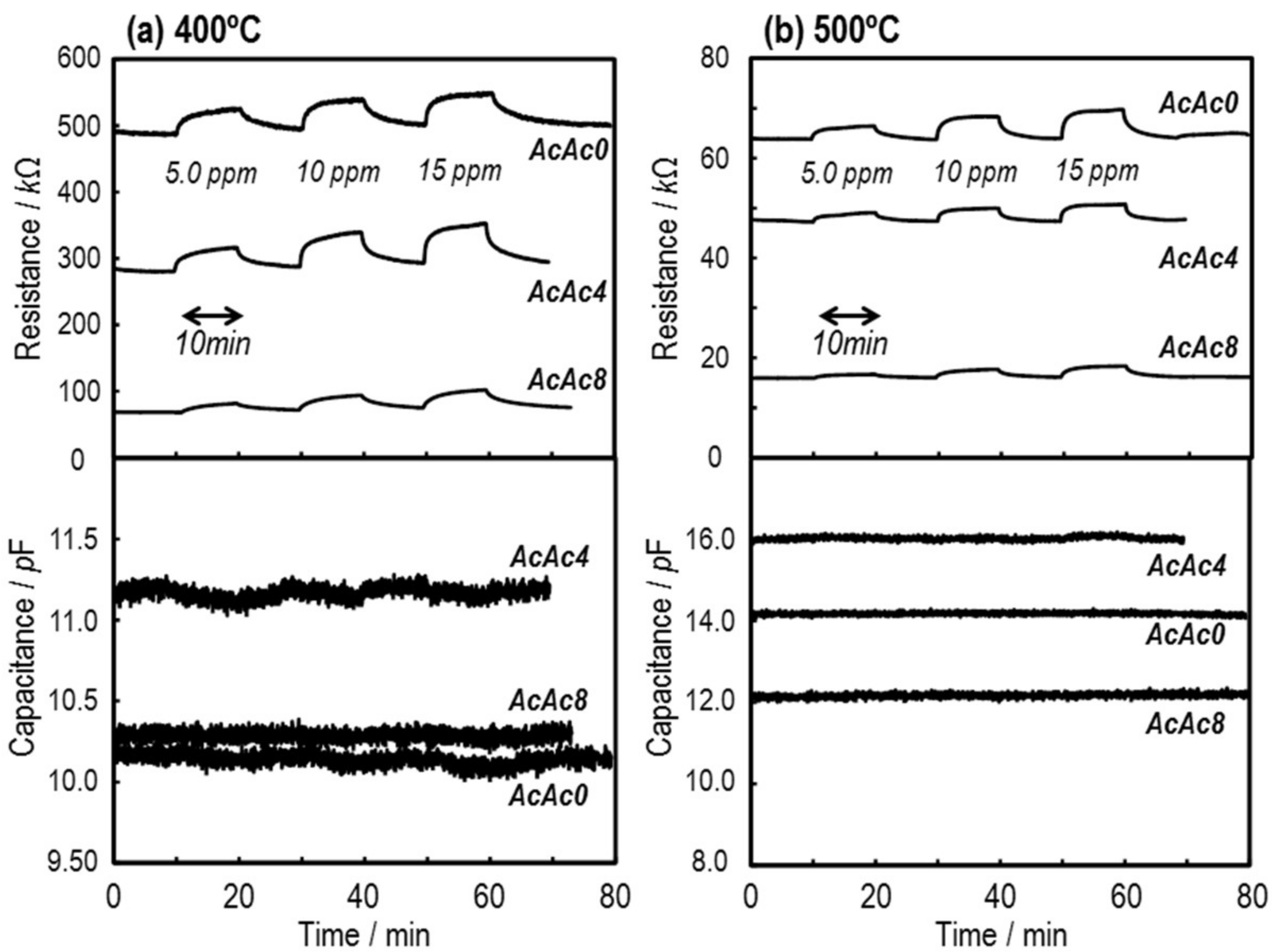
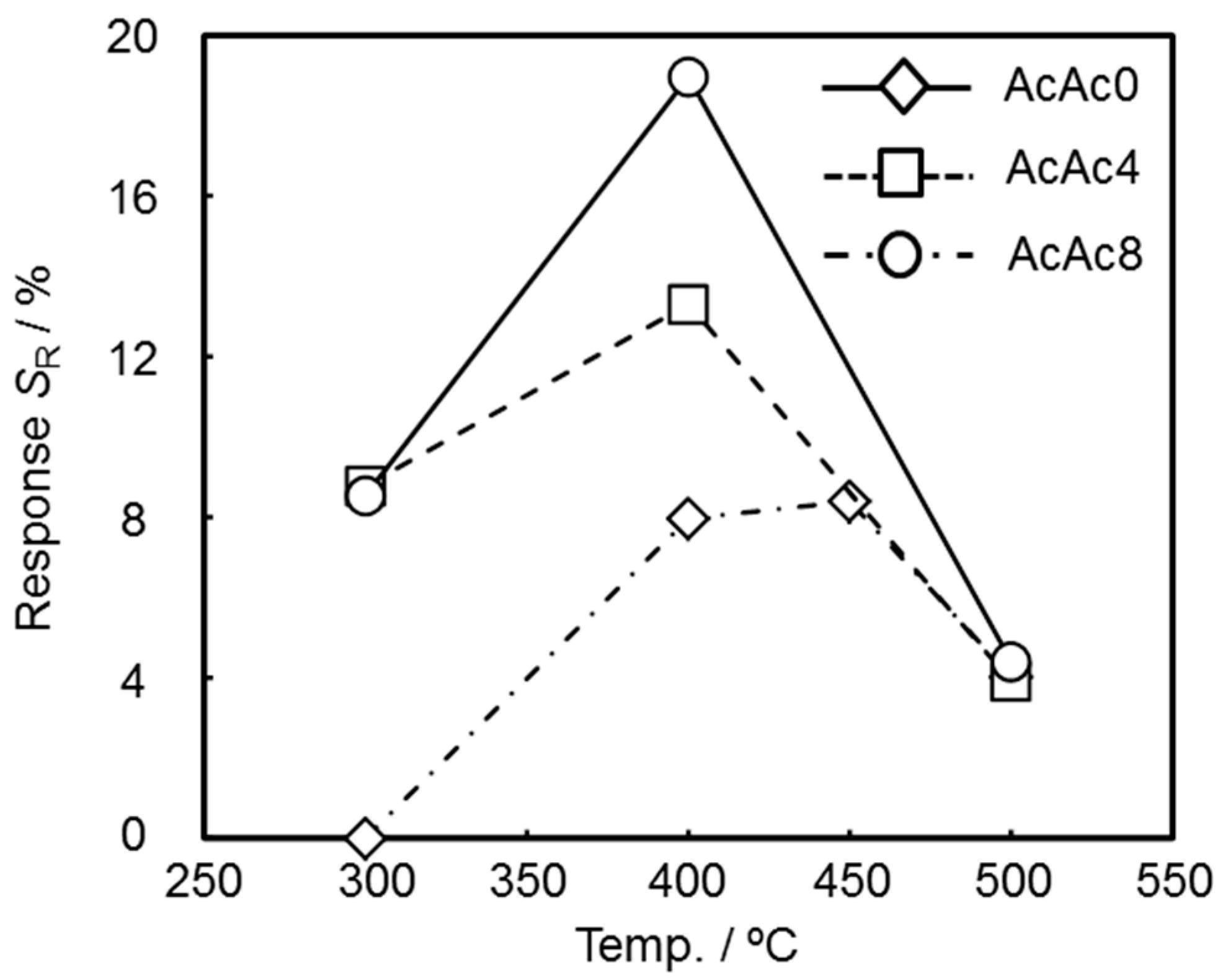
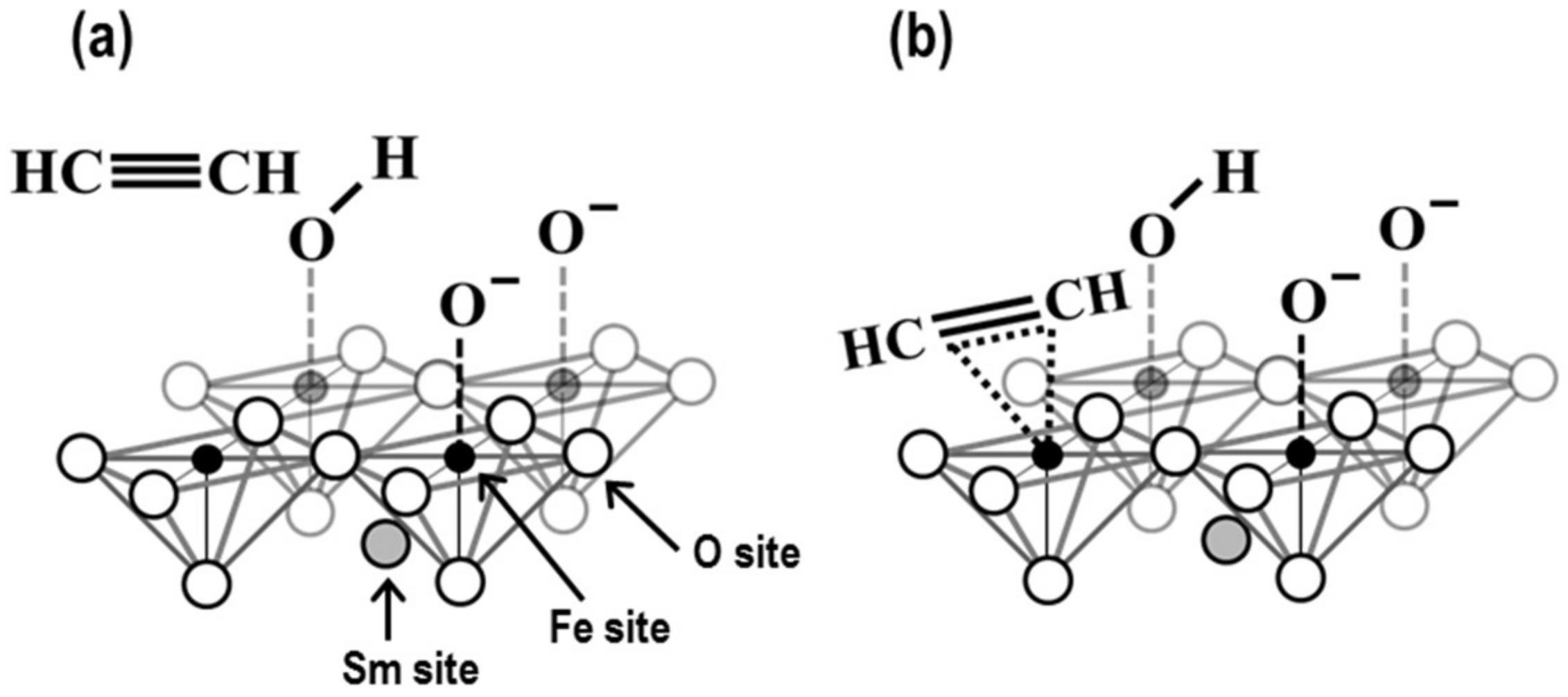
3.3. Sensing Properties of the SmFeO3 Thin-Film Devices
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pimtong-Ngam, Y.; Jiemsirilers; Supothina, S. Preparation of tungsten oxide–tin oxide nanocomposites and their ethylene sensing characteristics. Sens. Actuators A Phys. 2007, 139, 7–11. [Google Scholar] [CrossRef]
- Zevenbergen, M.A.G.; Wouter, D.; Dam, V.-A.T.; Brongersma, S.H.; Crego-Calama, M. Electrochemical Sensing of Ethylene Employing a Thin Ionic-Liquid Layer. Anal. Chem. 2011, 83, 6300–6307. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Su, Y.; Lv, X.; Xia, H.; Wang, Y. Electrochemical acetylene sensor based on Au/MWCNTs. Sens. Actuators B Chem. 2010, 149, 427–431. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Zheng, X.; Fan, H.; Liu, L.; Wang, R.; Zeng, Y. Electrical response of Sm2O3-doped SnO2 to C2H2 and effect of humidity interference. Sens. Actuators B Chem. 2008, 134, 36–42. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Zheng, J.; Li, L.; Zhu, Z. Hydrothermal synthesis of hierarchical nanoparticle-decorated ZnO microdisks and the structure-enhanced acetylene sensing properties at high temperatures. Sens. Actuators B Chem. 2011, 158, 144–150. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tanji, T.; Amezawa, K.; Kawada, T.; Uchimoto, Y.; Furuya, Y.; Sakai, T.; Matsuka, M.; Ishihara, T. Nanoprotonics in perovsikte-type oxides: Reversible changes in color and ion conductivity due to nanoionics phenomenon in platinum-containing perovskite oxide. Solid State Ionics 2011, 182, 13–18. [Google Scholar] [CrossRef]
- Meadowcroft, D.B. Low-cost carbon monoxide electrode material. Nature 1970, 226, 847–848. [Google Scholar] [CrossRef]
- Itagaki, Y.; Mori, M.; Hosoya, Y.; Aono, H.; Sadaoka, Y. O3 and NO2 sensing properties of SmFe1-xCoxO3 perovskite oxides. Sens. Actuators B Chem. 2007, 122, 315–320. [Google Scholar] [CrossRef]
- Tomoda, M.; Okano, S.; Itagaki, Y.; Aono, H.; Sadaoka, Y. Air quality prediction by using semiconducting gas sensor with newly fabricated SmFeO3 film. Sens. Actuators B Chem 2004, 97, 190–197. [Google Scholar] [CrossRef]
- Chaudhari, G.N.; Jagtap, S.V.; Gedam, N.N.; Pawar, M.J.; Sangawar, V.S. Sol-gel synthesized semiconducting LaCo0.8Fe0.2O3-based powder for thick film NH3 gas sensor. Talanta 2009, 78, 1136–1140. [Google Scholar] [CrossRef]
- Chai, Y.L.; Ray, D.T.; Chen, G.J.; Chang, Y.H. Synthesis of La0.8Sr0.2Co0.5Ni0.5 O3-δ thin films for high sensitivity CO sensing material using the Pechini process. J. Alloys Compd. 2002, 333, 147–153. [Google Scholar] [CrossRef]
- Sahner, K.; Moos, R.; Matam, M.; Tunney, J.J.; Post, M. Hydrocarbon sensing with thick and thin film p-type conducting perovskite materials. Sens. Actuators B Chem 2005, 108, 102–112. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Cheng, B.; Qin, H.; Zhao, M.; Yang, C. First-principles study of O2 adsorption on the LaFeO3 (0 1 0) surface. Sens. Actuators B Chem 2009, 139, 520–526. [Google Scholar] [CrossRef]
- Sun, L.; Hu, J.; Zhang, L.; Gao, F.; Zhang, Y.; Qin, H. Adsorption of CO on the O2 pre-adsorbed LaFeO3 (0 1 0) surface: A density functional theory study. Curr. Appl. Phys. 2011, 11, 1278–1281. [Google Scholar] [CrossRef]
- Sun, L.; Hu, J.; Qin, H.; Zhao, M.; Fan, K. Influences of Ca doping and oxygen vacancy upon adsorption of CO on the LaFeO3 (010) surface: A first-principles study. J. Phys. Chem. C 2011, 115, 5593–5598. [Google Scholar] [CrossRef]
- Tasaki, T.; Takase, S.; Shimizu, Y. Fabrication of Sm-Based Perovskite-Type Oxide Thin-Films and Gas Sensing Properties to Acetylene. J. Sens. Technol. 2012, 02, 75–81. [Google Scholar] [CrossRef]
- Tasaki, T.; Takase, S.; Shimizu, Y. Impedancemetric acetylene gas sensing properties of Sm-Fe-based perovskite-type oxide-based thick-film device. Sens. Actuators B Chem 2013, 187, 128–134. [Google Scholar] [CrossRef]
- Karageorgakis, N.I.; Heel, A.; Bieberle-Hütter, A.; Rupp, J.L.M.; Graule, T.; Gauckler, L.J. Flame spray deposition of La0.6Sr0.4CoO3-δ thin films: Microstructural characterization, electrochemical performance and degradation. J. Power Sources 2010, 195, 8152–8161. [Google Scholar] [CrossRef]
- Ueno, K.; Sakamoto, W.; Yogo, T.; Hirano, S. Synthesis of conductive LaNiO3 thin films by chemical solution deposition. Japanese J. Appl. Physics, Part 1 Regul. Pap. Short Notes Rev. Pap. 2001, 40, 6049–6054. [Google Scholar] [CrossRef]
- Shimizu, Y.; Murata, T. Sol-gel synthesis of perovskite-type lanthanum manganite thin films and fine powders using metal acetylacetonate and poly(vinyl alcohol). J. Am. Ceram. Soc. 1997, 80, 2702–2704. [Google Scholar] [CrossRef]
- Aono, H.; Tomida, M.; Sadaoka, Y. Conventional synthesis method for fine polymetallic LaFeO3 using ethylene glycol solvent addition. J. Ceram. Soc. Japan 2009, 117, 1048–1051. [Google Scholar] [CrossRef]
- Choppali, U.; Kougianos, E.; Mohanty, S.P.; Gorman, B.P. Polymeric precursor derived nanocrystalline ZnO thin films using EDTA as chelating agent. Sol. Energy Mater. Sol. Cells 2010, 94, 2351–2357. [Google Scholar] [CrossRef]
- Miller, J.B.; Ashok, T.; Lee, S.; Broitman, E. Zinc oxide-based thin film functional layers for chemiresistive sensors. Thin Solid Films 2012, 520, 6669–6676. [Google Scholar] [CrossRef]
- Keyes, B.M.; Gedvilas, L.M.; Li, X.; Coutts, T.J. Infrared spectroscopy of polycrystalline ZnO and ZnO:N thin films. J. Cryst. Growth 2005, 281, 297–302. [Google Scholar] [CrossRef]
- Carotta, M.C.; Martinelli, G.; Sadaoka, Y.; Nunziante, P.; Traversa, E. Gas-sensitive electrical properties of perovskite-type SmFeO3 thick films. Sens. Actuators B Chem 1998, B48, 270–276. [Google Scholar] [CrossRef]
- Vuong, D.D.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Hydrogen sulfide gas sensing properties of thin films derived from SnO2 sols different in grain size. Sens. Actuators B Chem 2005, 105, 437–442. [Google Scholar] [CrossRef]
- Hoffmann, R.; Chen, M.M.-L.; Thorn, D.L. Qualitative Discussion of Alternative Coordination Modes of Diatomic Ligands in Transition Metal Complexes. Inorg. Chem. 1977, 16, 503–511. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. New perspectives of gas sensor technology. Sens. Actuators B Chem 2009, 138, 100–107. [Google Scholar] [CrossRef]
- Zhang, R.; Villanueva, A.; Alamdari, H.; Kaliaguine, S. Cu- and Pd-substituted nanoscale Fe-based perovskites for selective catalytic reduction of NO by propene. J. Catal. 2006, 237, 368–380. [Google Scholar] [CrossRef]




| X in AcAcX | ||||
|---|---|---|---|---|
| 0 | 0.188 | 0.143 | 0.076 | 0.219 |
| 2 | 0.164 | 0.137 | 0.065 | 0.202 |
| 4 | 0.193 | 0.135 | 0.054 | 0.189 |
| 8 | 0.170 | 0.134 | 0.057 | 0.191 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasaki, T.; Takase, S.; Shimizu, Y. Improvement of Sensing Performance of Impedancemetric C2H2 Sensor Using SmFeO3 Thin-Films Prepared by a Polymer Precursor Method. Sensors 2019, 19, 773. https://doi.org/10.3390/s19040773
Tasaki T, Takase S, Shimizu Y. Improvement of Sensing Performance of Impedancemetric C2H2 Sensor Using SmFeO3 Thin-Films Prepared by a Polymer Precursor Method. Sensors. 2019; 19(4):773. https://doi.org/10.3390/s19040773
Chicago/Turabian StyleTasaki, Tomohisa, Satoko Takase, and Youichi Shimizu. 2019. "Improvement of Sensing Performance of Impedancemetric C2H2 Sensor Using SmFeO3 Thin-Films Prepared by a Polymer Precursor Method" Sensors 19, no. 4: 773. https://doi.org/10.3390/s19040773




