An Ultrahigh Sensitivity Acetone Sensor Enhanced by Light Illumination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Fabrication
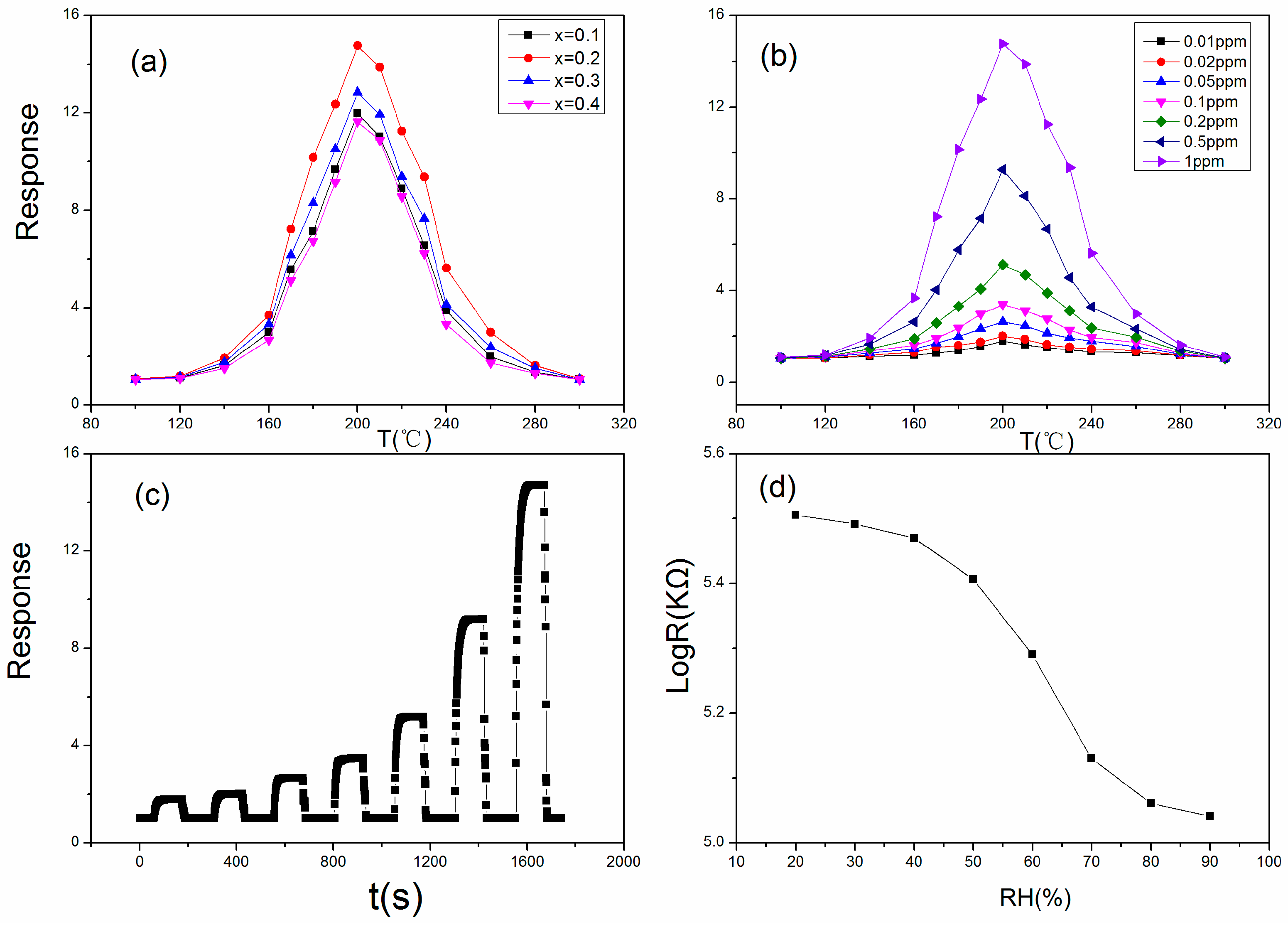
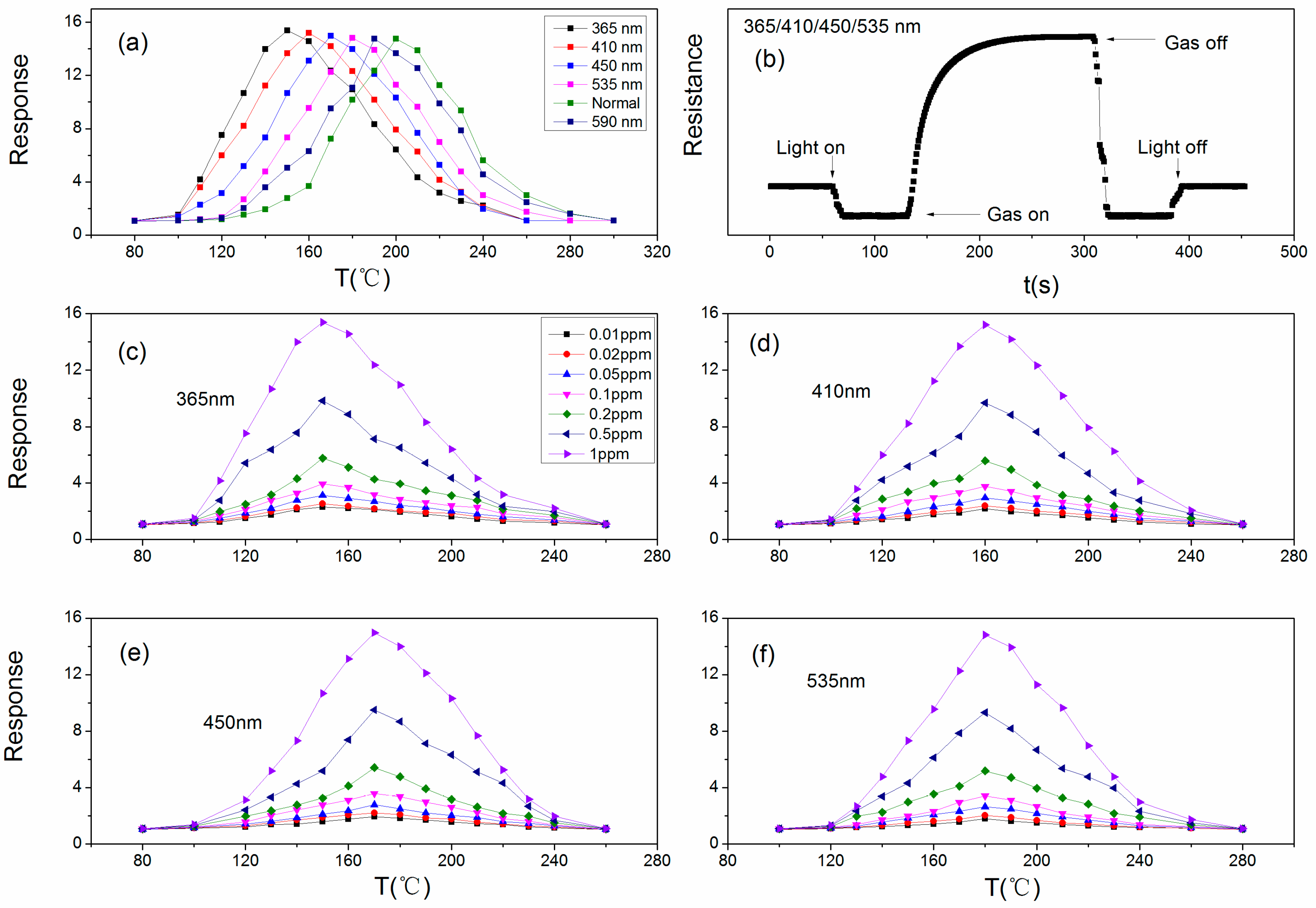
2.3. Gas-Sensing Test
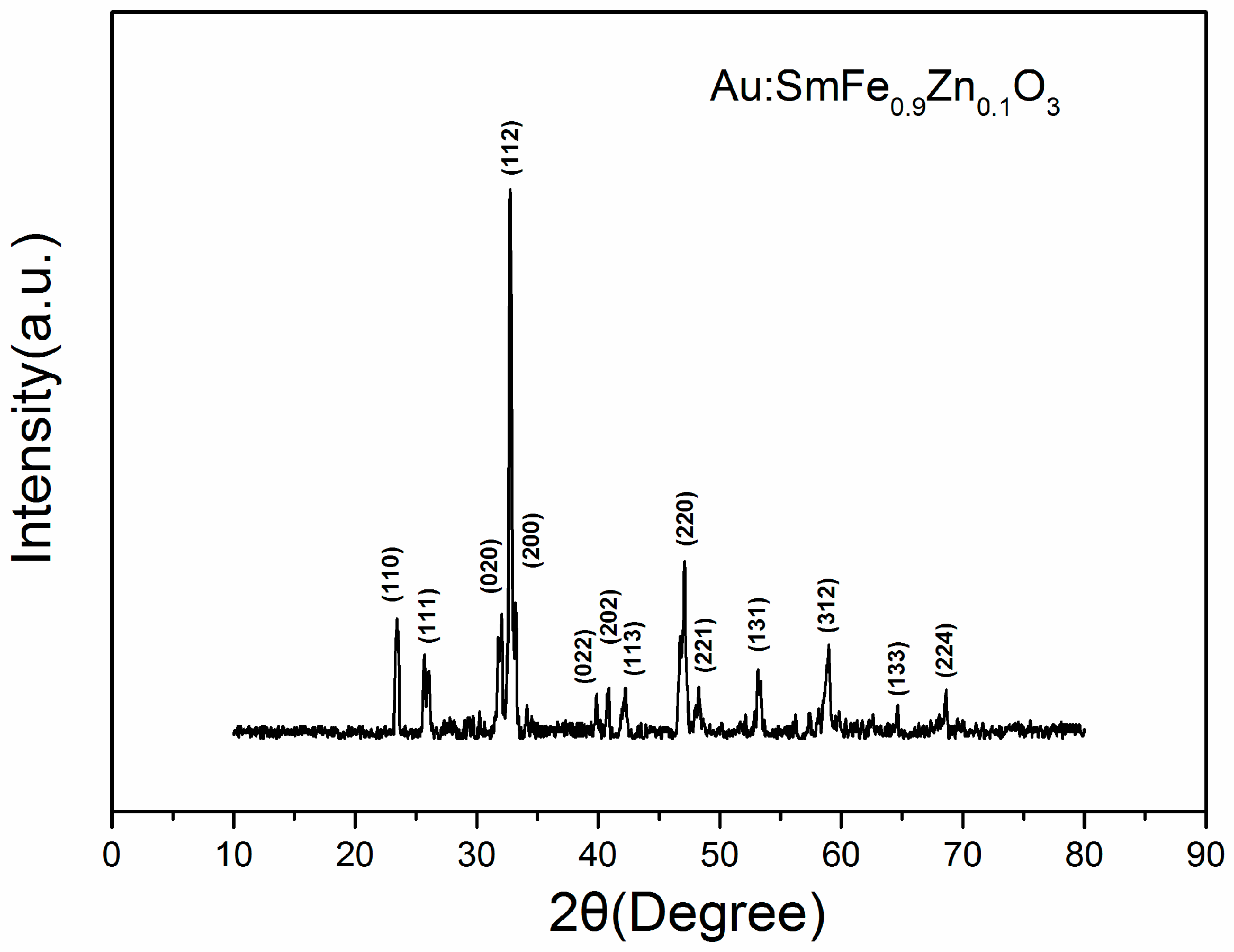
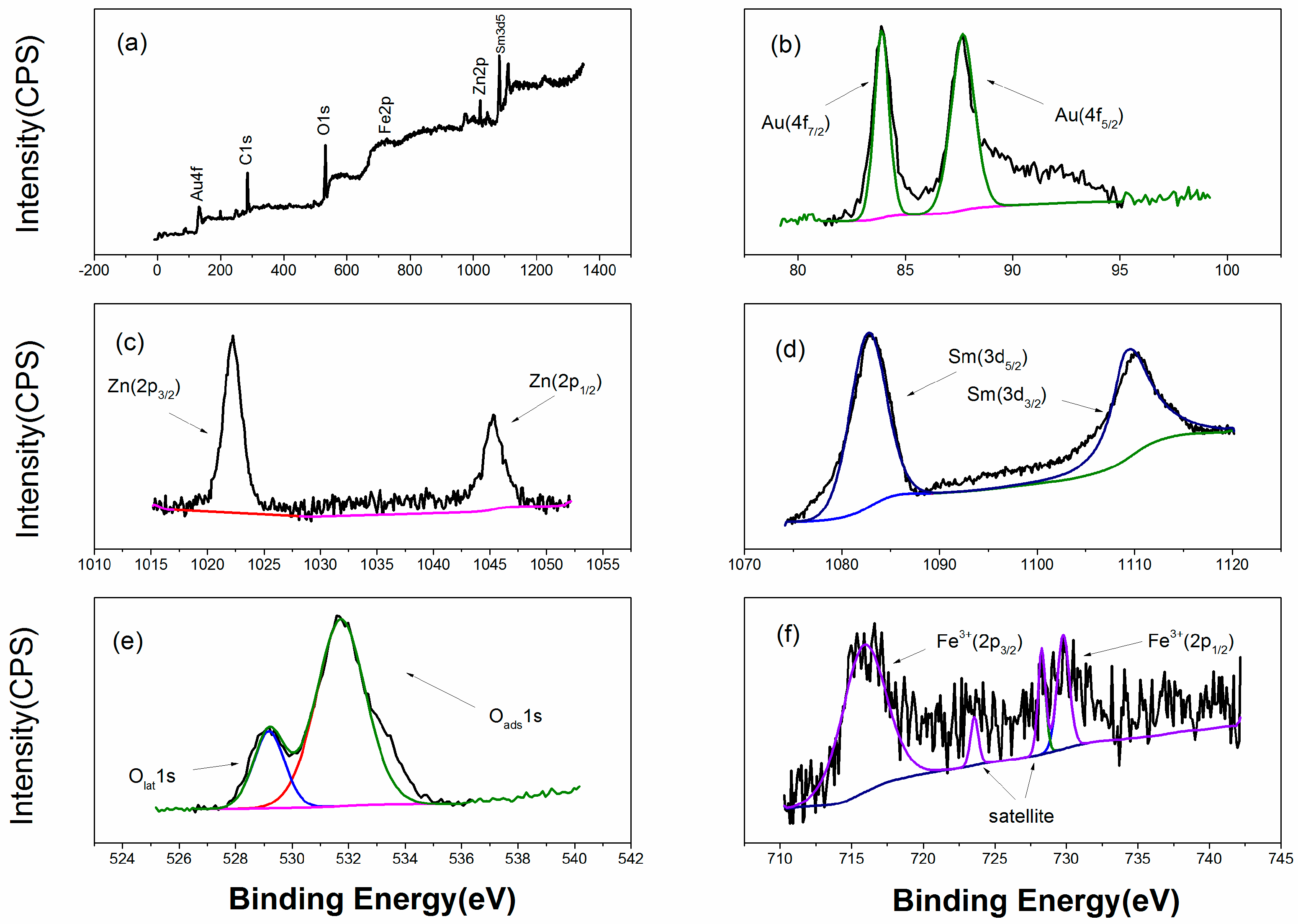
2.4. Characterization
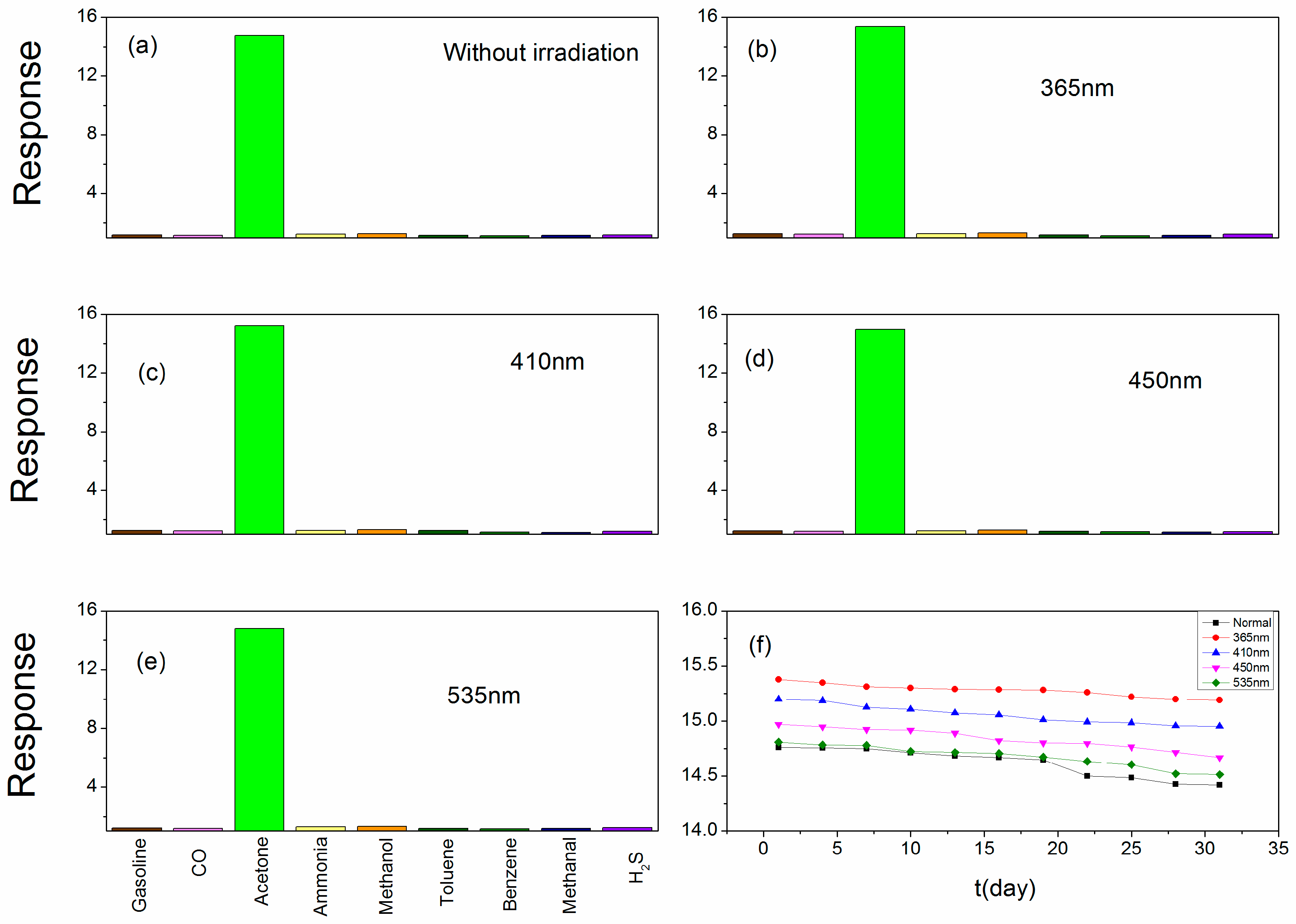
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, F.; Guan, Y.; Sun, R.; Liang, X.; Sun, P.; Liu, F.; Lu, G. Mixed potential type acetone sensor using stabilized zirconia and M3V2O8 (M: Zn, Co and Ni) sensing electrode. Sens. Actuators B Chem. 2015, 221, 673–680. [Google Scholar] [CrossRef]
- Jalili, R.; Hamed, A.S.; Esrafilzadeh, D.; Konstantinov, K.; Moulton, S.E.; Razal, J.M.; Wallace, G.G. Organic Solvent-Based Graphene Oxide Liquid Crystals: A Facile Route toward the Next Generation of Self-Assembled Layer-by-Layer Multifunctional 3D Architectures. ACS Nano 2013, 7, 3981–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-C.; Weng, Y.C.; Chou, T.C. Acetone sensor using lead foil as working electrode. Sens. Actuators B 2007, 122, 591–595. [Google Scholar] [CrossRef]
- Tomer, V.K.; Singh, K.; Kaur, H.; Shorie, M.; Sabherwal, P. Rapid acetone detection using indium loaded WO3/SnO2 nanohybrid sensor. Sens. Actuators B Chem. 2017, 253, 703–713. [Google Scholar] [CrossRef]
- Tomer, V.K.; Malik, R.; Kailasam, K. Near-Room-Temperature Ethanol Detection Using Ag-Loaded Mesoporous Carbon Nitrides. ACS Omega 2017, 2, 3658–3668. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Dankwort, T.; Mishra, Y.K.; Kienle, L. Cubic mesoporous Pd–WO3 loaded graphitic carbon nitride (g-CN) nanohybrids: Highly sensitive and temperature dependent VOC sensors. J. Mater. Chem. A 2018, 6, 10718–10730. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Kienle, L.; Chaudhary, V.; Nehra, S.; Duhan, S. Ordered Mesoporous Ag–ZnO@g-CN Nanohybrid as Highly Effcient Bifunctional Sensing Material. Adv. Mater. Interfaces 2018, 5, 1701357. [Google Scholar] [CrossRef]
- Makisimovich, N.; Vorottyntsev, V.; Nikitina, N.; Kaskevich, O.; Karabum, P.; Martynenko, F. Adsorption semiconductor sensor for diabetic ketoacidosis diagnosis. Sens. Actuators B Chem. 1996, 36, 419–421. [Google Scholar] [CrossRef]
- Madrolle, S.; Grangeat, P.; Jutten, C. A Linear-Quadratic Model for the Quantification of a Mixture of Two Diluted Gases with a Single Metal Oxide Sensor. Sensors 2018, 18, 1785. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Correlations between blood glucose and breath components from portable gas sensors and PTR-TOF-MS. J. Breath Res. 2013, 7, 037110. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Ma, S.; Sun, A.; Xu, X.; Yang, G.; Gao, J.; Zhang, Z.; Zhu, H. Characterization and acetone gas sensing properties of electrospun TiO2 nanorods. Superlattice Microstruct. 2015, 81, 107–113. [Google Scholar] [CrossRef]
- Bhowmik, B.; Hazra, A.; Dutta, K.; Bhattacharyya, P. Repeatability and Stability of Room-Temperature Acetone Sensor Based on Nanotubes: Influence of Stoichiometry Variation. IEEE Trans. Device Mater. Reliab. 2014, 14, 961–967. [Google Scholar] [CrossRef]
- Epifani, M.; Comini, E.; Díaz, R.; Genç, A.; Andreu, T.; Siciliano, P.; Morante, J.R. Acetone sensors based on TiO2 nanocrystals modified with tungsten oxide species. J. Alloys Compd. 2016, 665, 345–351. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Yang, Q.; Sun, P.; Gao, Y.; Liu, F.; Zheng, J.; Lu, G. Ultrasensitive and low detection limit of acetone gas sensor based on W-doped NiO hierarchical nanostructure. Sens. Actuators B Chem. 2015, 220, 59–67. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Fei, T.; Zhang, T. Enhanced acetone sensing performances of hierarchical hollow Au-loaded NiO hybrid structures. Sens. Actuators B Chem. 2012, 161, 178–183. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdullah, M.J.; Aziz, A.A. Performance of Cr-doped ZnO for acetone sensing. Appl. Surf. Sci. 2013, 270, 480–485. [Google Scholar] [CrossRef]
- Peng, C.; Guo, J.; Yang, W.; Shi, C.; Liu, M.; Zheng, Y.; Xu, J.; Chen, P.; Huang, T.; Yang, Y. Synthesis of three-dimensional flower-like hierarchical ZnO nanostructure and its enhanced acetone gas sensing properties. J. Alloys Compd. 2016, 654, 371–378. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, M.; Du, W. Improved acetone sensing properties of ZnO hollow nanofibers by single capillary electrospinning. Sens. Actuators B Chem. 2011, 160, 753–759. [Google Scholar] [CrossRef]
- An, D.; Tong, X.; Liu, J.; Wang, Q.; Zhou, Q.; Dong, J.; Li, Y. Template-free hydrothermal synthesis of ZnO micro/nano-materials and their application in acetone sensing properties. Superlattice Microstruct. 2015, 77, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, Z.; Ye, Z.; Zhu, L. Gas sensors based on ultrathin porous Co3O4 nanosheets to detect acetone at low temperature. RSC Adv. 2015, 5, 59976–59982. [Google Scholar] [CrossRef]
- Su, C.; Liu, C.; Liu, L.; Ni, M.; Li, H.; Bo, X.; Liu, L.; Chi, X. Excellent acetone sensing properties of Sm-doped alpha-Fe2O3. Appl. Surf. Sci. 2014, 314, 931–935. [Google Scholar] [CrossRef]
- Shan, H.; Liu, C.; Liu, L.; Li, S.; Wang, L.; Zhang, X.; Bo, X.; Chi, X. Highly sensitive acetone sensors based on La-doped alpha-Fe2O3 nanotubes. Sens. Actuators B Chem. 2013, 184, 243–247. [Google Scholar] [CrossRef]
- Chen, D.; Hou, X.; Li, T.; Yin, L.; Fan, B.; Wang, H.; Li, X.; Xu, H.; Lu, H.; Zhang, R.; et al. Effects of morphologies on acetone-sensing properties of tungsten trioxide nanocrystals. Sens. Actuators B Chem. 2011, 153, 373–381. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Park, S.; Lee, C. Acetone sensing of Au and Pd-decorated WO3 nanorod sensors. Sens. Actuators B 2015, 209, 180–185. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Gass, S.; Schmid, A.; Amann, A.; Pratsinis, S.E. Breath acetone monitoring by portable Si:WO3 gas sensors. Anal. Chim. Acta 2012, 738, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Patil, P.P.; More, M.A. Acetone vapour sensing characteristics of cobalt-doped SnO2 thin films. Sens. Actuators B 2007, 125, 126–130. [Google Scholar] [CrossRef]
- Jin, W.X.; Ma, S.Y.; Sun, A.M.; Luo, J.; Cheng, L.; Li, W.Q.; Tie, Z.Z.; Jiang, X.H.; Wang, T.T. Synthesis of hierarchical SnO2 nanoflowers and their high gas-sensing properties. Mater. Lett. 2015, 143, 283–286. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kushwaha, A.; Sahay, P.P. Cr-induced modifications in the structural, photoluminescence and acetone-sensing behaviour of hydrothermally synthesised SnO2 nanoparticles. J. Exp. Nanosci. 2014, 10, 1042–1056. [Google Scholar] [CrossRef]
- Singkammo, S.; Wisitsoraat, A.; Sriprachuabwong, C.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Electrolytically Exfoliated Graphene-Loaded Flame-Made Ni-Doped SnO2 Composite Film for Acetone Sensing. ACS Appl. Mater. Interfaces 2015, 7, 3077–3092. [Google Scholar] [CrossRef] [PubMed]
- Punginsang, M.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Effects of cobalt doping on nitric oxide, acetone and ethanol sensing performances of FSP-made SnO2 nanoparticles. Sens. Actuators B Chem. 2015, 210, 589–601. [Google Scholar] [CrossRef]
- Song, P.; Zhang, H.; Han, D.; Li, J.; Yang, Z.; Wang, Q. Preparation of biomorphic porous LaFeO3 by sorghum straw biotemplate method and its acetone sensing properties. Sens. Actuators B Chem. 2014, 196, 140–146. [Google Scholar] [CrossRef]
- Liu, X.; Ji, H.; Gu, Y.; Xu, M. Preparation and acetone sensitive characteristics of nano LaFeO3 semiconductor thin films by polymerization complex method. Mater. Sci. Eng. B Adv. 2006, 13, 98–101. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, H.; Lv, W.; Zhang, H.; Hu, J. Gas sensors based on ytterbium ferrites nanocrystalline powders for detecting acetone with low concentrations. Sens. Actuators B Chem. 2017, 246, 9–19. [Google Scholar] [CrossRef]
- Yang, M.; Huo, L.; Zhao, H.; Gao, S.; Rong, Z. Electrical properties and acetone-sensing characteristics of LaNi1−xTixO3 perovskite system prepared by amorphous citrate decomposition. Sens. Actuators B Chem. 2009, 143, 111–118. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Wang, Y. Surfactant CATB-assisted generation and gas-sensing characteristics of LnFeO3 (Ln = La, Sm, Eu) materials. Sens. Actuators B Chem. 2009, 143, 124–131. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Cheng, B.; Qin, H.; Jiang, M. Acetone gas sensing properties of SmFe1−xMgxO3 perovskite oxides. Sens. Actuators B Chem. 2008, 134, 483–487. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhang, R.; Zhao, M.; Fang, S.M.; Han, Z.X.; Hu, J.F.; Wang, K.Y. Effect of Pd doping on the acetone-sensing properties of NdFeO3. Int. J. Min. Met. Mater. 2012, 19, 141–145. [Google Scholar] [CrossRef]
- Fan, K.; Qin, H.; Zhang, Z.; Sun, L.; Sun, L.; Hu, J. Gas sensing properties of nanocrystalline La0.75Ba0.25FeO3 thick-film sensors. Sens. Actuators B 2012, 171, 302–308. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, Z.; Song, J.; Zheng, C. Improvement and mechanism for the fast response of a Pt/TiO2 gas sensor. Sens. Actuators B Chem. 2010, 148, 87–92. [Google Scholar] [CrossRef]
- Cabot, A.; Arbiol, J.; Morante, J.R.; Weimarb, U.; Bârsanb, N.; Göpel, W. Analysis of the noble metal catalytic additives introduced by impregnation of as obtained SnO2 sol–gel nanocrystals for gas sensors. Sens. Actuators B Chem. 2000, 70, 87–100. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, T.; Zhang, S.; Li, Z.; Yuan, Z.; Cao, Q. Response time and mechanism of Pd modified TiO2 gas sensor. Mater. Sci. Semicond. Process. 2014, 17, 149–154. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, L.; Qi, Q.; Li, S.; Lu, G. Development of microstructure In/Pd-doped SnO2 sensor for low-level CO detection. Sens. Actuators B Chem. 2009, 139, 287–291. [Google Scholar] [CrossRef]
- Yang, D.-J.; Kamienchick, I.; Youn, D.Y.; Rothschild, A.; Kim, I.-D. Ultrasensitive and Highly Selective Gas Sensors Based on Electrospun SnO2 Nanofibers Modified by Pd Loading. Adv. Funct. Mater. 2010, 20, 4258–4264. [Google Scholar] [CrossRef]
- Rajgure, A.V.; Patil, J.Y.; Pawar, R.C.; Lee, C.S.; Suryavanshi, S.S. Aqueous chemical route deposition of nanocrystalline ZnO thin films as acetone sensor: Effect of molarity. Ceram. Int. 2013, 39, 87–92. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Li, S.; Wang, L.; Tian, Y. Preparation, characterization, and gas-sensing properties of Pd-doped In2O3 nanofibers. Mater. Lett. 2009, 63, 1975–1977. [Google Scholar] [CrossRef]
- Menini, P.; Parret, F.; Guerrero, M.; Soulantica, K.; Erades, L.; Maisonnat, A.; Chaudret, B. CO response of a nanostructured SnO2 gas sensor doped with palladium and platinum. Sens. Actuators B Chem. 2004, 103, 111–114. [Google Scholar] [CrossRef]
- Vaishampayan, M.V.; Deshmukh, R.G.; Mulla, I.S. Influence of Pd doping on morphology and LPG response of SnO2. Sens. Actuators B Chem. 2008, 131, 665–672. [Google Scholar] [CrossRef]
- Lee, Y.C.; Huang, H.; Tan, O.K.; Tse, M.S. Semiconductor gas sensor based on Pd-doped SnO2 nanorod thin films. Sens. Actuators B Chem. 2008, 132, 239–242. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Lai, H.Y.; Chen, C.H. Highly sensitive room-temperature CO gas sensors: Pt and Pd nanoparticle-decorated In2O3 flower-like nanobundles. J. Mater. Chem. 2012, 22, 13204–13208. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, B.; Hu, J.; Qin, H.; Jiang, M. Semiconducting gas sensor for ethanol based on LaMgxFe1−xO3 nanocrystals. Sens. Actuators B Chem. 2008, 129, 53–58. [Google Scholar] [CrossRef]
- Wang, L.; Teleki, A.; Pratsinis, S.E.; Gouma, P.I. Ferroelectric WO3 Nanoparticles for Acetone Selective Detection. Chem. Mater. 2008, 20, 4794–4796. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1998; Volume 8, p. 122. [Google Scholar]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Qin, H.; Gao, C.; Hu, J. An Ultrahigh Sensitivity Acetone Sensor Enhanced by Light Illumination. Sensors 2018, 18, 2318. https://doi.org/10.3390/s18072318
Zhang H, Qin H, Gao C, Hu J. An Ultrahigh Sensitivity Acetone Sensor Enhanced by Light Illumination. Sensors. 2018; 18(7):2318. https://doi.org/10.3390/s18072318
Chicago/Turabian StyleZhang, Heng, Hongwei Qin, Chengyong Gao, and Jifan Hu. 2018. "An Ultrahigh Sensitivity Acetone Sensor Enhanced by Light Illumination" Sensors 18, no. 7: 2318. https://doi.org/10.3390/s18072318



