Development of a Function-Integrative Sleeve for Medical Applications
Abstract
:1. Introduction
1.1. Noradrenaline Kinetics
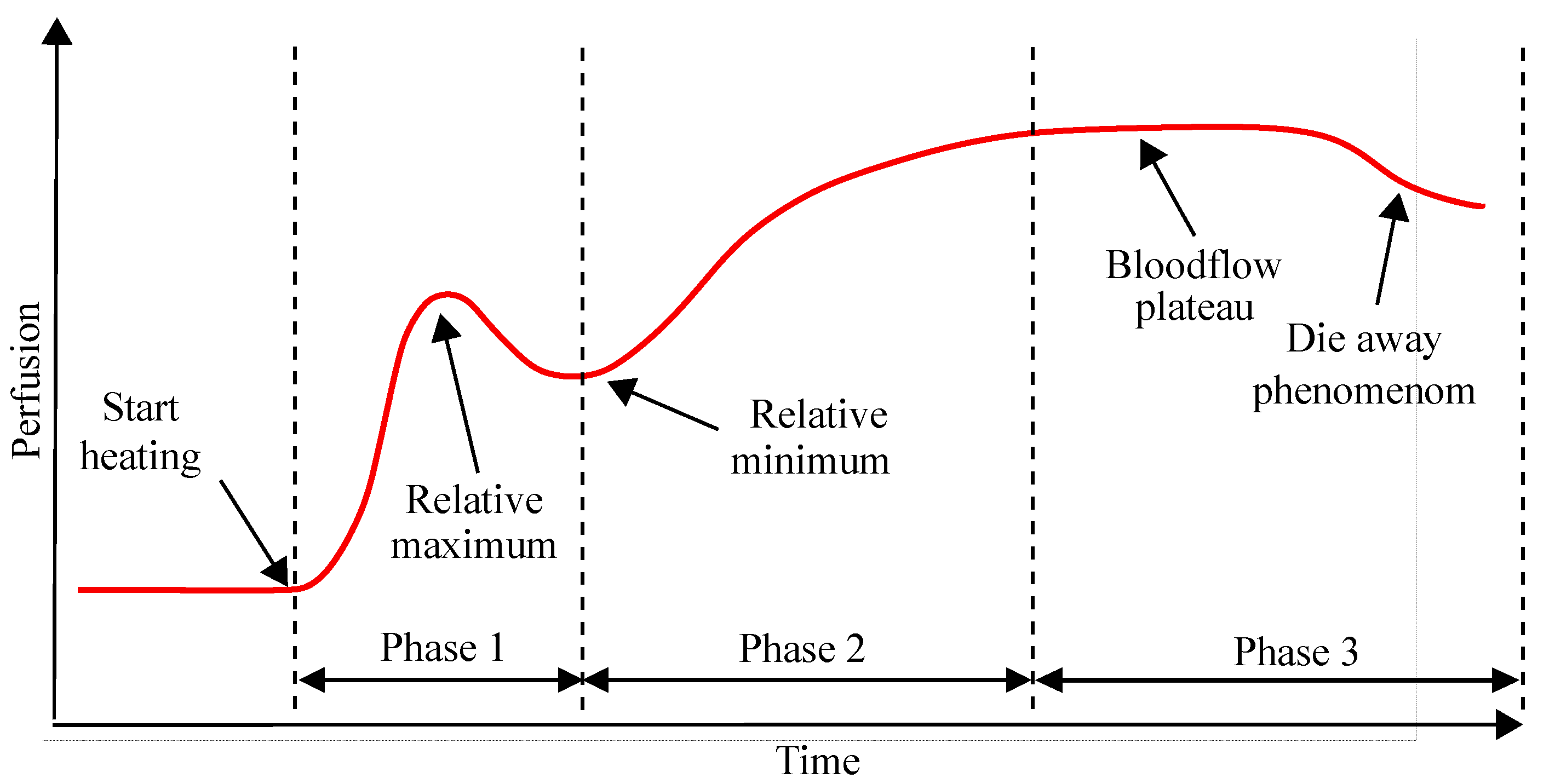
1.2. Physiology of Warming Induced Vasodilation of the Forearm
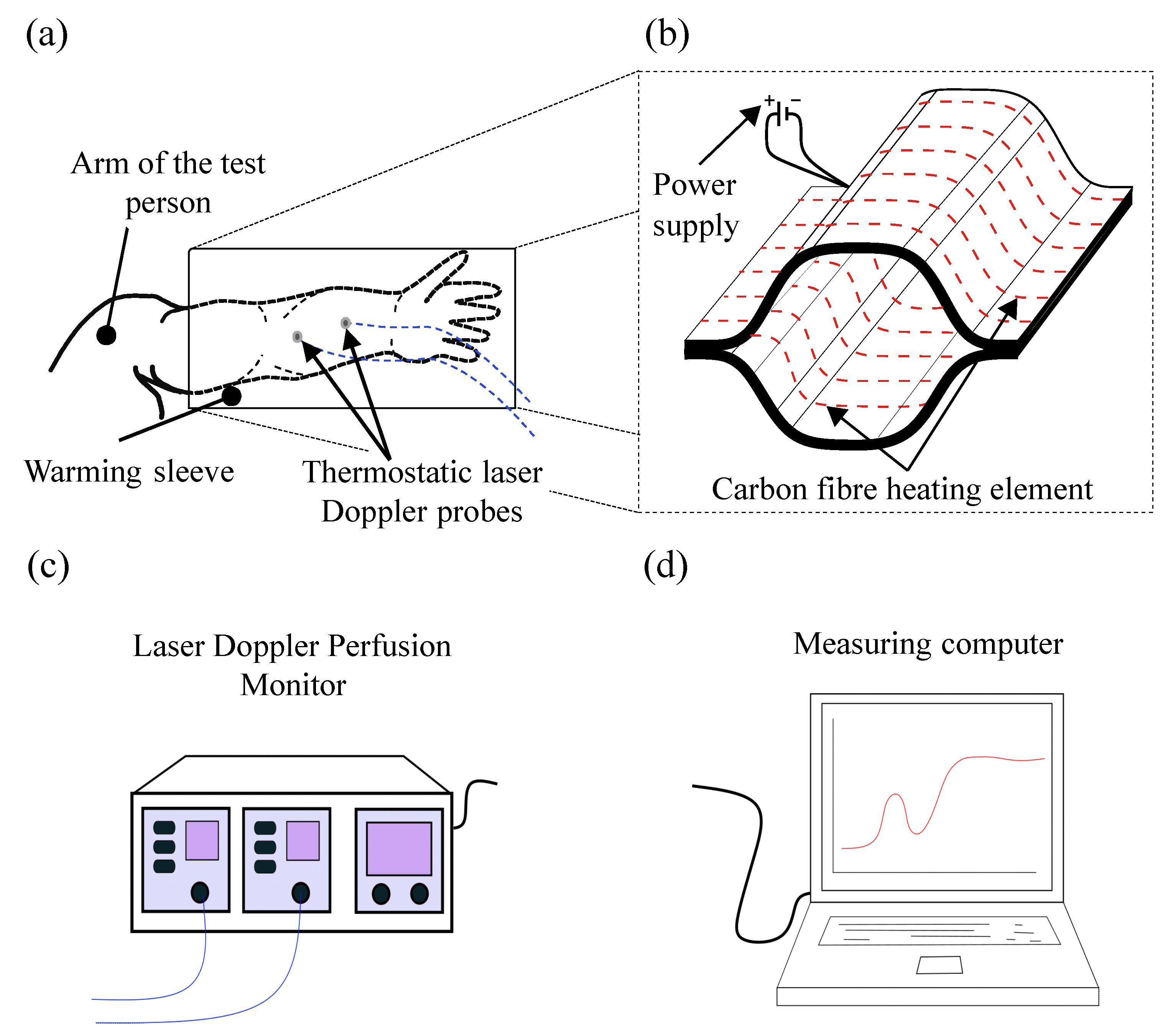
1.3. Current Approaches for Stimulating and Measuring the Blood Flow
2. Proof of Concept
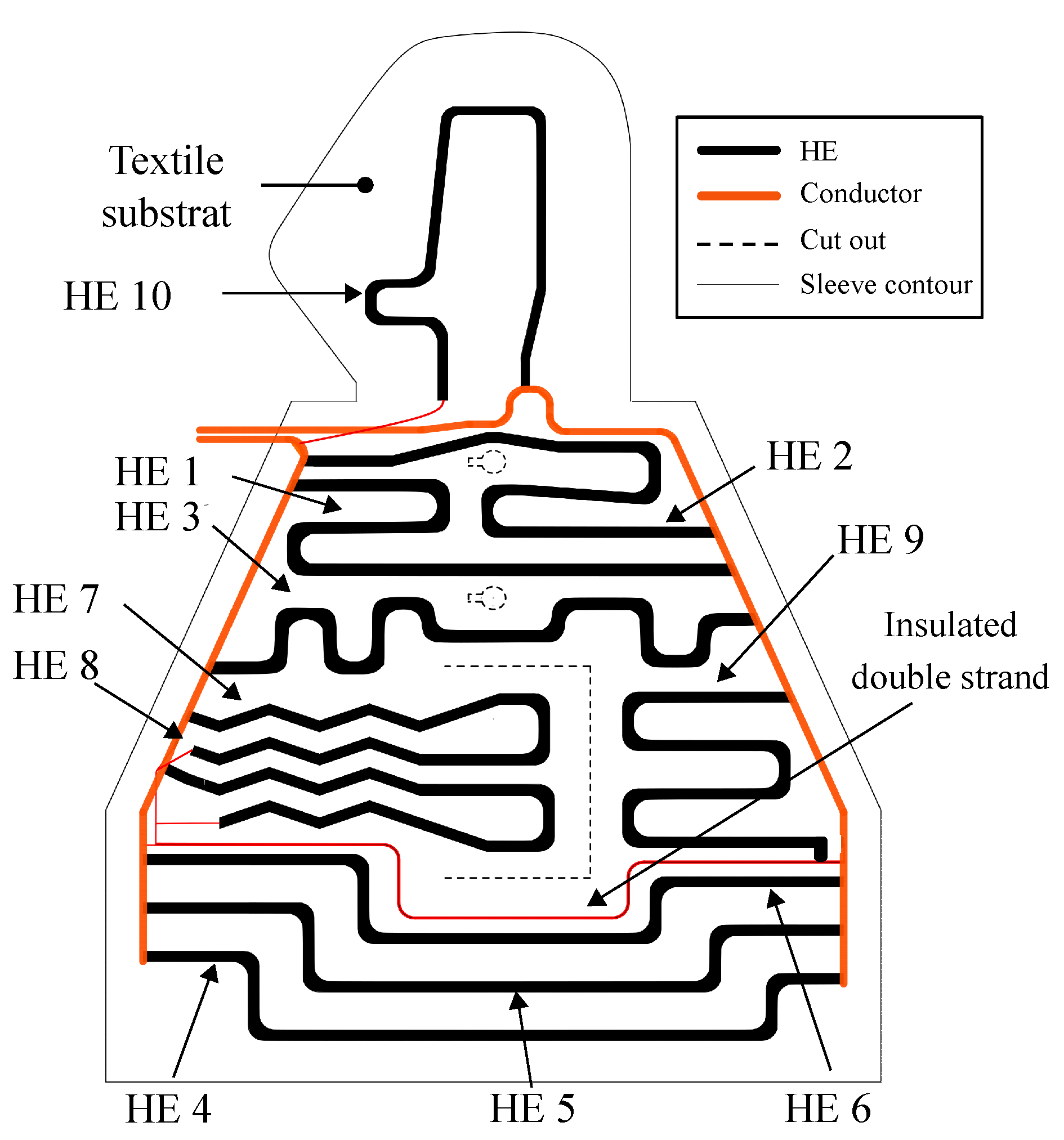
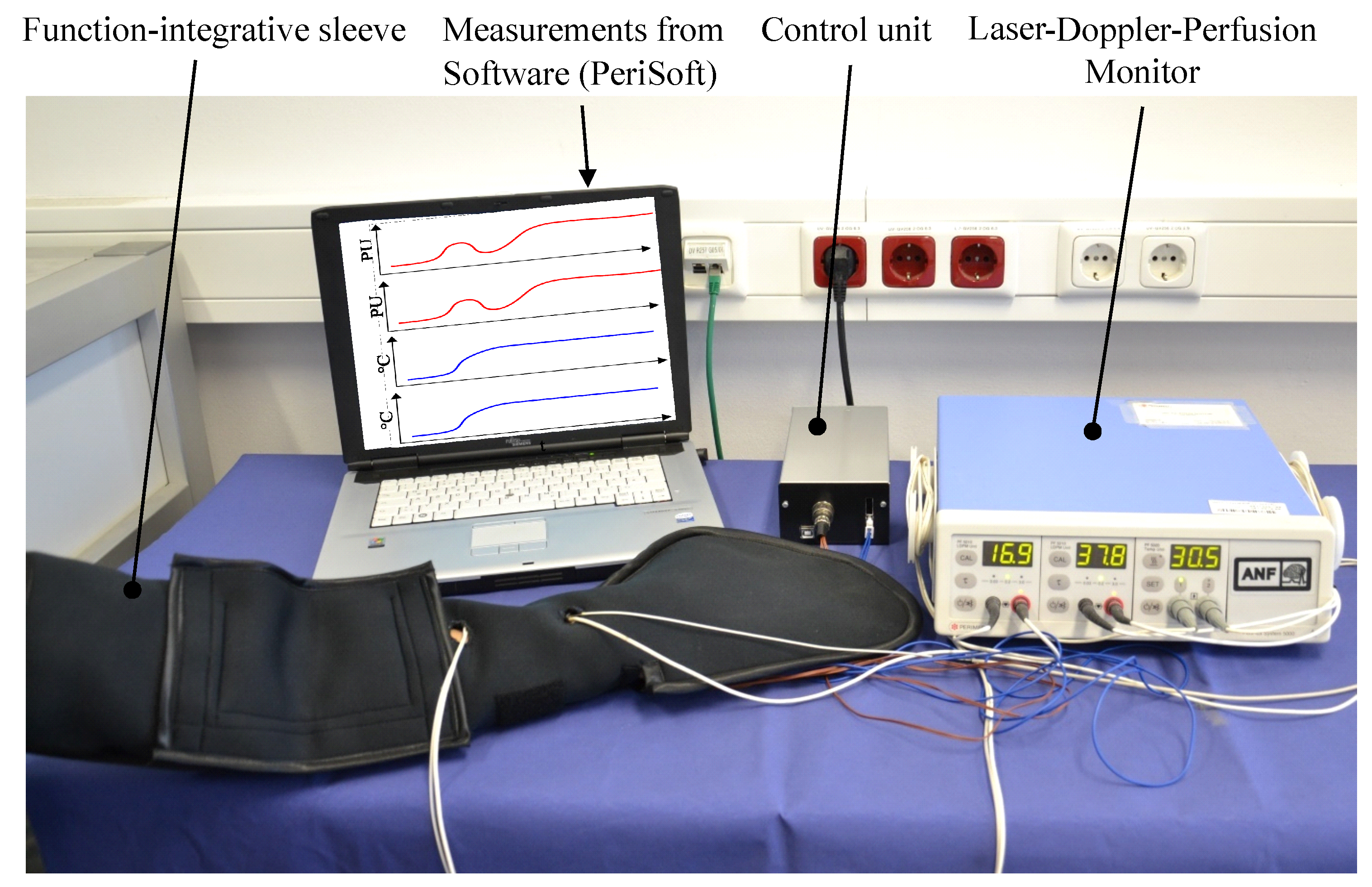
3. Development of the Function-Integrative Sleeve
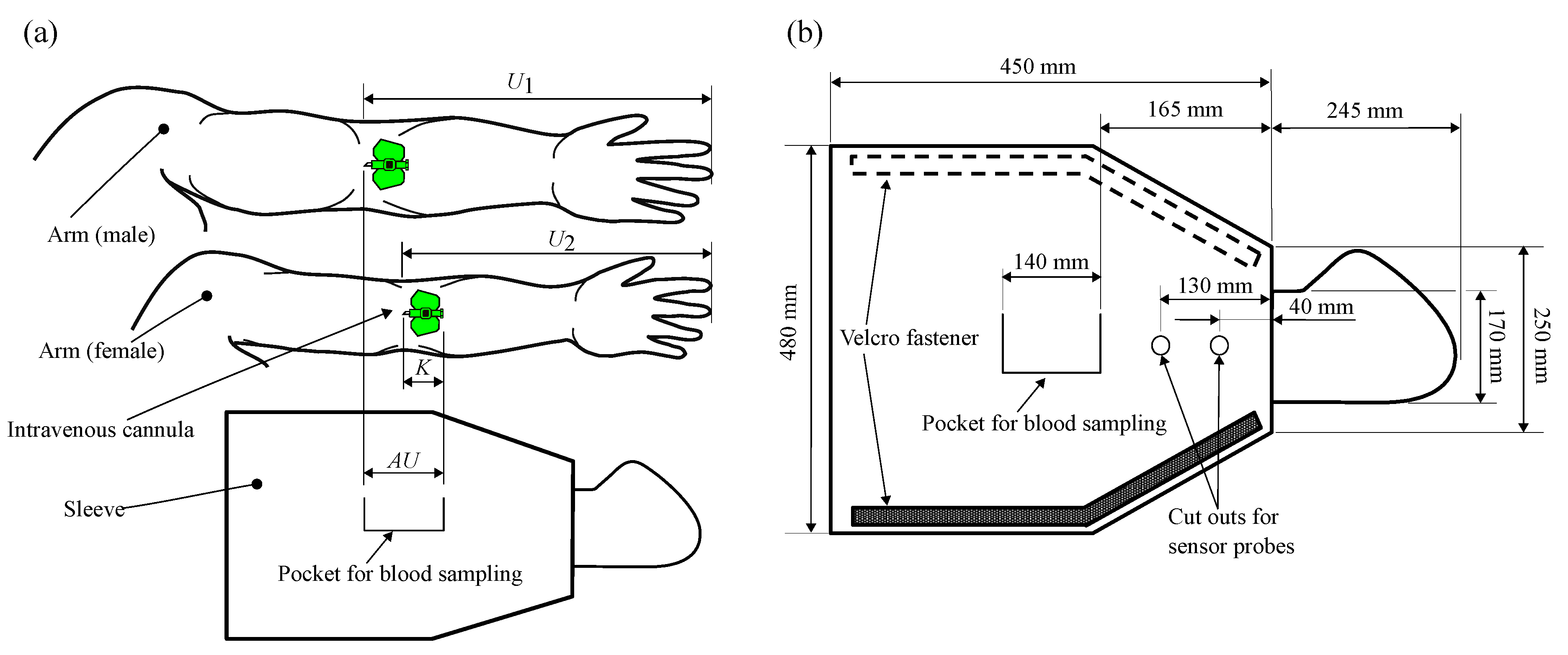
3.1. Design of the Function-Integrative Sleeve
3.2. Design and Arrangement of the Heating Elements
4. Manufacturing Process of the Prototype
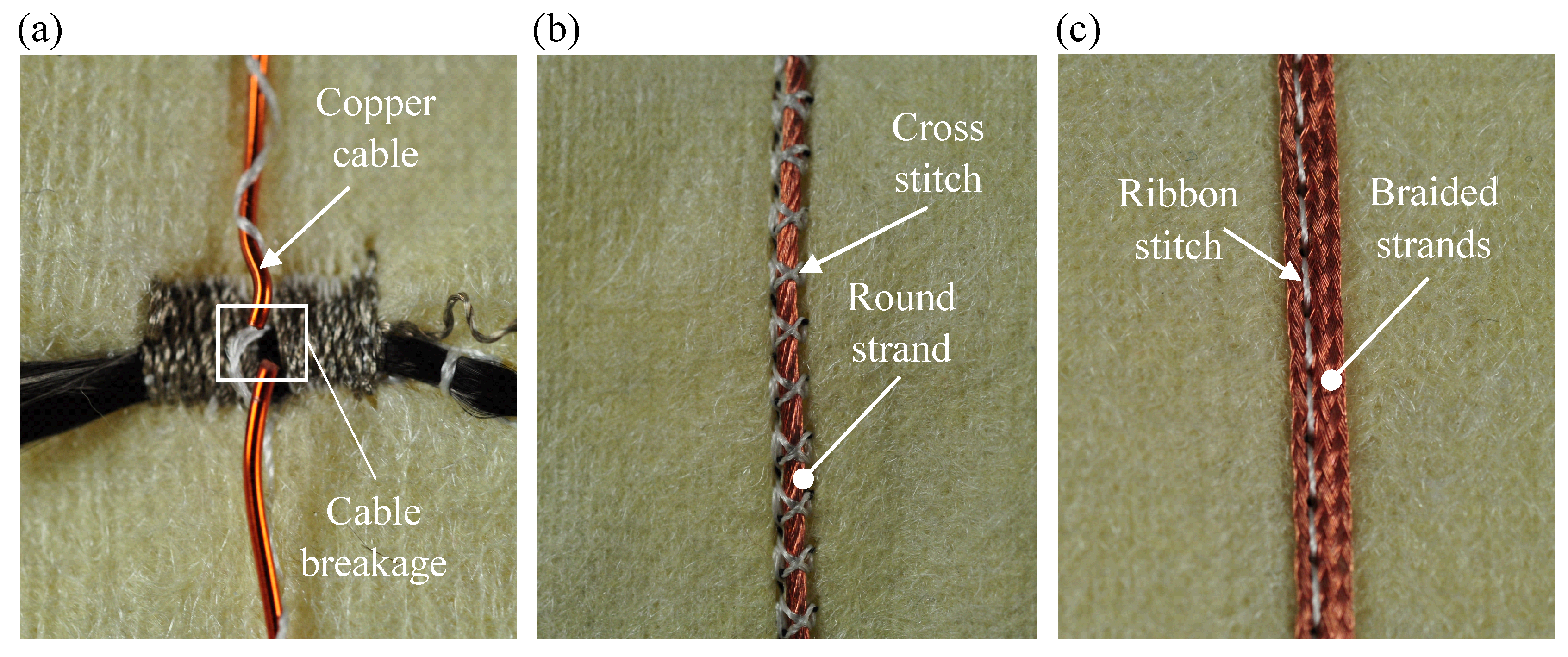
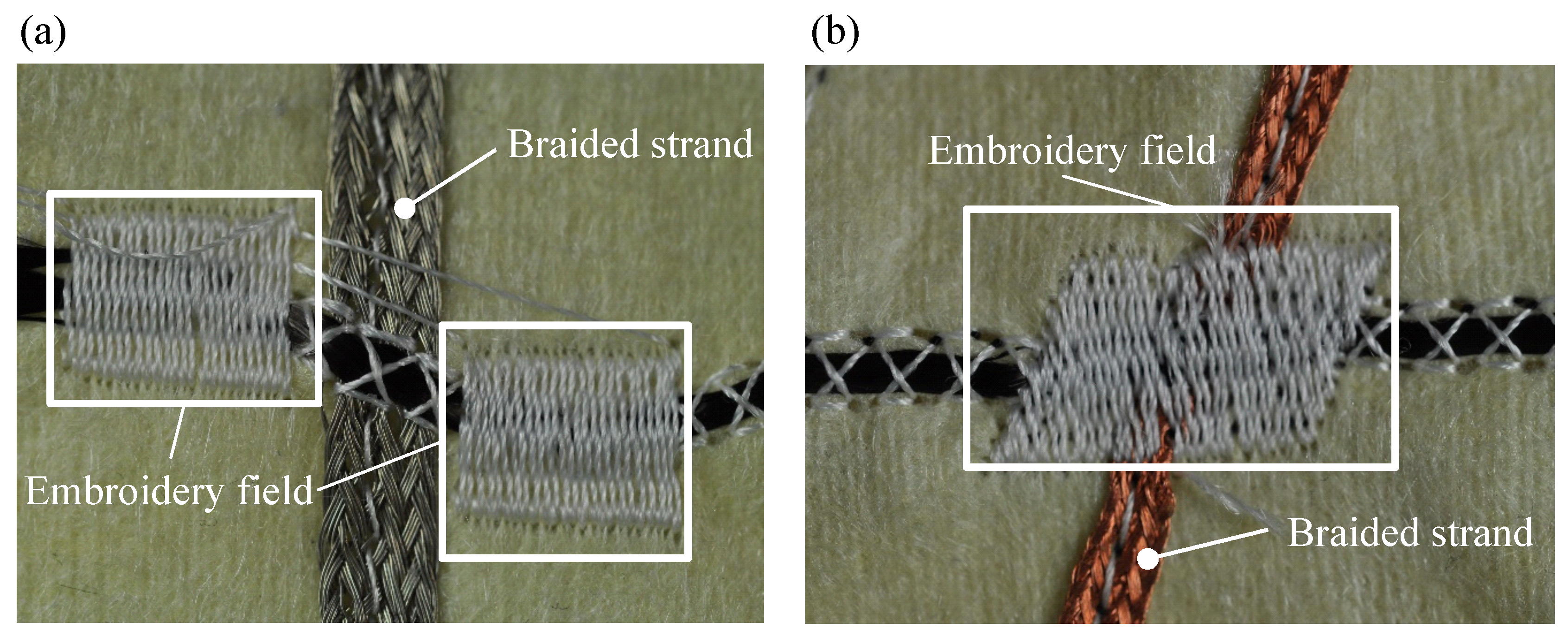
4.1. Manufacturing of the Contact and the Isolation Layer
4.2. Manufacturing of the Functional Warming Layer
5. Testing of the Prototype
5.1. Function Test of the Heating Elements
5.2. Approach for Automated Blood Flow Determination
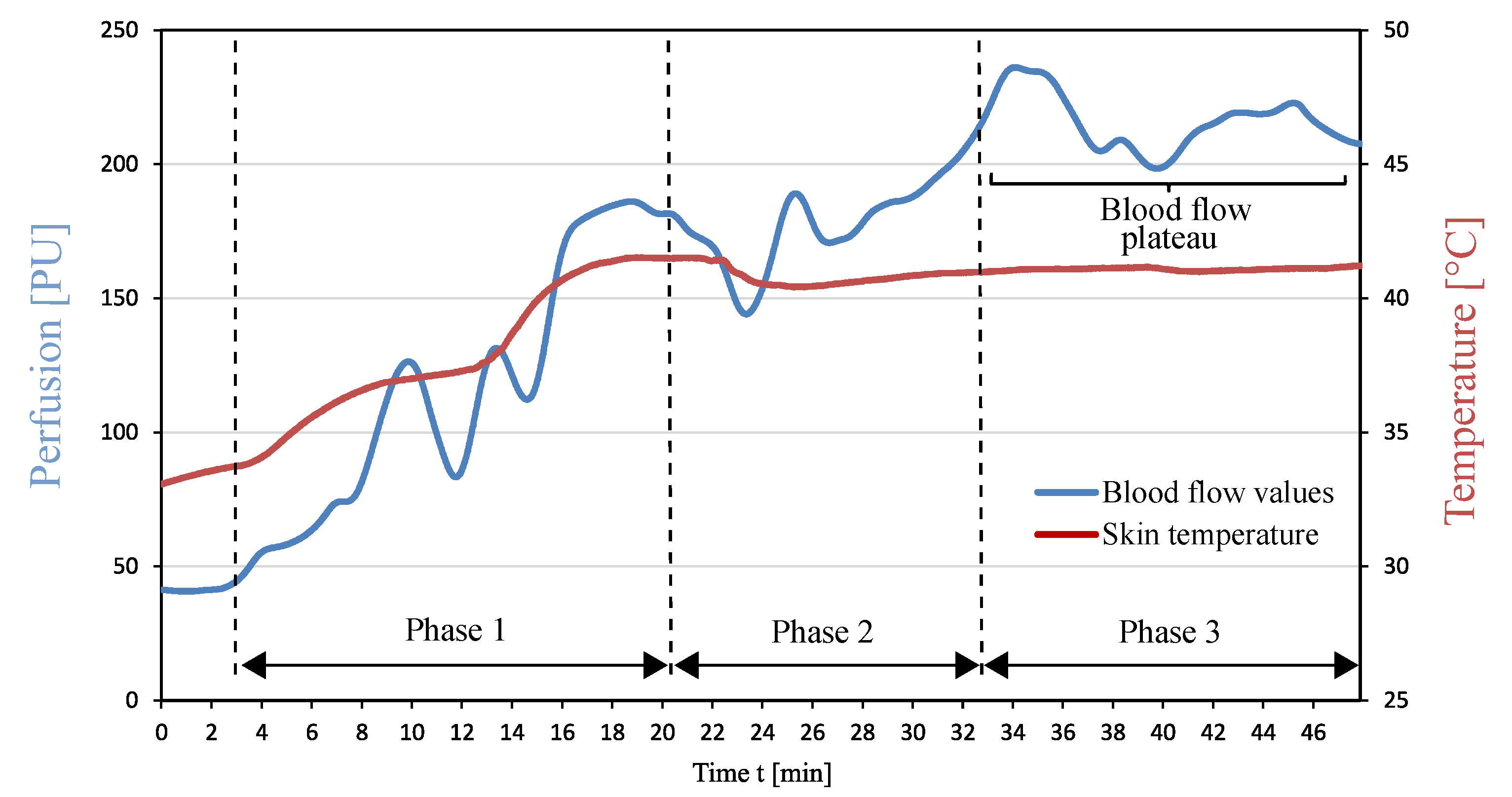
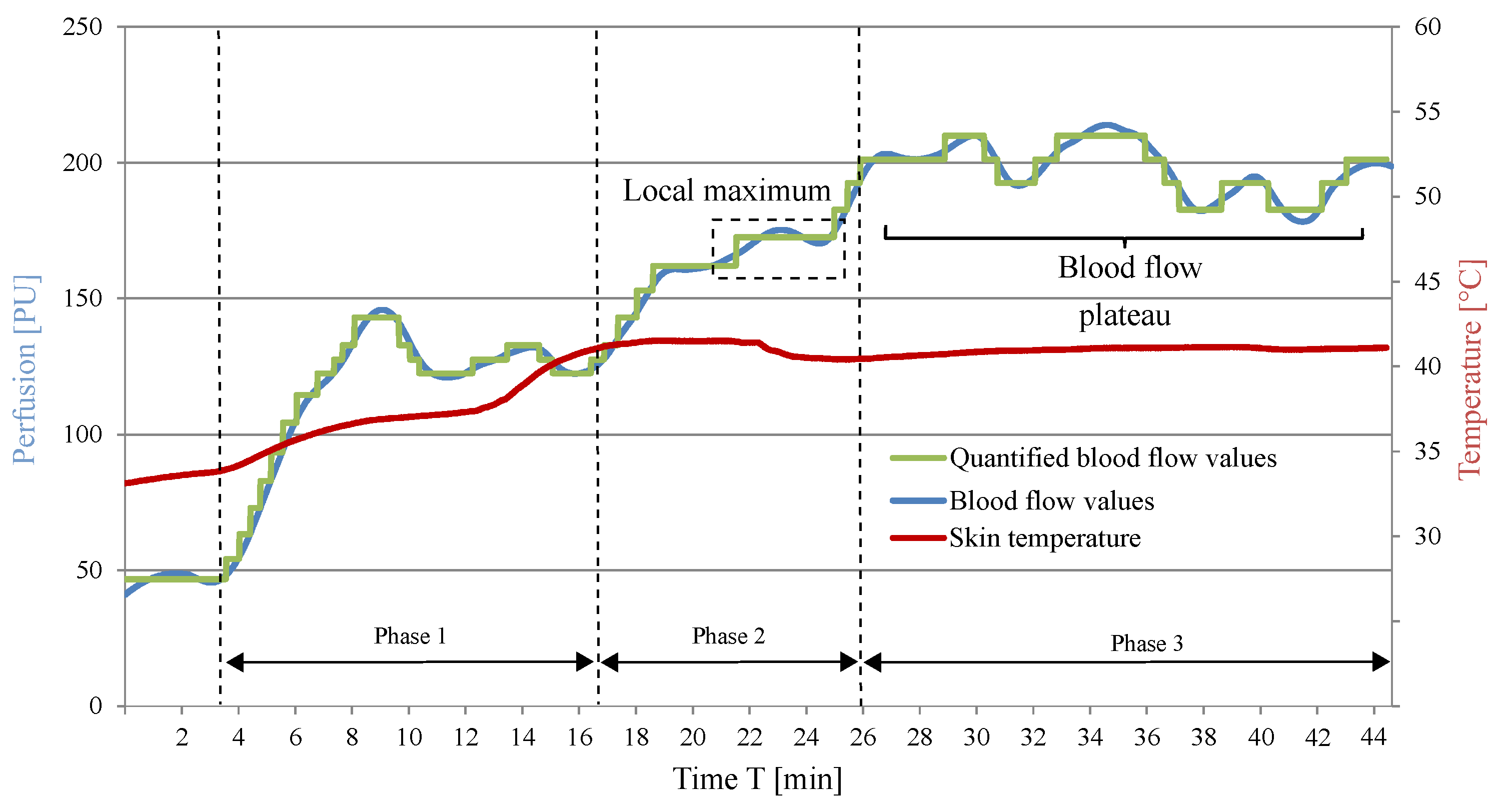
5.3. Adjustment and Determination of the Blood Flow Plateau
5.4. Future Works
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BFP | Blood flow plateau |
| CF | Carbon fibre |
| HE | Heating element |
| LDPM | Laser Doppler Perfusion Monitor |
| PU | Perfusion units |
References
- Hume, D.M. Pheochromocytoma in the adult and in the child. Am. J. Surg. 1960, 99, 458–496. [Google Scholar] [CrossRef]
- Barontini, M.; Levin, G.; Sanso, G. Characteristics of pheochromocytoma in a 4-to 20-year-old population. Ann. N. Y. Acad. Sci. 2006, 1073, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Robles, J.F.; Mercado-Asis, L.B.; Pacak, K. Pheochromocytoma: Unmasking the Chameleon. In Endocrine Hypertension: Underlying Mechanisms and Therapy; Koch, C.A., Chrousos, G.P., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 123–148. [Google Scholar]
- Fonkalsrud, E. Pheochromocytoma in childhood. In Surgery for Endocrinological Diseases and Malformations in Childhood; Springer: Berlin, Germany, 1991; pp. 103–111. [Google Scholar]
- Bravo, E.L. Evolving concepts in the pathophysiology, diagnosis, and treatment of pheochromocytoma. Endocr. Rev. 1994, 15, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Manger, W.M.; Gifford, R.W. Pheochromocytoma: Current diagnosis and management. Clevel. Clin. J. Med. 1993, 60, 365. [Google Scholar]
- Mannelli, M. Diagnostic problems in pheochromocytoma. J. Endocrinol. Investig. 1989, 12, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Bravo, E.L.; Tarazi, R.C.; Gifford, R.W.; Stewart, B.H. Circulating and Urinary Catecholamines in Pheochromocytoma. N. Engl. J. Med. 1979, 301, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Därr, R.; Pamporaki, C.; Peitzsch, M.; Miehle, K.; Prejbisz, A.; Peczkowska, M.; Weismann, D.; Beuschlein, F.; Sinnott, R.; Bornstein, S.R.; et al. Biochemical diagnosis of phaeochromocytoma using plasma-free normetanephrine, metanephrine and methoxytyramine: Importance of supine sampling under fasting conditions. Clin. Endocrinol. 2014, 80, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Pamporaki, C.; Bursztyn, M.; Reimann, M.; Ziemssen, T.; Bornstein, S.R.; Sweep, F.C.; Timmers, H.; Lenders, J.W.; Eisenhofer, G. Seasonal variation in plasma free normetanephrine concentrations: implications for biochemical diagnosis of pheochromocytoma. Eur. J. Endocrinol. 2014, 170, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Eisenhofer, G.; Pamporaki, C.; Filippatos, A. Device for Adjusting and Determining the Blood Flow Plateau. U.S. Patent No. 15/127 558, 2017. [Google Scholar]
- Eisenhofer, G. Sympathetic nerve function. Clin. Auton. Res. 2005, 15, 264–283. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Eisenhofer, G.; Sax, F.L.; Keiser, H.R.; Kopin, I.J. Plasma norepinephrine pharmacokinetics during mental challenge. Psychosom. Med. 1987, 49, 591–605. [Google Scholar] [CrossRef]
- Best, J.D.; Halter, J.B. Release and clearance rates of epinephrine in man: Importance of arterial measurements. J. Clin. Endocrinol. Metab. 1982, 55, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Marker, J.C.; Cryer, P.E.; Clutter, W.E. Simplified measurement of norepinephrine kinetics: Application to studies of aging and exercise training. Am. J. Physiol.-Endocrinol. Metab. 1994, 267, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Rundquist, B.; Aneman, A.; Friberg, P.; Dakak, N.; Kopin, I.J.; Jacobs, M.; Lenders, J. Regional release and removal of catecholamines and extraneuronal metabolism to metanephrines. J. Clin. Endocrinol. Metab. 1995, 80, 3009–3017. [Google Scholar] [PubMed]
- Eisenhofer, G.; Lenders, J. Rapid circulatory clearances and half-lives of plasma free metanephrines. Clin. Endocrinol. 2012, 77, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Minson, C.T.; Berry, L.T.; Joyner, M.J. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J. Appl. Physiol. 2001, 91, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.L.; Liu, Y.; Kosiba, I.F.; O’Donnell, D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J. Appl. Physiol. 1999, 86, 1185–1190. [Google Scholar] [CrossRef]
- A Carberry, P.; Shepherd, A.; Johnson, J. Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension 1992, 20, 349–355. [Google Scholar] [CrossRef]
- Sandeman, D.D.; Pym, C.A.; Green, E.M.; Seamark, C.; Shore, A.C.; Tooke, J.E. Microvascular vasodilatation in feet of newly diagnosed non-insulin dependent diabetic patients. BMJ Br. Med. J. 1991, 302, 1122–1123. [Google Scholar] [CrossRef]
- Roustit, M.; Cracowski, J.L. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol. Sci. 2013, 34, 373–384. [Google Scholar] [CrossRef]
- Sokolnicki, L.A.; Roberts, S.K.; Wilkins, B.W.; Basu, A.; Charkoudian, N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E314–E318. [Google Scholar] [CrossRef] [Green Version]
- Kane, J.R.; Christensen, S.A.; Hamilton, N.; Williams, S.J. Methods and Apparatus for Adjusting Blood Circulation. U.S. Patent 8,603,150, 10 December 2013. [Google Scholar]
- Christensen, S.A.; Kane, J.R.; Hamilton, N. Methods and Apparatus for Enhancing Vascular Access in an Appendage to Enhance Therapeutic and Interventional Procedures. U.S. Patent 8,771,329, 8 July 2014. [Google Scholar]
- Peret, J.; Grisanti, L.C.; Kedge, G.E.; Cazzini, K.; McGee, J. Heat Transfer Device: Seal and Thermal Energy Contact Units. U.S. Patent 11/893,446, 19 February 2009. [Google Scholar]
- Chen, C.C. Electro-Thermal Protecting Device for Heat Treatment. U.S. Patent 12/379,313, 19 August 2010. [Google Scholar]
- Bonner, L.H. Thermal, Outer Layer Leg Wrap Device. U.S. Patent App. 12/378,562, 17 February 2009. [Google Scholar]
- Stoppa, M.; Chiolerio, A. Wearable electronics and smart textiles: A critical review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed]
- Tabrizchi, R.; Pugsley, M.K. Methods of blood flow measurement in the arterial circulatory system. J. Pharmacol. Toxicol. Methods 2000, 44, 375–384. [Google Scholar] [CrossRef]
- Perimed, A. Periflux System 5000 Instrument Version 1.70 a 1.79 Extended User Manual, Instrument version 1; Perimed AB: Järfälla, Sweden, 2012. [Google Scholar]
- Shepherd, A.P.; Öberg, P.Å. Laser-Doppler Blood Flowmetry; Springer Science & Business Media: Berlin, Germany, 2013; Volume 107. [Google Scholar]
- Brandes, J.; Funke, H.; Meyer, J. Elektrisch beheizbares Formwerkzeug in Kunststoffbauweise. Fachhochschule Dortmund. Germany Patent No. DE102006058198 Al, 12 June 2008. [Google Scholar]
- Göbel, M. Faser-Kunststoff-Metall-Glas-Hybridsysteme und deren Einsatz in Tragenden Konstruktionen: Beitrag zur Entwicklung Hocheffizienter Struktureller Hybridsysteme unter Anwendung Moderner Faserwerkstoffe, Kunststoffe und Klebstoffe. Ph.D. Thesis, Bauhaus-Universität Weimar, Weimar, Germany, July 2013. [Google Scholar]
- Rip Curl H-Bomb Wetsuit. Available online: https://blog.kingofwatersports.com/2008/11/rip-curl-h-bomb-wetsuit/ (accessed on 26 November 2018).
- Morin, P.K.; Tomalesky, S.G. Carbon Fiber Heating Element. U.S. Patent 9,968,214, 15 May 2018. [Google Scholar]
- Li, C.L.; Hsiung, H.H.; Huang, C.T.; Chu, C.K. Electric Heating Textile. U.S. Patent 7,884,307, 2011. [Google Scholar]
- Hao, J.; Ghosh, P.; Li, S.K.; Newman, B.; Kasting, G.B.; Raney, S.G. Heat effects on drug delivery across human skin. Expert Opin. Drug Deliv. 2016, 13, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.; Kellogg, D., Jr.; Johnson, J.; Kosiba, W.; Solomon, D. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am. J. Physiol.-Heart Circ. Physiol. 1993, 265, H785–H792. [Google Scholar] [CrossRef] [PubMed]
- Barcroft, H.; Edholm, O. The effect of temperature on blood flow and deep temperature in the human forearm. J. Physiol. 1943, 102, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, H.; Lang, K.H.; Schäfer, A.; Schultetus, W. Anthropometrische Daten in Normen: Bestandsaufnahme und Bedarfsanalyse unter besonderer Berücksichtigung des Arbeitsschutzes; Kommission Arbeitsschutz und Normung: Sankt Augustin, Germany, 2009. [Google Scholar]
- Jürgens, H. Erhebung Anthropometrischer Maße zur Aktualisierung der DIN 33 402-Teil 2; Bundesanstalt für Arbeitsschutz und Arbeitsmedizin: Dortmund, Germany, 2004. [Google Scholar]
- Schade, M. Embroidered Semi-finished Products and Embroidery Techniques. In Textile Materials for Lightweight Constructions; Springer: Berlin, Germany, 2016; pp. 347–360. [Google Scholar]






| Criteria | Description of the Criteria |
|---|---|
| Criterion 1 | Warming of the complete upper arm up to the shoulder attachment of the patient |
| Criterion 2 | Easy adaptation of the sleeve to the patient’s arm circumference to ensure heat transfer to the patient’s skin surface |
| Criterion 3 | Freedom of movement in the area surrounding the hand |
| Criterion 4 | Cut-outs for two Laser Doppler Probes of the LDPM in the area of the forearm |
| Criterion 5 | Integration of a blood sampling zone providing access to the required permanent cannula |
| Criterion 6 | Customisability to different arm measurements |
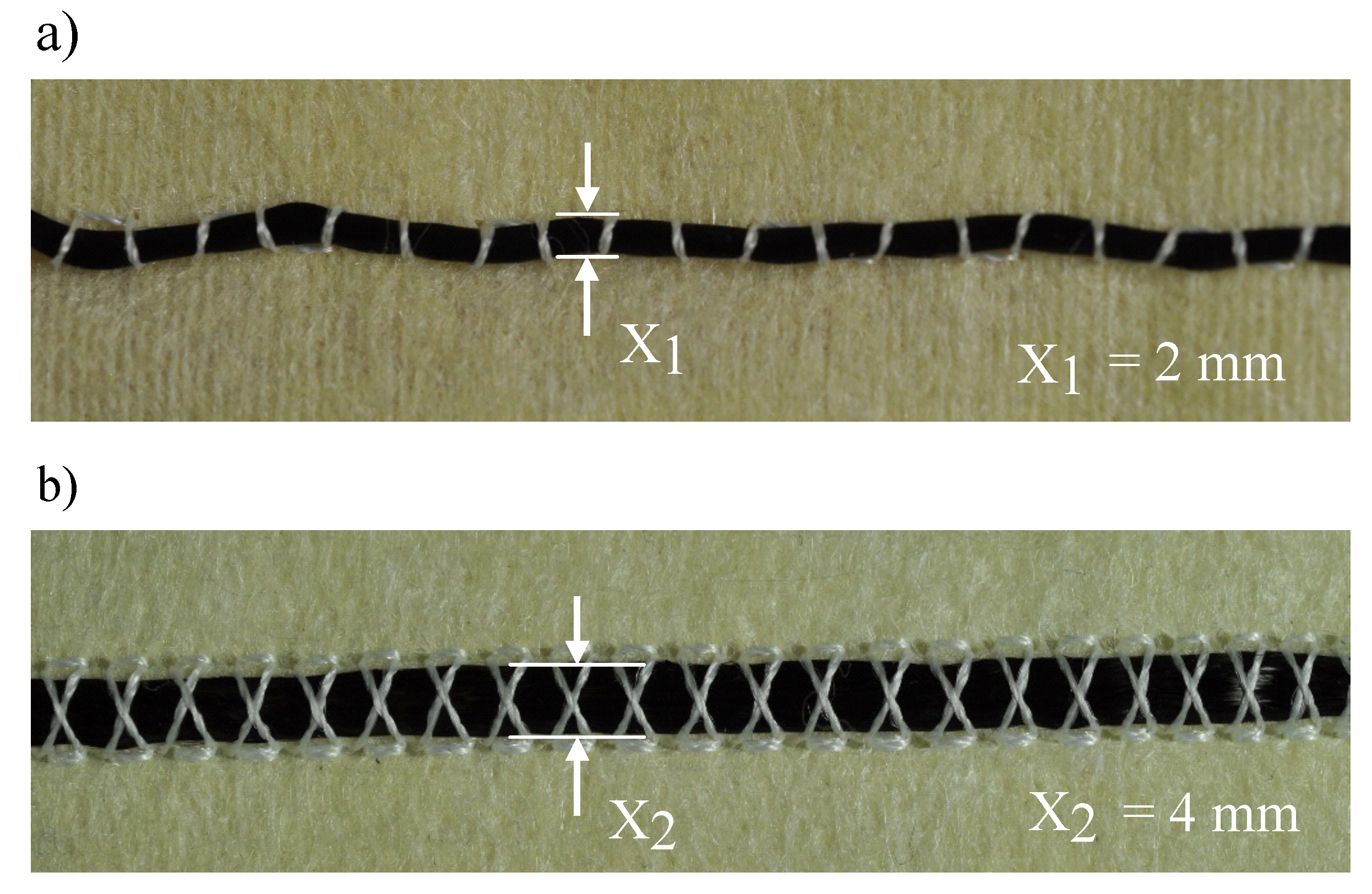
| Combination | Type of Strand | Upper Thread | (Textile) | (Crimp) |
|---|---|---|---|---|
| 1 | round strand | conductive | 0.96 | 1.31 |
| 2 | round strand | non-conductive | 1.00 | 1.29 |
| 3 | braided strand | conductive | 0.56 | 1.27 |
| 4 | braided strand | non-conductive | 0.87 | 1.29 |
| Conditions | Description |
|---|---|
| Condition 1 | At least 25 min must elapse between the beginning of the warming process and the time of possible blood collection, in order to prevent premature blood sample collection. |
| Condition 2 | The blood flow remains constant for at least 3 min after 25 min of the monitoring procedure went by. |
| Condition 3 | If the blood flow drops after the 3 min as defined in Condition 2, the previously measured blood flow value is identified as the BFP. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neubauer, M.; Häntzsche, E.; Pamporaki, C.; Eisenhofer, G.; Dannemann, M.; Nocke, A.; Modler, N.; Filippatos, A. Development of a Function-Integrative Sleeve for Medical Applications. Sensors 2019, 19, 2588. https://doi.org/10.3390/s19112588
Neubauer M, Häntzsche E, Pamporaki C, Eisenhofer G, Dannemann M, Nocke A, Modler N, Filippatos A. Development of a Function-Integrative Sleeve for Medical Applications. Sensors. 2019; 19(11):2588. https://doi.org/10.3390/s19112588
Chicago/Turabian StyleNeubauer, Moritz, Eric Häntzsche, Christina Pamporaki, Graeme Eisenhofer, Martin Dannemann, Andreas Nocke, Niels Modler, and Angelos Filippatos. 2019. "Development of a Function-Integrative Sleeve for Medical Applications" Sensors 19, no. 11: 2588. https://doi.org/10.3390/s19112588









