Novel Operation Strategy to Obtain a Fast Gas Sensor for Continuous ppb-Level NO2 Detection at Room Temperature Using ZnO—A Concept Study with Experimental Proof
Abstract
:1. Introduction
2. Pre-Considerations
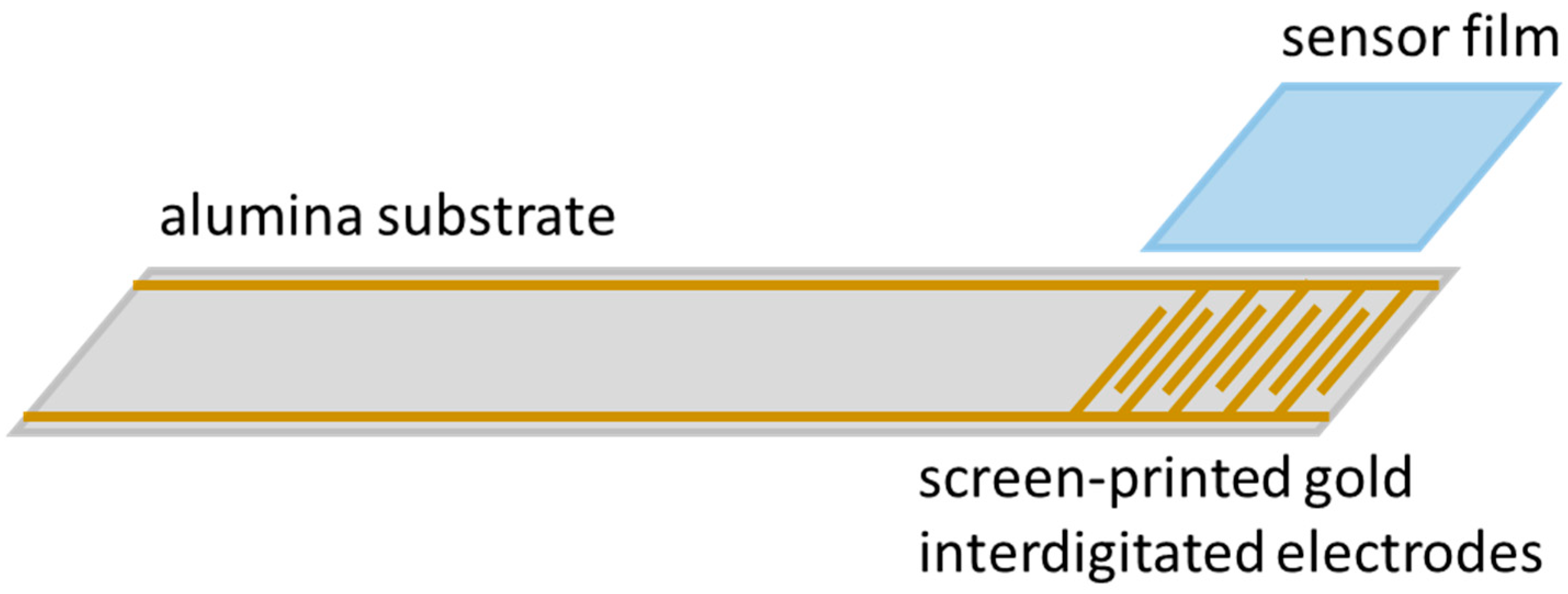
3. Experimental
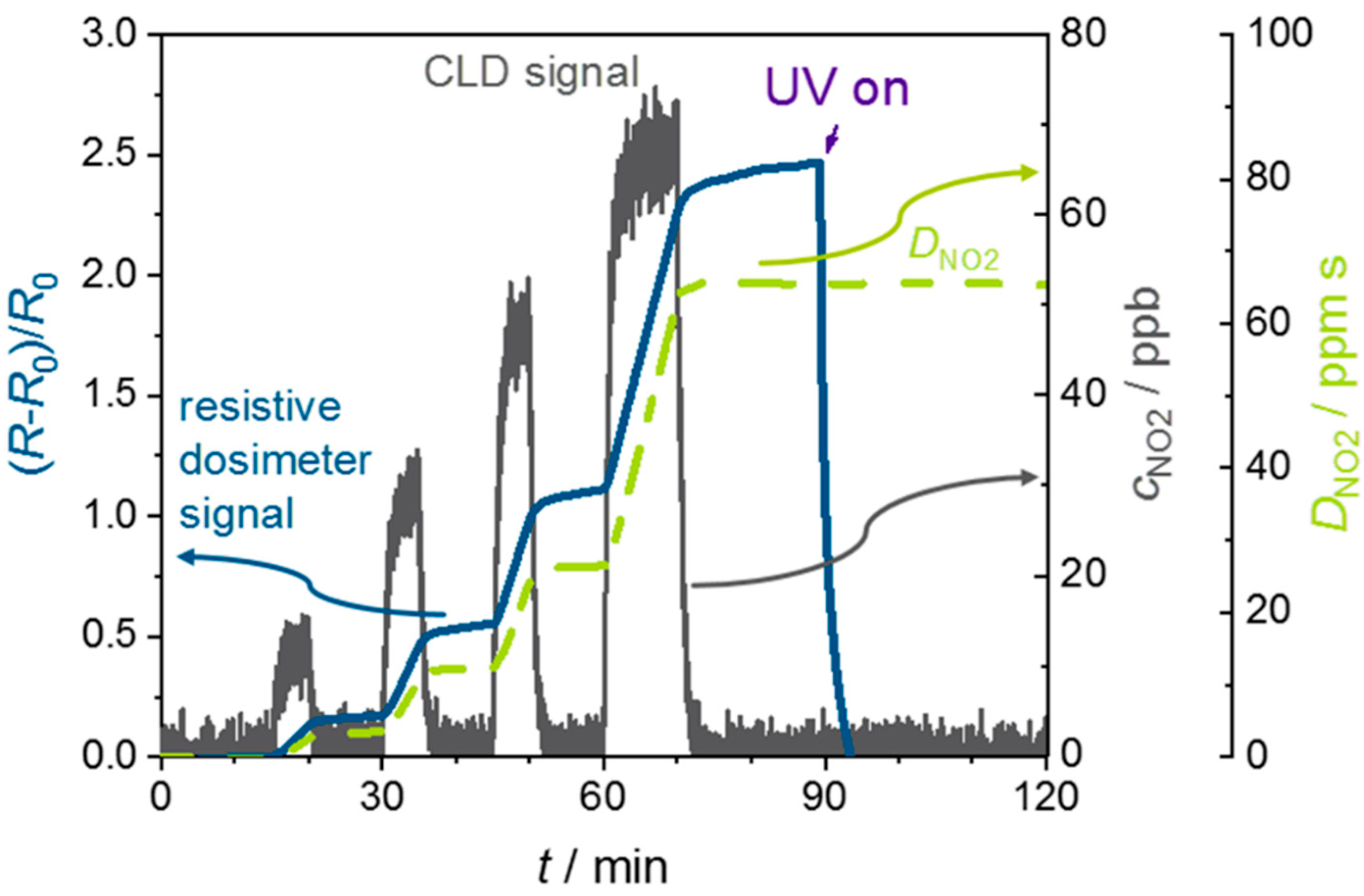
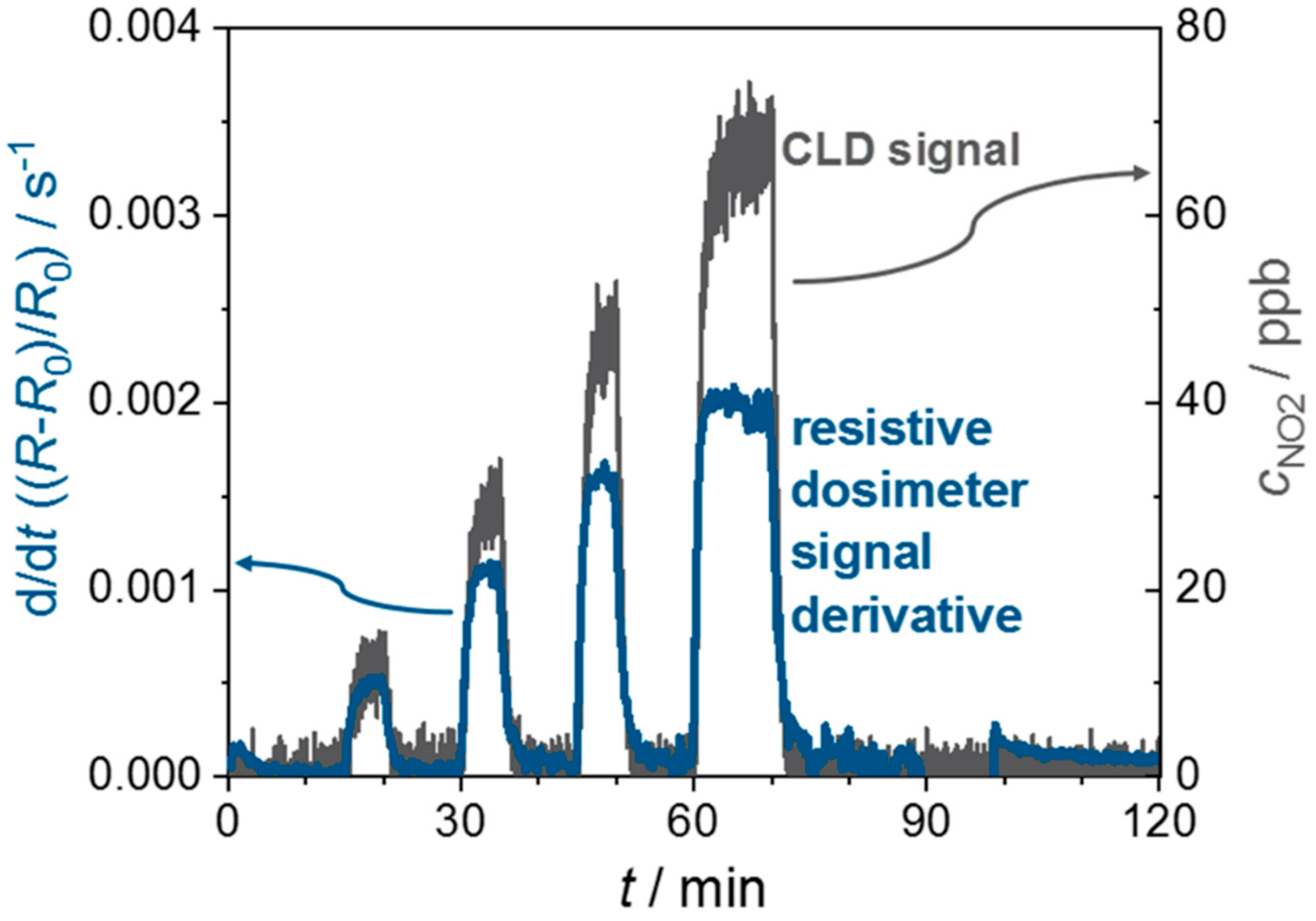
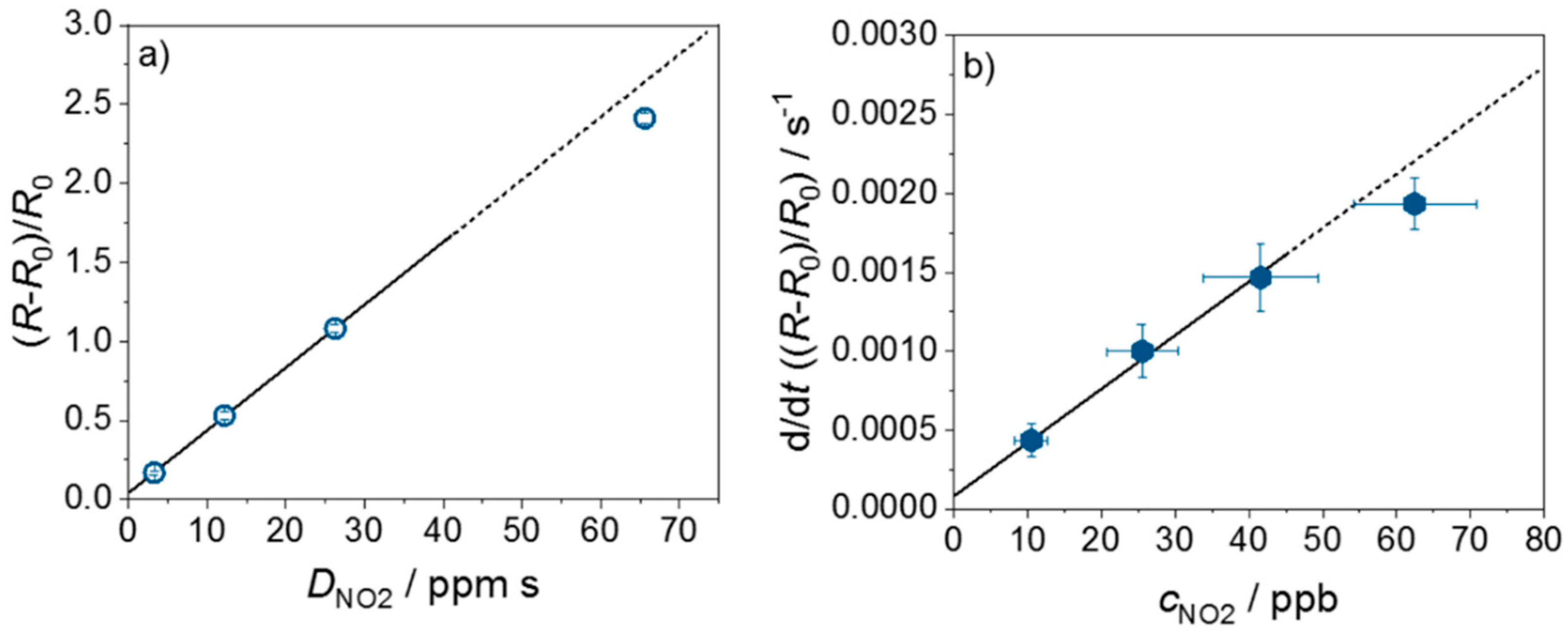
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas-Sensor Applications: A Review. Nano Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Neununddreißigste Verordnung zur Durchführung des Bundes-Immissionsschutzgesetzes (Verordnung über Luftqualitätsstandards und Emissionshöchstmengen—39); Ein Service des Bundesministeriums der Justiz und für Verbraucherschutz sowie des Bundesamts für Justiz: Berlin, Germany, 2010. Available online: https://www.gesetze-im-internet.de/bimschv_39/BJNR106510010.html (accessed on 20 September 2019).
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient air Quality and Cleaner Air for Europe. Available online: https://eur-lex.europa.eu/eli/dir/2008/50/oj (accessed on 20 September 2019).
- Bârsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. High-sensitivity NO2 gas sensors based on flower-like and tube-like ZnO nanomaterials. Sens. Actuators B Chem. 2011, 157, 565–574. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Liao, H.; Li, C.-J.; Debliquy, M. Room-temperature nitrogen-dioxide sensors based on ZnO1−x coatings deposited by solution precursor plasma spray. Sens. Actuators B Chem. 2017, 242, 102–111. [Google Scholar] [CrossRef]
- Baratto, C.; Sberveglieri, G.; Onischuk, A.; Caruso, B.; Di Stasio, S. Low temperature selective NO2 sensors by nanostructured fibres of ZnO. Sens. Actuators B Chem. 2004, 100, 261–265. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B Chem. 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Groppelli, S.; Nelli, P. Highly sensitive and selective NOx and NO2 sensor based on Cd-doped SnO2 thin films. Sens. Actuators B Chem. 1991, 4, 457–461. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G. UV light activation of tin oxide thin films for NO2 sensing at low temperatures. Sens. Actuators B Chem. 2001, 78, 73–77. [Google Scholar] [CrossRef]
- Carotta, M.C.; Ferroni, M.; Gnani, D.; Guidi, V.; Merli, M.; Martinelli, G.; Casale, M.C.; Notaro, M. Nanostructured pure and Nb-doped TiO2 as thick film gas sensors for environmental monitoring. Sens. Actuators B Chem. 1999, 58, 310–317. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuator A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Borrego, C.; Costa, A.M.; Ginja, J.; Amorim, M.; Coutinho, M.; Karatzas, K.; Sioumis, T.; Katsifarakis, N.; Konstantinidis, K.; de Vito, S.; et al. Assessment of air quality microsensors versus reference methods: The EuNetAir joint exercise. Atmos. Environ. 2016, 147, 246–263. [Google Scholar] [CrossRef] [Green Version]
- Carotta, M.C.; Cervi, A.; Fioravanti, A.; Gherardi, S.; Giberti, A.; Vendemiati, B.; Vincenzi, D.; Sacerdoti, M. A novel ozone detection at room temperature through UV-LED-assisted ZnO thick film sensors. Thin Solid Film. 2011, 520, 939–946. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-enhanced NO2 gas sensing properties of SnO2-core/ZnO-shell nanowires at room temperature. ACS Appl. Mater. Interfaces 2013, 5, 4285–4292. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Li, J.; Luo, Y.; Lu, P. Role of oxygen vacancy in tuning of optical, electrical and NO2 sensing properties of ZnO1−x coatings at room temperature. Sens. Actuators B Chem. 2017, 248, 886–893. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chang, L.-F.; Hsueh, T.-J. Light-activated humidity and gas sensing by ZnO nanowires grown on LED at room temperature. Sens. Actuators B Chem. 2017, 249, 265–277. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Facile Synthesis and UV-Activated Gas Sensing Performance of Ag: ZnO Nano-Ellipsoids. ECS J. Solid State Sci. Technol. 2018, 7, Q3089–Q3093. [Google Scholar] [CrossRef]
- Markiewicz, N.; Casals, O.; Fabrega, C.; Gràcia, I.; Cané, C.; Wasisto, H.S.; Waag, A.; Prades, J.D. Micro light plates for low-power photoactivated (gas) sensors. Appl. Phys. Lett. 2019, 114, 53508. [Google Scholar] [CrossRef] [Green Version]
- Casals, O.; Markiewicz, N.; Fabrega, C.; Gràcia, I.; Cané, C.; Wasisto, H.S.; Waag, A.; Prades, J.D. A parts per billion (ppb) sensor for NO2 with microwatt (μW) power requirements based on micro light plates. ACS Sens. 2019, 4, 822–826. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R. 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Xiang, L. Facile synthesis of mesoporous ZnO sheets assembled by small nanoparticles for enhanced NO2 sensing performance at room temperature. Sens. Actuators B Chem. 2018, 270, 207–215. [Google Scholar] [CrossRef]
- Gonzalez-Chavarri, J.; Parellada-Monreal, L.; Castro-Hurtado, I.; Castaño, E.; Mandayo, G.G. ZnO nanoneedles grown on chip for selective NO2 detection indoors. Sens. Actuators B Chem. 2018, 255, 1244–1253. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Alshammari, A.S.; Jayawardena, K.I.; Beliatis, M.J.; Henley, S.J.; Silva, S.R.P. Role of the Exposed Polar Facets in the Performance of Thermally and UV Activated ZnO Nanostructured Gas Sensors. J. Phys. Chem. C 2013, 117, 17850–17858. [Google Scholar] [CrossRef] [Green Version]
- Tai, W.-P.; Oh, J.-H. Humidity sensing behaviors of nanocrystalline Humidity sensing behaviors of nanocrystalline Al-doped ZnO thin films prepared by sol-gel process sol-gel process. J. Mater. Sci. Mater. Electr. 2002, 13, 391–394. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter 2003, 15, R813–R839. [Google Scholar] [CrossRef]
- Chang, J.F.; Kuo, H.H.; Leu, I.C.; Hon, M.H. The effects of thickness and operation temperature on ZnO:Al thin film CO gas sensor. Sens. Actuators B Chem. 2002, 84, 258–264. [Google Scholar] [CrossRef]
- Oh, E.; Choi, H.-Y.; Jung, S.-H.; Cho, S.; Kim, J.C.; Lee, K.-H.; Kang, S.-W.; Kim, J.; Yun, J.-Y.; Jeong, S.-H. High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation. Sens. Actuators B Chem. 2009, 141, 239–243. [Google Scholar] [CrossRef]
- Gaiardo, A.; Fabbri, B.; Giberti, A.; Guidi, V.; Bellutti, P.; Malagù, C.; Valt, M.; Pepponi, G.; Gherardi, S.; Zonta, G.; et al. ZnO and Au/ZnO thin films: Room-temperature chemoresistive properties for gas sensing applications. Sens. Actuators B Chem. 2016, 237, 1085–1094. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, G.; Xu, M.; Su, Y.; Tai, H.; Du, H.; Jiang, Y. Visible light-assisted room temperature gas sensing with ZnO-Ag heterostructure nanoparticles. Sens. Actuators B Chem. 2018, 259, 269–281. [Google Scholar] [CrossRef]
- Stănoiu, A.; Simion, C.E.; Somăcescu, S. NO2 sensing mechanism of ZnO–Eu2O3 binary oxide under humid air conditions. Sens. Actuators B Chem. 2013, 186, 687–694. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens Actuator B Chem. 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, C.; Debliquy, M. Cadmium sulfide activated zinc oxide coatings deposited by liquid plasma spray for room temperature nitrogen dioxide detection under visible light illumination. Ceram. Int. 2016, 42, 4845–4852. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Zhang, Z.; Yue, J.; Zakaria, Q.M.D.; Chen, C.; Jiang, X.; Yu, A. Crystal plane-dependent gas-sensing properties of zinc oxide nanostructures: Experimental and theoretical studies. Phys. Chem. Chem. Phys. 2014, 16, 11471–11480. [Google Scholar] [CrossRef]
- Yan, D.; Hu, M.; Li, S.; Liang, J.; Wu, Y.; Ma, S. Electrochemical deposition of ZnO nanostructures onto porous silicon and their enhanced gas sensing to NO2 at room temperature. Electrochim. Acta 2014, 115, 297–305. [Google Scholar] [CrossRef]
- Su, X.; Duan, G.; Xu, Z.; Zhou, F.; Cai, W. Structure and thickness-dependent gas sensing responses to NO2 under UV irradiation for the multilayered ZnO micro/nanostructured porous thin films. J. Colloid Interface Sci. 2017, 503, 150–158. [Google Scholar] [CrossRef]
- Chen, Z.; Zhan, G.; Lu, Z. Solvothermal synthesis and conductive properties of nanorod-constructed Al-doped ZnO microflowers. J. Mater. Sci. Mater. Electr. 2014, 25, 1724–1730. [Google Scholar] [CrossRef]
- Chouchene, B.; Chaabane, T.B.; Mozet, K.; Girot, E.; Corbel, S.; Balan, L.; Medjahdi, G.; Schneider, R. Porous Al-doped ZnO rods with selective adsorption properties. Appl. Surf. Sci. 2017, 409, 102–110. [Google Scholar] [CrossRef]
- Gurav, K.V.; Gang, M.G.; Shin, S.W.; Patil, U.M.; Deshmukh, P.R.; Agawane, G.L.; Suryawanshi, M.P.; Pawar, S.M.; Patil, P.S.; Lokhande, C.D.; et al. Gas sensing properties of hydrothermally grown ZnO nanorods with different aspect ratios. Sens. Actuators B Chem. 2014, 190, 439–445. [Google Scholar] [CrossRef]
- Mun, Y.; Park, S.; An, S.; Lee, C.; Kim, H.W. NO2 gas sensing properties of Au-functionalized porous ZnO nanosheets enhanced by UV irradiation. Ceram. Int. 2013, 39, 8615–8622. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. Porous ZnO Polygonal Nanoflakes: Synthesis, Use in High-Sensitivity NO2 Gas Sensor, and Proposed Mechanism of Gas Sensing. J. Phys. Chem. C 2011, 115, 12763–12773. [Google Scholar] [CrossRef]
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx sensors based on semiconducting metal oxide nanostructures: Progress and perspectives. Sens. Actuators B Chem. 2012, 171–172, 25–42. [Google Scholar] [CrossRef]
- Anothainart, K.; Burgmair, M.; Karthigeyan, A.; Zimmer, M.; Eisele, I. Light enhanced NO2 gas sensing with tin oxide at room temperature: Conductance and work function measurements. Sens. Actuators B Chem. 2003, 93, 580–584. [Google Scholar] [CrossRef]
- Fan, S.-W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B Chem. 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Li, Y.; Della Valle, F.; Simonnet, M.; Yamada, I.; Delaunay, J.-J. Competitive surface effects of oxygen and water on UV photoresponse of ZnO nanowires. Appl. Phys. Lett. 2009, 94, 23110. [Google Scholar] [CrossRef] [Green Version]
- Helwig, A.; Müller, G.; Garrido, J.A.; Eickhoff, M. Gas sensing properties of hydrogen-terminated diamond. Sens. Actuators B Chem. 2008, 133, 156–165. [Google Scholar] [CrossRef]
- Shu, J.H.; Wikle, H.C.; Chin, B.A. Passive chemiresistor sensor based on iron (II) phthalocyanine thin films for monitoring of nitrogen dioxide. Sens. Actuators B Chem. 2010, 148, 498–503. [Google Scholar] [CrossRef]
- Groß, A.; Beulertz, G.; Marr, I.; Kubinski, D.J.; Visser, J.H.; Moos, R. Dual mode NOx sensor: Measuring both the accumulated amount and instantaneous level at low concentrations. Sensors 2012, 12, 2831–2850. [Google Scholar] [CrossRef]
- Marr, I.; Groß, A.; Moos, R. Overview on conductometric solid-state gas dosimeters. J. Sens. Sens. Syst. 2014, 3, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Marr, I.; Moos, R. Resistive NOx dosimeter to detect very low NOx concentrations—Proof-of-principle and comparison with classical sensing devices. Sens. Actuators B Chem. 2017, 248, 848–855. [Google Scholar] [CrossRef]
- Groß, A.; Hanft, D.; Beulertz, G.; Marr, I.; Kubinski, D.J.; Visser, J.H.; Moos, R. The effect of SO2 on the sensitive layer of a NOx dosimeter. Sens. Actuators B Chem. 2013, 187, 153–161. [Google Scholar] [CrossRef]
- Giese, U.; Stenner, H.; Ludwig, E.; Kettrup, A. Determination of chlorinated hydrocarbons in single and multi component test gases. Fresenius J. Anal. Chem. 1990, 338, 610–614. [Google Scholar] [CrossRef]
- Seethapathy, S.; Górecki, T.; Li, X. Passive sampling in environmental analysis. J. Chromatogr. A 2008, 1184, 234–253. [Google Scholar] [CrossRef]
- Ferber, B.I.; Sharp, F.A.; Freedman, R.W. Dosimeter for oxides of nitrogen. Am. Ind. Hyg. Assoc. J. 1976, 37, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.; Helwig, A.; Müller, G. Room-temperature dosimeter-type gas sensors with periodic reset. Sens. Actuators B Chem. 2017, 244, 701–708. [Google Scholar] [CrossRef]
- Vasiliev, R.B.; Rumyantseva, M.N.; Ryabova, L.I.; Akimov, B.A.; Labeau, M.; Langlet, M.; Gaskov, A.M. Memory effect and its switching by electric field in solid-state gas sensors. Mater. Sci. Eng. B 2000, 77, 106–109. [Google Scholar] [CrossRef]
- Novikov, S.; Lebedeva, N.; Satrapinski, A. Ultrasensitive NO2 Gas Sensor Based on Epitaxial Graphene. J. Sens. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Diodati, S.; Hennemann, J.; Fresno, F.; Gialanella, S.; Dolcet, P.; Lavrenčič Štangar, U.; Smarsly, B.M.; Gross, S. Easy and Green Route towards Nanostructured ZnO as an Active Sensing Material with Unexpected H2S Dosimeter-Type Behaviour. Eur. J. Inorg. Chem. 2019, 2019, 837–846. [Google Scholar] [CrossRef]
- Müller, G.; Krstev, I.; Maier, K.; Helwig, A.; Stutzmann, M.; Garrido, J. Resettable, Low-temperature Accumulation Gas Sensors Based on Hydrogenated Diamond Transducers. Proc. Eng. 2015, 120, 590–593. [Google Scholar] [CrossRef] [Green Version]
- Offermans, P.; Vitushinsky, R. NO2 Detection with AlGaN/GaN 2DEG Channels for Air Quality Monitoring. IEEE Sens. J. 2013, 13, 2823–2827. [Google Scholar] [CrossRef]
- Vogel, L.; Wagner, R.; Moos, R.; Schönauer-Kamin, D. Investigations on the crystal growth mechanism of one-pot-synthesized Al-doped ZnO and its UV-enhanced room temperature NO2 gas sensing characteristics. Funct. Mater. Lett. 2018, 11, 1850087. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Hille, P.; Eickhoff, M. Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors. Materials 2015, 8, 6570–6588. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kumar, V.; Li, Y.; Zhang, H.; Marks, T.J.; Chang, R.P.H. Fabrication of ZnO Nanorods and Nanotubes in Aqueous Solutions. Chem. Mater. 2005, 17, 1001–1006. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, R.; Schönauer-Kamin, D.; Moos, R. Novel Operation Strategy to Obtain a Fast Gas Sensor for Continuous ppb-Level NO2 Detection at Room Temperature Using ZnO—A Concept Study with Experimental Proof. Sensors 2019, 19, 4104. https://doi.org/10.3390/s19194104
Wagner R, Schönauer-Kamin D, Moos R. Novel Operation Strategy to Obtain a Fast Gas Sensor for Continuous ppb-Level NO2 Detection at Room Temperature Using ZnO—A Concept Study with Experimental Proof. Sensors. 2019; 19(19):4104. https://doi.org/10.3390/s19194104
Chicago/Turabian StyleWagner, Ricarda, Daniela Schönauer-Kamin, and Ralf Moos. 2019. "Novel Operation Strategy to Obtain a Fast Gas Sensor for Continuous ppb-Level NO2 Detection at Room Temperature Using ZnO—A Concept Study with Experimental Proof" Sensors 19, no. 19: 4104. https://doi.org/10.3390/s19194104




