Fast Hydrogenation and Dehydrogenation of Pt/Pd Bimetal Decorated over Nano-Structured Ag Islands Grown on Alumina Substrates
Abstract
:1. Introduction
2. Experimental Section
2.1. Device Fabrication
2.2. Characterization
2.3. Sensing Measurement
3. Results and Discussion
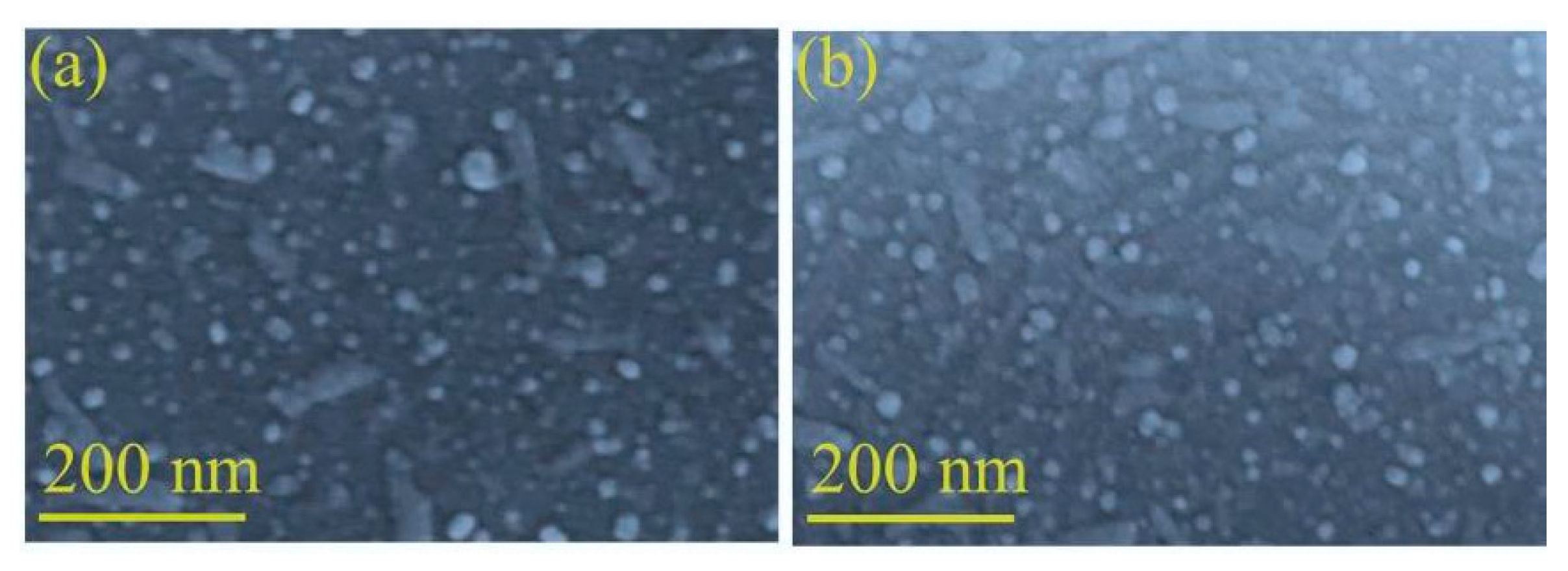
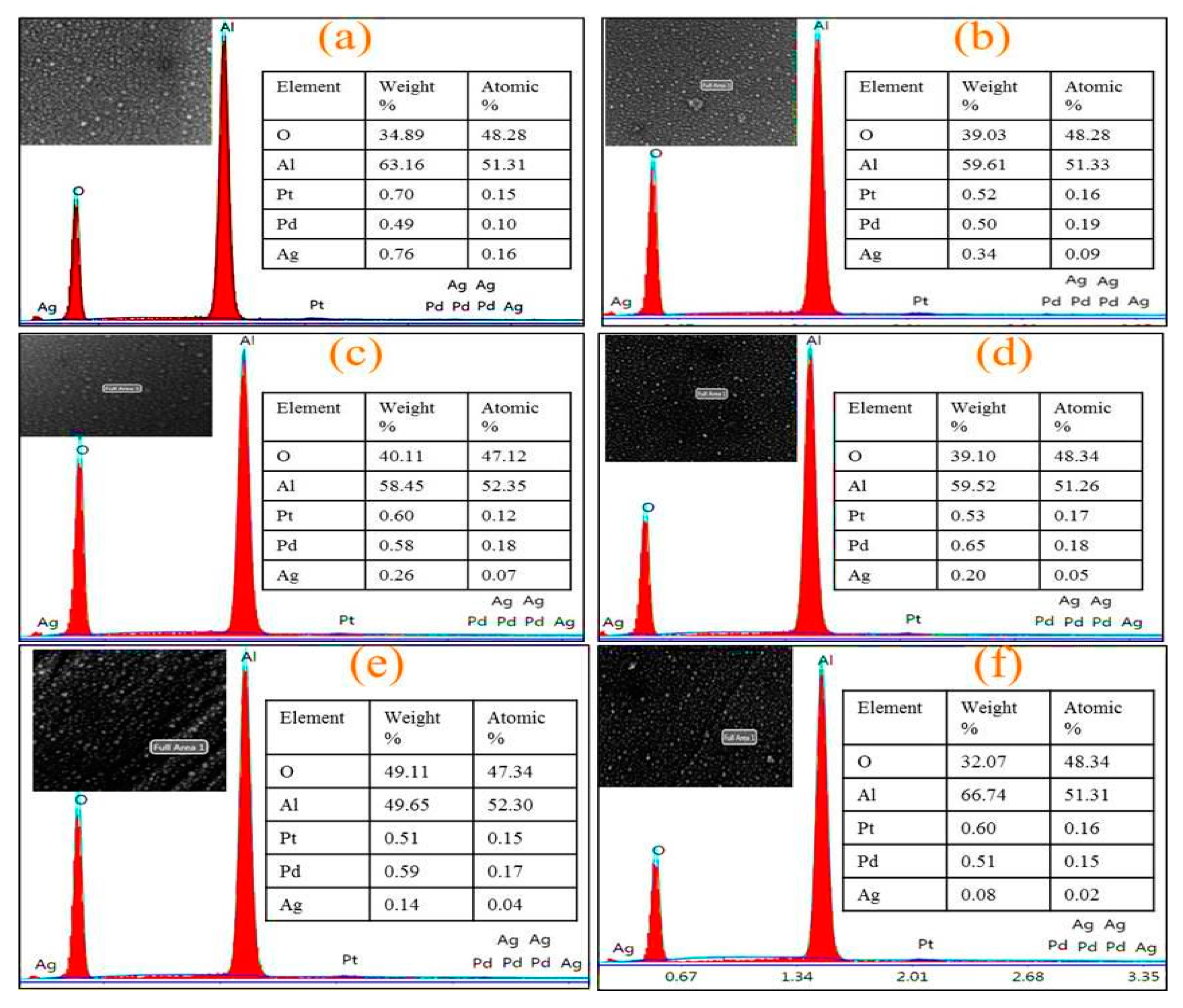
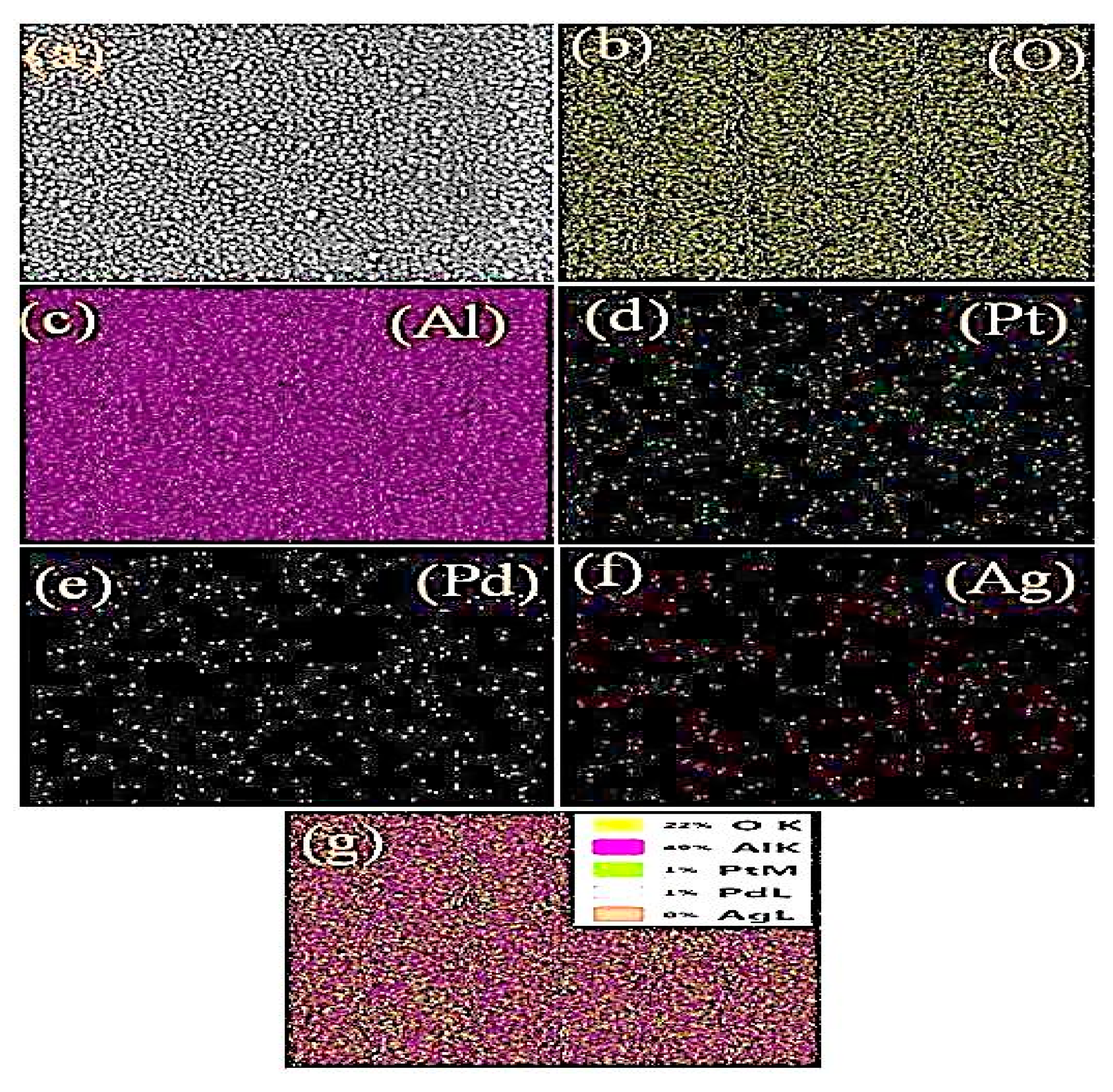
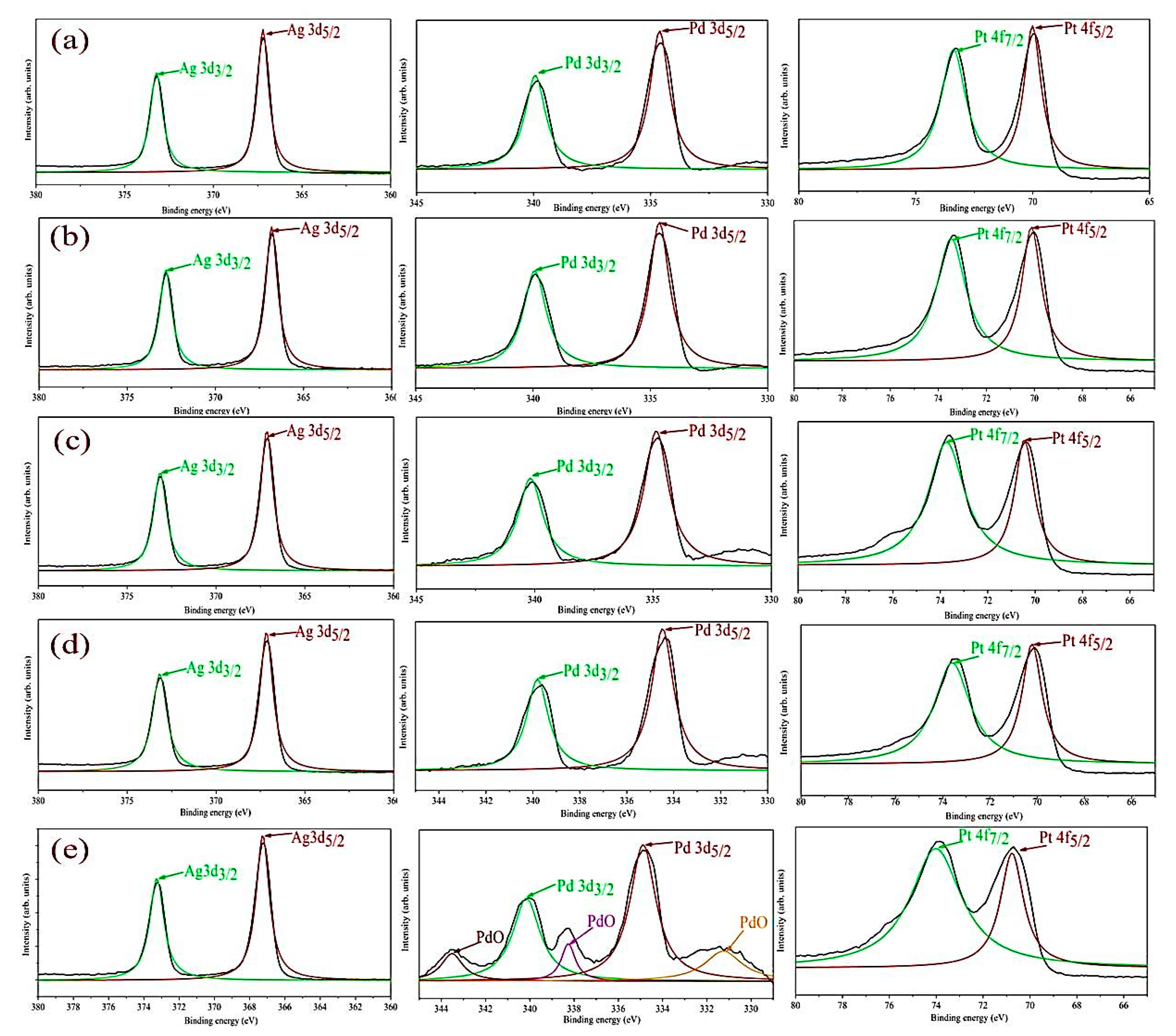
3.1. Materials Structure and Morphology
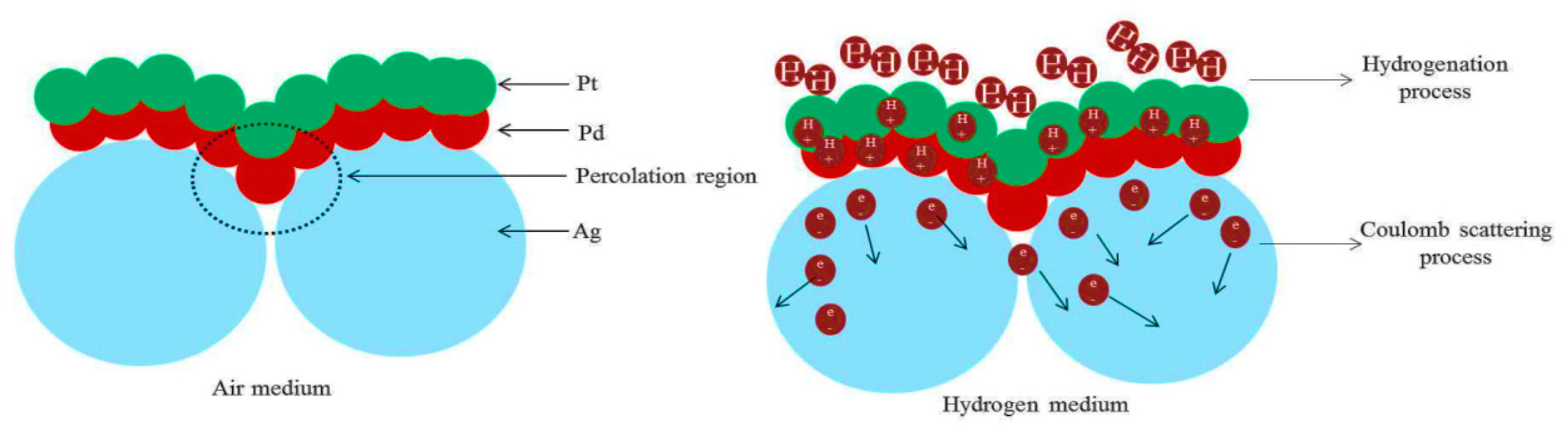
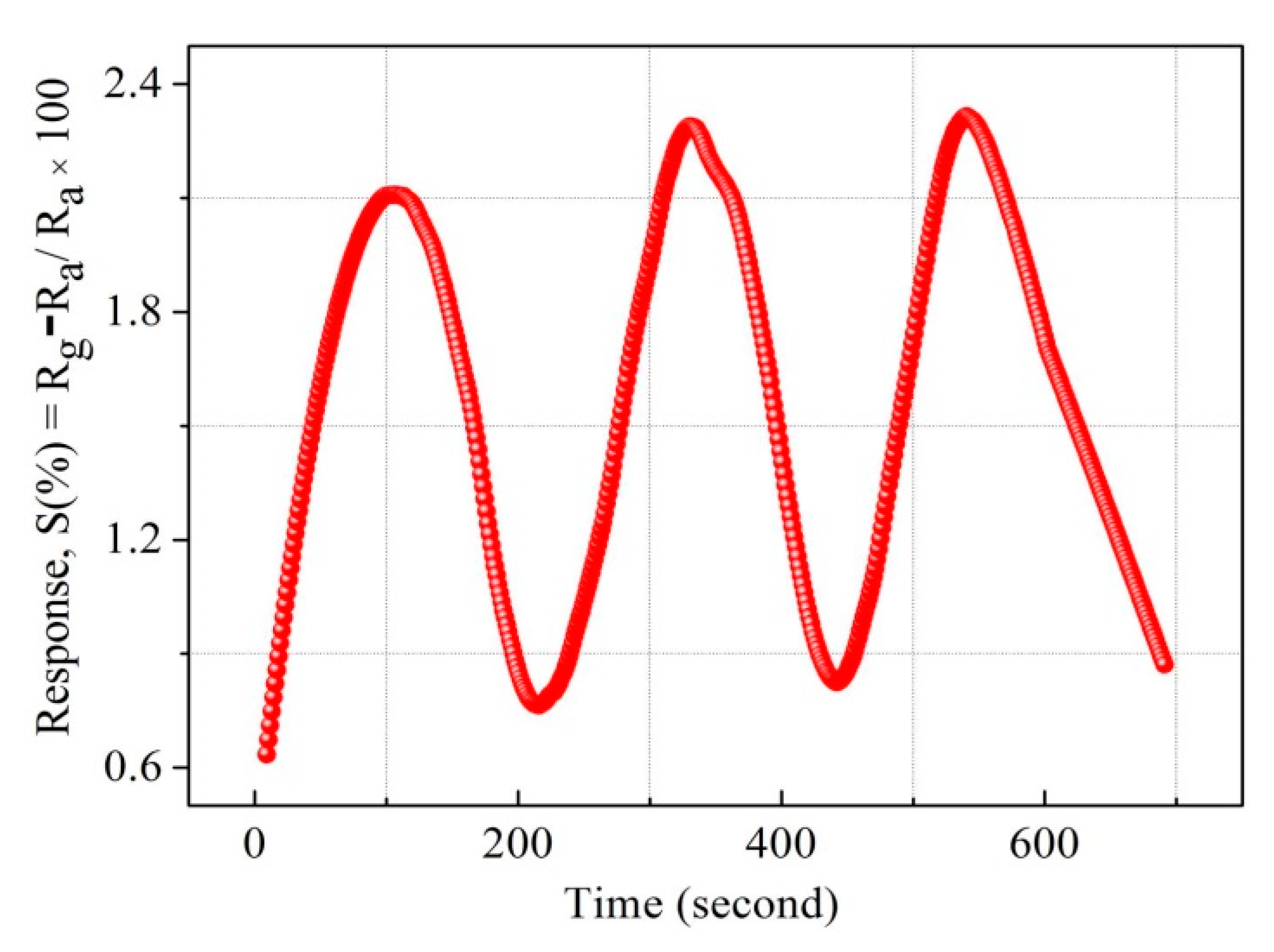
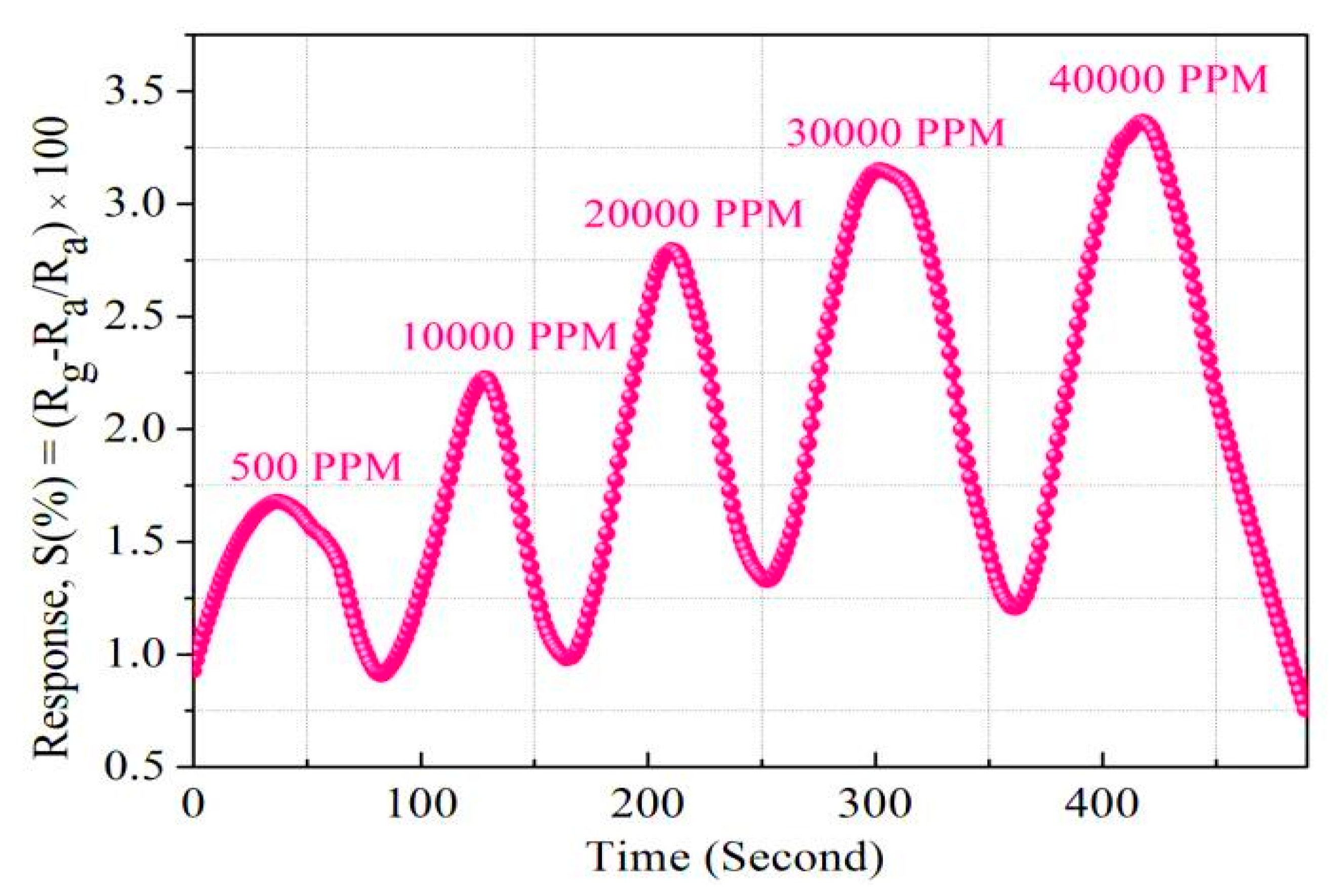
3.2. Hydrogenation and Dehydrogenation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grochala, W. First Tere was Hydrogen. Nat. Chem. 2015, 7, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.T.; Sutapun, B.; Petrick, R.; Kazemi, A. Highly sensitive hydrogen sensors using palladium coated fiber optics with exposed cores and evanescent filed interaction. Sens. Actuators B 1999, 56, 158–163. [Google Scholar] [CrossRef]
- Karim, G.A. Hydrogen as a spark ignition engine fuel. Int. J. Hydrog. Energy 2003, 28, 569–577. [Google Scholar] [CrossRef]
- Firth, J.G.; Jones, A.; Jones, T.A. The principles of the detection of flammable atmospheres by catalytic devices. Combust. Flame 1973, 20, 303–311. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Q.; Zhang, S.; Zong, P.; Yang, F. Highly sensitive and selective hydrogen gas sensor using mesoporous SnO2 modified layers. Sensors 2017, 10, 2351. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.K.; Gong, D.; Paulose, M.; Ong, K.G.; Grimes, C.A. Hydrogen sensing using titania nanotubes. Sens. Actuators B 2003, 93, 338–344. [Google Scholar] [CrossRef]
- Rout, C.S.; Krishna, S.H.; Vivekchand, S.R.C.; Govindaraj, A.; Rao, C.N.R. Hydrogen and ethanol sensors based on ZnO nanorods, nanowires and nanotubes. Chem. Phys. Lett. 2006, 418, 586–590. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, L.F.; Yang, Y.H.; Xu, N.S.; Yang, G.W. Fabrication of a SnO2 nanowire gas sensor and sensor performance for hydrogen. J. Phys. Chem. C 2008, 112, 6643–6647. [Google Scholar] [CrossRef]
- Shegai, T.; Langhammer, C. Hydride formation in single palladium and magnesium nanoparticles studied by nanoplasmonic dark-field scattering spectroscopy. Adv. Mater. 2011, 23, 4409–4414. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.A. The Palladium Hydrogen System; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Ingham, B.; Toney, M.F.; Hendy, S.C.; Cox, T.; Fong, D.D.; Eastman, J.A.; Fuoss, P.H.; Stevens, K.J.; Lassesson, A.; Brown, S.A.; et al. Particle size effect of hydrogen-induced lattice expansion of palladium nanoclusters. Phys. Rev. B 2008, 78, 245408. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yang, M.; Hong, S.H. H2 sensing characteristics of highly textured Pd-doped SnO2 thin films. Sens. Actuators B 2008, 134, 117–121. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Phanichphant, S. H2 sensing response of flame spray-made Ru/SnO2 thick films fabricated from spin-coated nanoparticles. Sensors 2009, 9, 8996–9010. [Google Scholar] [CrossRef]
- Kiefer, T.; Favier, F.; Vazquez-Mena, O.; Villanueva, G.; Brugger, J. A single nanotrench in a palladium microwire for hydrogen detection. Nanotechnology 2008, 19, 125502. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.J. Highly sensitive MEMS-type micro sensor for hydrogen gas detection by modifying the surface morphology of Pd catalytic metal. Korean J. Mater. Res. 2014, 24, 527–532. [Google Scholar] [CrossRef]
- Hughes, R.C.; Schubert, W.K.; Zipperian, T.E.; Rodriguez, J.L.; Plut, T.A. Thin-film palladium and silver alloys and layers for metal-insulator-semiconductor sensors. J. Appl. Phys. 1987, 62, 1074–1083. [Google Scholar] [CrossRef]
- Othonos, A.; Kalli, K.; Tsai, D.P. Optically thin palladium films on silicon-based substrates and nanostructure formation: Effects of hydrogen. Appl. Surf. Sci. 2000, 161, 54–60. [Google Scholar] [CrossRef]
- Goltsova, M.V.; Artemenko, Y.A.; Zaitsev, V.I. Kinetics and morphology of the reverse β/α hydride transformation in thermodynamically open Pd-H system. J. Alloys Compd. 1999, 293–295, 379–384. [Google Scholar] [CrossRef]
- Randeniya, L.K.; Martin, P.J.; Bendavid, A. Detection of hydrogen from 5 ppm to 4 % using multi-walled carbon nanotube yarns coated with nanocrystalline Pd and Pd/Pt layered structures. Carbon 2011, 12, 26. [Google Scholar]
- Chen, H.-S.; Chiu, J.-J.; Perng, T.-P. On the photoluminescence of si nanoparticles. Mater. Phys. Mech. 2001, 4, 62–66. [Google Scholar]
- Pundt, A. Hydrogen in nano-sized metals. Adv. Eng. Mater. 2004, 6, 11–22. [Google Scholar] [CrossRef]
- Phan, D.-T.; Chung, G.-S. A novel Pd nanocube–graphene hybrid for hydrogen detection. Sensors Actuators B 2014, 199, 354–360. [Google Scholar] [CrossRef]
- Offermans, P.; Tong, H.D.; Rijn, C.J.M.V.; Merken, P.; Brongersma, S.H.; Calama, M.C. Ultralow-power hydrogen sensing with single palladium nanowires. Appl. Phys. Lett. 2009, 94, 223110. [Google Scholar] [CrossRef]
- Jeon, K.J.; Lee, J.M.; Lee, E.; Lee, W. Individual Pd nanowire hydrogen sensors fabricated by electron-beam lithography. Nanotechnology 2009, 20, 135502. [Google Scholar] [CrossRef] [PubMed]
- Favier, F.; Walter, E.C.; Zach, M.P.; Benter, T.; Penner, R.M. Hydrogen sensors and switches from electrodeposited palladium mesowire arrays. Science 2001, 293, 2227–2231. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, T.; Villanueva, L.; Fargier, F.; Favier, F.; Brugger, J. Fast and robust hydrogen sensors based on discontinuous palladium films on polyimide, fabricated on a wafer scale. Nanotechnology 2010, 21, 505501. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Varandani, D.; Mehta, B.R.; Singh, V.N.; Wen, Z.H.; Feng, X.L.; Mullen, K. Fast response and recovery of hydrogen sensing in Pd-Pt nanoparticle-graphene composite layers. Nanotechnology 2011, 22, 275719. [Google Scholar] [CrossRef]
- Yang, F.; Taggart, D.K.; Penner, R.M. Fast, sensitive hydrogen gas detection using single palladium nanowires that resist fracture. Nano Lett. 2009, 9, 2177–2182. [Google Scholar] [CrossRef]
- Yang, F.; Kung, S.C.; Taggart, D.K.; Penner, R.M. Hydrogen sensing with a single palladium nanowires. Sens. Lett. 2010, 8, 534–538. [Google Scholar] [CrossRef]
- Peng, Y.; Ye, J.; Zhenga, L.; Zouab, K. The hydrogen sensing properties of Pt–Pd/reduced graphene oxide based sensor under different operating conditions. RSC Adv. 2016, 6, 24880. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.-K. Pd/Ag alloy as an application for hydrogen sensing. Int. J. Hydrog. Energy 2017, 42, 25446–25452. [Google Scholar] [CrossRef]
- Gryaznov, V. Metal containing membranes for the production of ultrapure hydrogen and the recovery of hydrogen isotopes. Sep. Purif. Methods Rev. 2000, 29, 171–187. [Google Scholar] [CrossRef]
- Quan, J.; Zhang, J.; Qi, X.; Li, J.; Wang, N.; Zhu, Y. A study on the corre;lation between the dewetting temperature of Ag film and SERS intensity. Sci. Rep. 2017, 7, 14771. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Martinez-Gil, A.; Agnus, G.; Moyen, E.; Maroutian, T.; Bartenlian, B.; Mégy, R.; Hanbücken, M.; Beauvillain, P. Au island growth on a si(1 1 1) vicinal surface. Surf. Sci. 2016, 600, 1207–1212. [Google Scholar] [CrossRef]
- Rufno, F.; Cacciato, G.; Grimaldi, M.G. Surface difusion coefcient of au atoms on single layer graphene grown on cu. J. Appl. Phys. 2014, 115, 666. [Google Scholar]
- Jiran, E.; Tompson, C.V. Capillary instabilities in thin flms. J. Electron. Mater. 1990, 19, 1153–1160. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Wang, L.; Polyakova Stolyarova, E.; Taniguchi, T.; Watanabe, K.; Hone, J.; Flynn, G.W.; Brus, L.E. Slow gold adatom difusion on graphene: Efect of silicon dioxide and hexagonal boron nitride substrates. J. Phys. Chem. B 2013, 117, 4305. [Google Scholar] [CrossRef] [PubMed]
- Rha, J.J.; Park, J.K. Stability of the grain confgurations of thin flms—A model for agglomeration. J. Appl. Phys. 1997, 82, 1608–1616. [Google Scholar] [CrossRef]
- Huang, C.W.; Lin, B.J.; Lin, H.Y.; Huang, C.H.; Shih, F.Y.; Wang, W.H.; Liu, C.Y.; Chui, H.C. Surface-enhanced raman scattering of suspended monolayer graphene. Nanoscale Res. Lett. 2013, 82, 480. [Google Scholar] [CrossRef]
- Qiu, C.; Zhou, H.; Cao, B.; Sun, L.; Yu, T. Raman spectroscopy of morphology-controlled deposition of au on graphene. Carbon 2013, 59, 487–494. [Google Scholar] [CrossRef]
- Zhang, J.; Pengyue, Z.; Yimin, D.; Xiaolei, Z.; Jiamin, Q.; Yong, Z. Ag-cu nanoparticles encaptured by graphene with magnetron sputtering and cvd for surface-enhanced raman scattering. Plasmonics 2016, 11, 1495–1504. [Google Scholar]
- Mo, Y.W.; Kleiner, J.; Webb, M.B.; Lagally, M.G. Activation energy for surface difusion of si on si(001): A scanning-tunnelingmicroscopy study. Phys. Rev. Lett. 1991, 66, 1998. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Alford, T.L.; Allee, D.R. Tickness dependence on the thermal stability of silver thin flms. Appl. Phys. Lett. 2002, 81, 4287–4289. [Google Scholar] [CrossRef]
- Duyne, R.P.V.; Hulteen, J.C.; Treichel, D.A. Atomic force microscopy and surface-enhanced raman spectroscopy. I. Ag island flms and ag flm over polymer nanosphere surfaces supported on glass. J. Chem. Phys. 1993, 99, 2101–2115. [Google Scholar] [CrossRef]
- Chiu, S.M.; Hwang, S.J.; Chu, C.W.; Gan, D. The influence of Cr-based coating on the adhesion force between epoxy molding compounds and IC encapsulation mold. Thin Solid Films 2006, 515, 285–292. [Google Scholar] [CrossRef]
- Jung, D.; Han, M.; Lee, G.S. Fast-Response Room Temperature Hydrogen Gas Sensors Using Platinum-Coated Spin-Capable Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 3050–3057. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C.; Owens, D.K.; Wendt, R.C. Estimation of surface free energy of polymers. Appl. Polym. Sci. 1969, 13, 1741. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kobayashi, H.; Kitagawa, H. Hydrogen storage mediated by Pd and Pt nanoparticles. Chem. Phys. Chem. 2009, 10, 2566–2576. [Google Scholar] [CrossRef]
- Temsta, K.; van Baela, M.J.; van Haesendoncka, C.; Bruynseraedea, Y.; de Grootb, D.G.; Koemanb, N.; Griessenb, R. An X-ray diffraction study of interface roughness and diffusion in Ag/Pd superlattices. Thin Solid Films 1999, 342, 174–179. [Google Scholar] [CrossRef]
- Dorranian, D.; Tajmir, S.; Khazanehfar, F. Effect of Laser Fluence on the Characteristics of Ag Nanoparticles Produced by Laser Ablation. Soft Nanosci. Lett. 2013, 3, 93–100. [Google Scholar] [CrossRef]
- Salem, M.A.; Bakr, E.A.; El-Attar, H.G. Pt@Ag and Pd@Ag core/shell nanoparticles for catalytic degradation of Congo red in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cao, G. Synthesis and structural investigation of Pd/Ag bimetallic nanoparticles prepared by the solvothermal method. J. Nanopart. Res. 2007, 9, 1153–1161. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Yan, Z.; Jing, J.; Xie, J.; Chen, M. Improved catalytic activity of cobalt core–platinum shell nanoparticles supported on surface functionalized graphene for methanol electro-oxidation. Electrochim. Acta 2015, 158, 81–88. [Google Scholar]
- Alfonso, E.; Olaya, J.; Cubillos, G. Thin Film Growth Through Sputtering Technique and Its Application; INTECH Open Access Publisher: New York, NY, USA, 2012. [Google Scholar]
- Li, Y.; Wang, Z.W.; Chiu, C.Y.; Ruan, L.; Yang, W.; Yang, Y.; Palmer, R.E.; Huang, Y. Synthesis of bimetallic Pt-Pd core-shell nanocrystals and their high electrocatalytic activity modulated by Pd shell thickness. Nanoscale 2012, 4, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, G.; Chen, Y.; Lu, T.; Tang, Y.; Xing, W. A strategy for fabricating porous PdNi@ Pt core-shell nanostructures and their enhanced activity and durability for the methanol electrooxidation. Sci. Rep. 2015, 5, 7619. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shim, W.; Lee, E.; Noh, J.S.; Lee, W. Highly mobile palladium thin films on an elastomeric substrate: Nano gap based hydrogen gas sensors. Angew. Chem. Int. Ed. 2011, 50, 5301–5305. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Ogawa, H.; Yamazaki, Y.; Ishizaki, A.; Nakagiri, T. Quantum size effects on photoluminescence in ultrafine Si particles. Appl. Phys. Lett. 1990, 56, 2379. [Google Scholar] [CrossRef]
- Morisaki, H.; Ping, F.W.; Ono, H.; Yazawa, K. Above-band-gap photoluminescence from Si fine particles with oxide shell. J. Appl. Phys. 1991, 70, 1869. [Google Scholar] [CrossRef]
- Morisaki, H. Above-band-gap photoluminescence from Si fine particles. Nanotechnology 1992, 3, 196. [Google Scholar] [CrossRef]
- Zakaria, R.; Hamdan, K.S.; Noh, S.M.C.; Supangat, A.; Sookhakian, M. Surface plasmon resonance and photoluminescence studies of Au and Ag micro-flowers. Opt. Mater. Express 2015, 5, 943–950. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Y.; Fang, Y. Spectroscopy property of ag nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Suna, T.; Xua, Z.; Zhaoa, W.; Wua, X.; Liua, S.; Zhanga, Z.; Wanga, S.; Liua, W.; Liub, S.; Peng, J. Fabrication of the similar porous alumina silicon template for soft uv nanoimprint lithography. Appl. Surf. Sci. 2013, 276, 363–368. [Google Scholar] [CrossRef]
- Wadell, C.; Syrenova, S.; Langhammer, C. Plasmonic hydrogen sensing with nanostructured metal hydrides. ACS Nano 2014, 8, 11925. [Google Scholar] [CrossRef] [PubMed]
- Kaniyoor, A.; Imran Jafri, R.; Arockiadoss, T.; Ramaprabhu, S. Nanostructured Pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor. Nanoscale 2009, 1, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Donavan, K.C.; Kung, S.C.; Penner, R.M. The surface scattering-based detection of hydrogen in air using a platinum nanowire. Nano Lett. 2012, 12, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Luntz, A.C.; Williams, M.D.; Bethune, D.S. Oxygen interactions with the Pt (111) surface. Surf. Sci. 1980, 12, 656. [Google Scholar]
- Fisher, G.B.; Gland, J.L. The interaction of water with the Pt (111) surface. Surf. Sci. 1980, 94, 446–455. [Google Scholar] [CrossRef]
- Obayashi, H.; Yamauchi, M.; Kitagawa, H.; Kubota, Y.; Kato, K.; Takata, M. Hydrogen absorption in the core/shell interface of Pd/Pt nanoparticles. J. Am. Chem. Soc. 2008, 130, 1818–1819. [Google Scholar] [CrossRef]
- Ruths, P.F.; Ashok, S.; Fonash, S.J.; Ruths, J.M. A study of Pd/Si MIS schottky barrier diode hydrogen detector. IEEE Trans. Electron. Devices 1981, 28, 1003–1009. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Hemminger, J.C.; Penner, R.M. Catalytically activated palladium@platinum nanowires for accelerated hydrogen gas detection. ACS Nano 2015, 9, 3215–3225. [Google Scholar] [CrossRef]
- Gland, J.L.; Sexton, B.A.; Fisher, G.B. Oxygen interactions with the Pt (111) surface. Surf. Sci. 1980, 95, 587–602. [Google Scholar] [CrossRef]
- Johansson, M.; Ekedahl, L. Hydrogen adsorbed on palladium during water formation studied with palladium membranes. Appl. Surf. Sci. 2001, 173, 122–133. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.M.; Lee, E.; Noh, J.-S.; Joe, J.H.; Jung, B.; Lee, W. Hydrogen gas sensing performance of Pd–Ni alloy thin films. Thin Solid Films 2010, 519, 880–884. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.-S. MEMS based highly sensitive dual FET gas sensor using graphene decorated Pd-Ag alloy nanoparticles for H2 detection. Sci. Rep. 2018, 8, 5902. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.; Uddin, A.S.M.I.; Chung, G.-S. Fast-response hydrogen sensors based on discrete Pt/Pd bimetallic ultra-thin films. Sens. Actuators B 2016, 234, 435–445. [Google Scholar] [CrossRef]
- Zeng, X.-Q.; Wang, Y.-L.; Deng, H.; Latimer, M.L.; Xiao, Z.-L.; Pearson, J.; Xu, T.; Wang, H.-H.; Welp, U.; Crabtree, G.W.; et al. Networks of ultrasmall Pd/Cr nanowires as high performance hydrogen sensors. ACS Nano 2011, 5, 7443–7452. [Google Scholar] [CrossRef] [PubMed]
- Uddin, A.S.M.I.; Yaqoob, U.; Hassan, K.; Chung, G.-S. Effects of Pt shell thickness on self-assembly monolayer Pd@Pt core-shell nanocrystals based hydrogen sensing. Int. J. Hydrog. Energy 2016, 41, 15399–15410. [Google Scholar] [CrossRef]
- Yoshimura, K.; Nakano, S.; Uchinashi, S.; Yamaura, S.; Kimura, H.; Inoue, A. A hydrogen sensor based on Mg–Pd alloy thin film. Meas. Sci. Technol. 2007, 18, 3335–3338. [Google Scholar] [CrossRef]











| Sample | RMS Grain Size (nm) |
|---|---|
| Ag@alumina (non-annealed) | 18.50 |
| Ag@alumina at 200 °C | 6.5 |
| Ag@alumina at 300 °C | 4.96 |
| Ag@alumina at 350 °C | 2.63 |
| Ag@alumina at 400 °C | 2.45 |
| Ag@alumina at 500 °C | 1.76 |
| Ag@alumina at 600 °C | 30 |
| Sample | Contact Angle | |||
|---|---|---|---|---|
| Ag@alumina (non-annealed) | 112.5° | 28.79 | 28.11 | 0.68 |
| Ag@alumina at 200 °C | 106.7° | 28.72 | 28.575 | 0.14 |
| Ag@alumina at 300 °C | 105° | 29.88 | 29.72 | 0.16 |
| Ag@alumina at 350 °C | 104.2° | 31.78 | 31.44 | 0.34 |
| Ag@alumina at 400 °C | 101.3° | 32.816 | 32.58 | 0.236 |
| Pt/Pd@200 °C annealed Ag nanoislands on alumina | 127.3° | 24.210 | 22.128 | 2.08 |
| Sample | Ag 3d3/2 (eV) | Ag 3d5/2 (eV) | Pd 3d3/2 (eV) | Pd 3d5/2 (eV) | Pt 4f5/2 (eV) | Pt 4f7/2 (eV) |
|---|---|---|---|---|---|---|
| Pt/Pd@ non-annealed Ag nanoislands on alumina | 373.5 | 367.5 | 340 | 334.5 | 70 | 73.5 |
| Pt/Pd@200 °C annealed Ag nanoislands on alumina | 374 | 368 | 340.5 | 335 | 70.5 | 74 |
| Pt/Pd@300 °C annealed Ag nanoislands on alumina | 373.8 | 367.7 | 340.5 | 334.8 | 70.2 | 73.7 |
| Pt/Pd@350 °C annealed Ag nanoislands on alumina | 373.5 | 367.3 | 340 | 335 | 70 | 73.8 |
| Pt/Pd@400 °C annealed Ag nanoislands on alumina | 373 | 367.4 | 340 | 335 | 70 | 74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahaman, M.H.; Yaqoob, U.; Kim, H.C. Fast Hydrogenation and Dehydrogenation of Pt/Pd Bimetal Decorated over Nano-Structured Ag Islands Grown on Alumina Substrates. Sensors 2019, 19, 86. https://doi.org/10.3390/s19010086
Rahaman MH, Yaqoob U, Kim HC. Fast Hydrogenation and Dehydrogenation of Pt/Pd Bimetal Decorated over Nano-Structured Ag Islands Grown on Alumina Substrates. Sensors. 2019; 19(1):86. https://doi.org/10.3390/s19010086
Chicago/Turabian StyleRahaman, Md Habibur, Usman Yaqoob, and Hyeon Cheol Kim. 2019. "Fast Hydrogenation and Dehydrogenation of Pt/Pd Bimetal Decorated over Nano-Structured Ag Islands Grown on Alumina Substrates" Sensors 19, no. 1: 86. https://doi.org/10.3390/s19010086





