Substituted 2-Aminobenzothiazoles Salicylidenes Synthesis and Characterization as Cyanide Sensors in Aqueous Medium
Abstract
:1. Introduction
2. Experimental
Reagents and Instruments
3. Synthesis
3.1. Synthesis of 1
3.2. Synthesis of 2
4. Results and Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chaicham, A.; Kulchat, S.; Tumcharern, G.; Tuntulani, T.; Tomapatanaget, B. Synthesis, photophysical properties, and cyanide detection in aqueous solution of BF2-curcumin dyes. Tetrahedron 2010, 66, 6217–6223. [Google Scholar] [CrossRef]
- Ballhorn, D.J. Cyanogenic glycosides in nuts and seeds. In Nuts & Seeds in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 129–136. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 1996; Volume 2, pp. 6–8. [Google Scholar]
- Sun, Y.; Liu, Y.; Chen, M.; Guo, W. A novel fluorescent and chromogenic probe for cyanide detection in water based on the nucleophilic addition of cyanide to imine group. Talanta 2009, 80, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Duke, R.M.; Veale, E.B.; Pfeffer, F.M.; Kruger, P.E.; Gunnlaugsson, T. Colorimetric and fluorescent anion sensors: An overview of recent developments in the use of 1,8-naphthalimide-based chemosensors. Chem. Soc. Rev. 2010, 39, 3936–3953. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, W.; Hui, Y.; Sun, X.; Xu, L.; Feng, L.; Xie, Z. A new highly selective fluorescent turn-on chemosensor for cyanide anion. Talanta 2015, 137, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Kaloo, M.A.; Sankar, J. Reusable and specific proton transfer signalling by inorganic cyanide in solution and solid phase. Chem. Commun. 2015, 51, 14528–14531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.Y.; Nam, S.W.; Lim, J.; Park, S.; Yoon, J. A highly selective cyanide sensing in water via fluorescence change and its application to in vivo imaging. Chem. Commun. 2009, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.T.; Su, D.; Jiang, X.; Yang, W.; Yin, Z.; He, J.; Cheng, J.P. A simple yet highly selective colorimetric sensor for cyanide anion in an aqueous environment. Org. Biomol. Chem. 2008, 6, 3038–3040. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-K.; Tae, J. Acridinium Salt Based Fluorescent and Colorimetric Chemosensor for the Detection of Cyanide in Water. Org. Lett. 2006, 8, 5721–5723. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Cho, D.-G. The Benzil Rearrangement Reaction: Trapping of a Hitherto Minor Product and Its Application to the Development of a Selective Cyanide Anion Indicator. Org. Lett. 2008, 10, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Barare, B.; Babahan, I.; Hijji, Y.M.; Bonyi, E.; Tadesse, S.; Aslan, K. A Highly Selective Sensor for Cyanide in Organic Media and on Solid Surfaces. Sensors 2016, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Huo, F.; Xu, M.; Barnes, C.L.; Glass, T.E. A NIR, special recognition on HS−/CN− colorimetric and fluorescent imaging material for endogenous H2S based on nucleophilic addition. Sens. Actuators B Chem. 2017, 252, 592–599. [Google Scholar] [CrossRef]
- Hijji, Y.M.; Barare, B.; Kennedy, A.P.; Butcher, R. Synthesis and photophysical characterization of a Schiff base as anion sensor. Sens. Actuators B Chem. 2009, 136, 297–302. [Google Scholar] [CrossRef]
- Reena, V.; Suganya, S.; Velmathi, S. Synthesis and anion binding studies of azo-Schiff bases: Selective colorimetric fluoride and acetate ion sensors. J. Fluor. Chem. 2013, 153, 89–95. [Google Scholar] [CrossRef]
- Zang, L.; Wei, D.; Wang, S.; Jiang, S. A phenolic Schiff base for highly selective sensing of fluoride and cyanide via different channels. Tetrahedron 2012, 68, 636–641. [Google Scholar] [CrossRef]
- Lee, M.; Moon, J.H.; Swamy, K.M.K.; Jeong, Y.; Kim, G.; Choi, J.; Lee, J.Y.; Yoon, J. A new bis-pyrene derivative as a selective colorimetric and fluorescent chemosensor for cyanide and fluoride and anion-activated CO2 sensing. Sens. Actuators B Chem. 2014, 199, 369–376. [Google Scholar] [CrossRef]
- Sivakumar, R.; Reena, V.; Ananthi, N.; Babu, M.; Anandan, S.; Velmathi, S. Colorimetric and fluorescence sensing of fluoride anions with potential salicylaldimine based schiff base receptors. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Singh, A.K.; Gupta, B. A cerium(III) selective polyvinyl chloride membrane sensor based on a Schiff base complex of N,N’-bis[2-(salicylideneamino)ethyl]ethane-1,2-diamine. Anal. Chim. Acta 2006, 575, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, V.K.; Gupta, B. Chromium(III) selective membrane sensors based on Schiff bases as chelating ionophores. Anal. Chim. Acta 2007, 585, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Bhatnagar, J.M. Copper(II) selective electrochemical sensor based on Schiff Base complexes. Talanta 2004, 64, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Figueira, E.C.; Neres, L.C.S.; Ruy, M.R.S.; Troiano, G.F.; Sotomayor, M.D.P.T. Development of a biomimetic sensor for selective identification of cyanide. Anal. Methods 2016, 8, 6353–6360. [Google Scholar] [CrossRef]
- Kaur, K.; Mittal, S.K.; Kumar, S.K.A.; Kumar, A.; Kumar, S. Viologen substituted anthrone derivatives for selective detection of cyanide ions using voltammetry. Anal. Methods 2013, 5, 5565. [Google Scholar] [CrossRef]
- Destanoğlu, O.; Gümüş Yılmaz, G.; Apak, R. Selective Determination of Free Cyanide in Environmental Water Matrices by Ion Chromatography with Suppressed Conductivity Detection. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1537–1545. [Google Scholar] [CrossRef]
- Tomasulo, M.; Raymo, F.M. Colorimetric Detection of Cyanide with a Chromogenic Oxazine. Org. Lett. 2005, 7, 4633–4636. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jang, J.H.; Velusamy, N.; Jung, H.S.; Bhuniya, S.; Kim, J.S. An intramolecular crossed-benzoin reaction based KCN fluorescent probe in aqueous and biological environments. Chem. Commun. 2015, 51, 7709–7712. [Google Scholar] [CrossRef] [PubMed]
- Dagiliene, M.; Martynaitis, V.; Krisciuniene, V.; Krikstolaityte, S.; Sackus, A. Colorimetric Cyanide Chemosensor Based on 1′,3,3′,4-Tetrahydrospiro[chromene-2,2′-indole]. ChemistryOpen 2015, 4, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Hu, J.W.; Chen, K.Y. A ratiometric chemodosimeter for highly selective naked-eye and fluorogenic detection of cyanide. Anal. Chim. Acta 2015, 893, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, X.; Cao, D. A cyanide-selective colorimetric “naked-eye” and fluorescent chemosensor based on a diketopyrrolopyrrole–hydrazone conjugate and its use for the design of a molecular-scale logic device. RSC Adv. 2016, 6, 96676–96685. [Google Scholar] [CrossRef]
- Long, L.; Zhou, L.; Wang, L.; Meng, S.; Gong, A.; Du, F.; Zhang, C. A highly selective and sensitive fluorescence ratiometric probe for cyanide and its application for the detection of cyanide in natural water and biological samples. Anal. Methods 2013, 5, 6605. [Google Scholar] [CrossRef]
- Beck, H.P.; Zhang, B.; Bordeanu, A. Fluorimetric Determination of Free Cyanide by Flow-Injection Analysis. Anal. Lett. 2003, 36, 2211–2228. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Fluorescence intensity and lifetime-based cyanide sensitive probes for physiological safeguard. Anal. Chim. Acta 2004, 522, 9–17. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Yang, Q.; Song, Y.; Li, Y. A triphenylamine-based colorimetric and “turn-on” fluorescent probe for detection of cyanide anions in live cells. RSC Adv. 2015, 5, 47990–47996. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kim, H.-J.; Kim, G.-H.; Shin, I.; Hong, J.-I. Fluorescent Chemodosimeter for Selective Detection of Cyanide in Water. Org. Lett. 2008, 10, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Barare, B.; Yıldız, M.; Alpaslan, G.; Dilek, N.; Ünver, H.; Tadesse, S.; Aslan, K. Synthesis, characterization, theoretical calculations, DNA binding and colorimetric anion sensing applications of 1-[(E)-[(6-methoxy-1,3-benzothiazol-2-yl)imino]methyl]naphthalen-2-ol. Sens. Actuators B Chem. 2015, 215, 52–61. [Google Scholar] [CrossRef]
- Albayrak, C.; Kastas, G.; Odabasoglu, M.; Frank, R. Probing the compound (E)-5-(diethylamino)-2-[(4-methylphenylimino)methyl]phenol mainly from the point of tautomerism in solvent media and the solid state by experimental and computational methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 81, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.R.; Kamounah, F.S. Mass Spectral Study of Tautomerism in Some 1-Hydroxy-2-Naphthaldehyde Schiff Bases. Spectrosc. Lett. 2002, 35, 327–335. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Enhanced Fluorescence Cyanide Detection at Physiologically Lethal Levels: Reduced ICT-Based Signal Transduction. J. Am. Chem. Soc. 2005, 127, 3635–3641. [Google Scholar] [CrossRef] [PubMed]

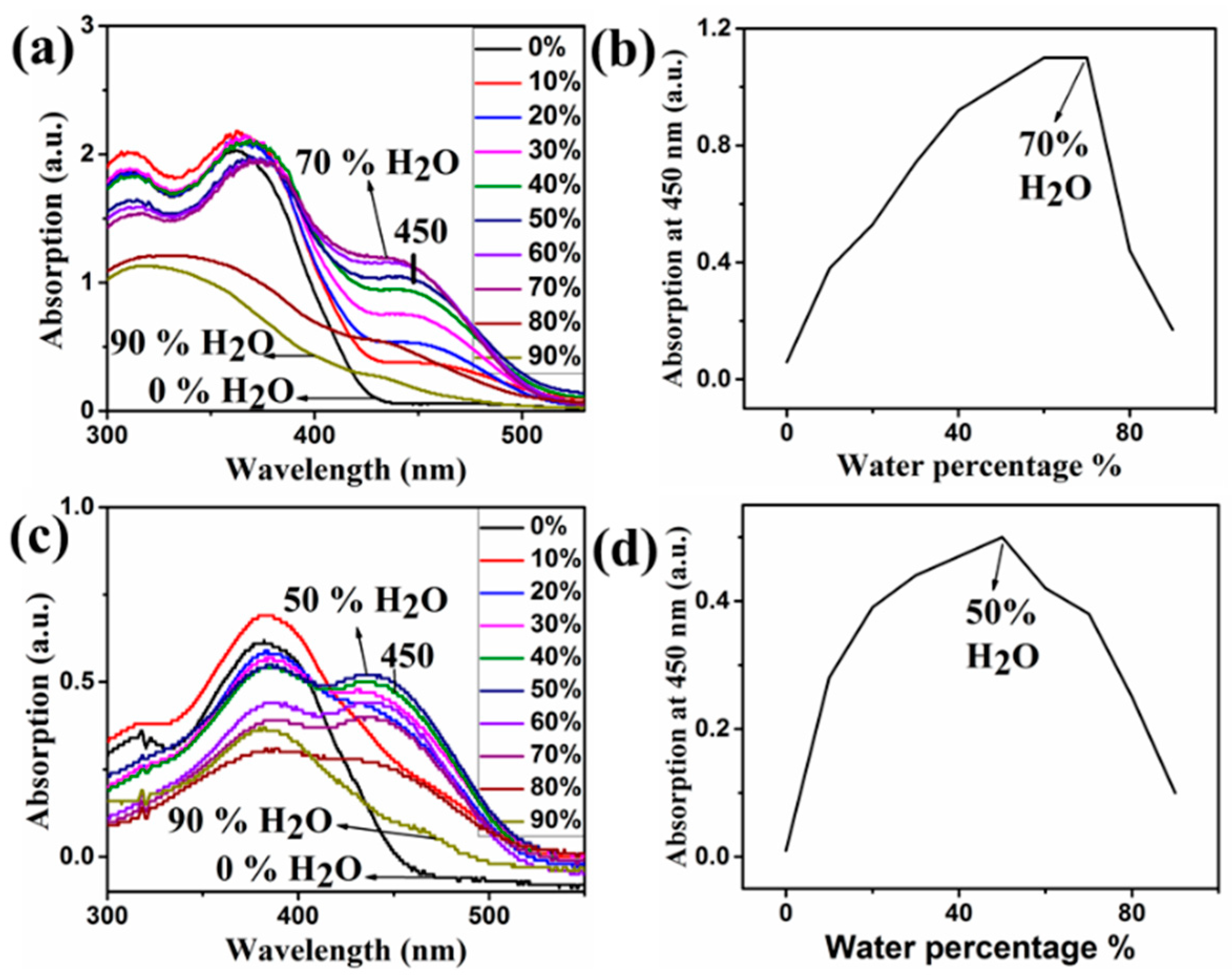
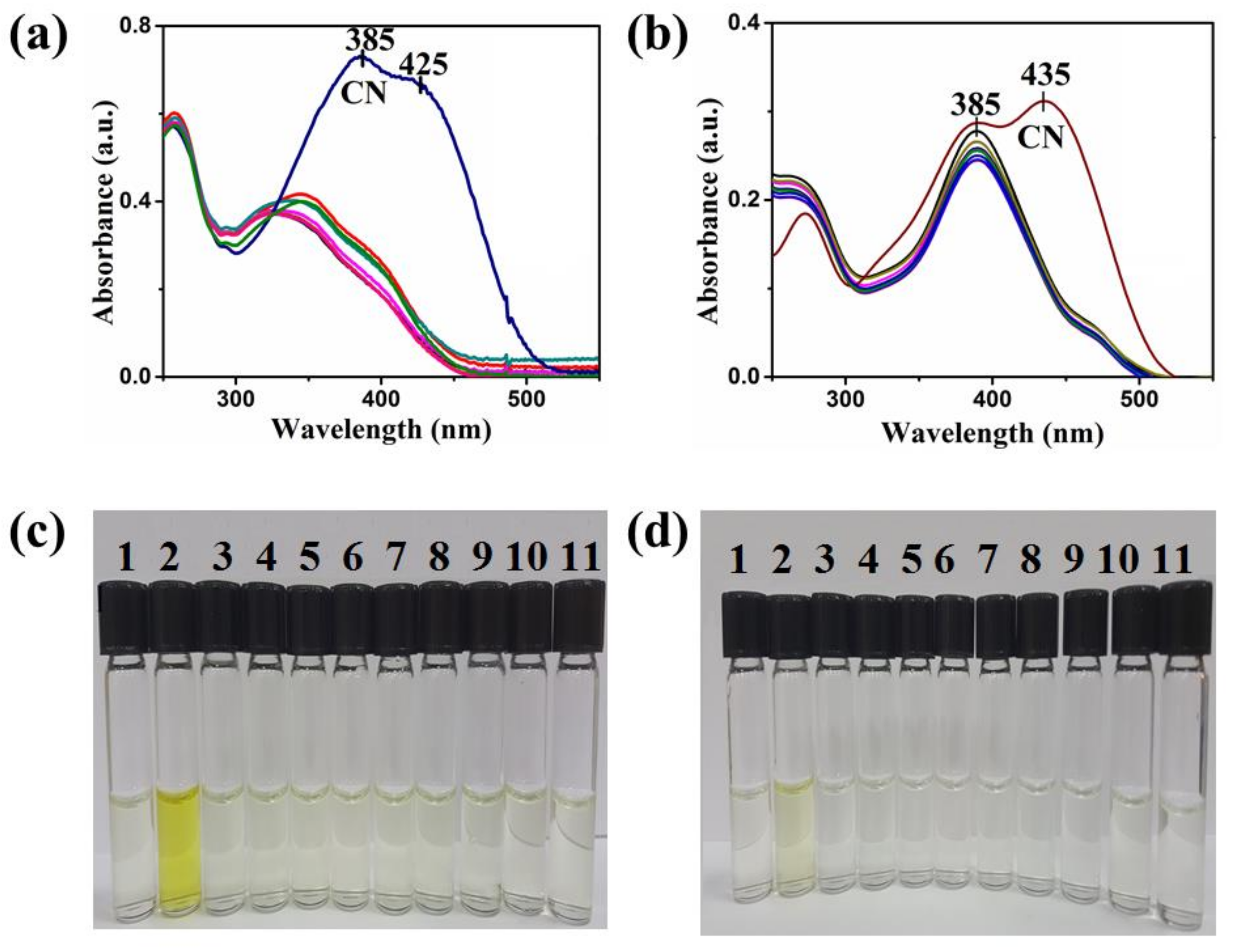
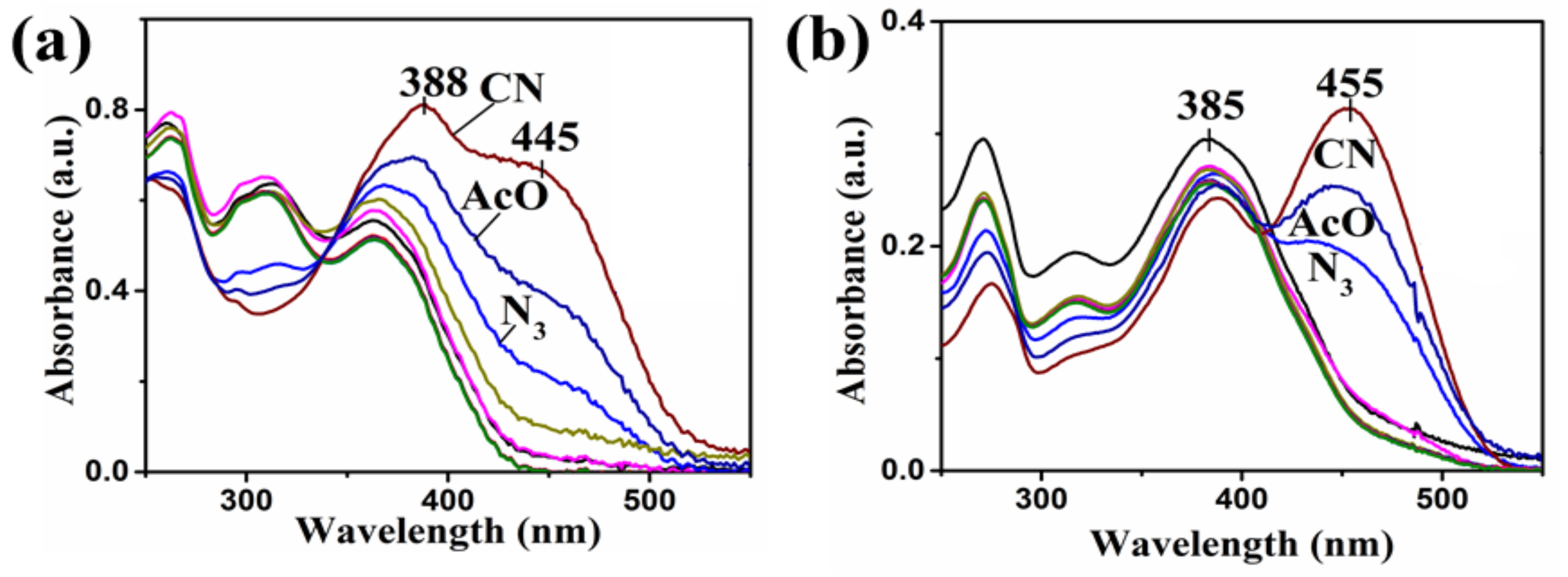
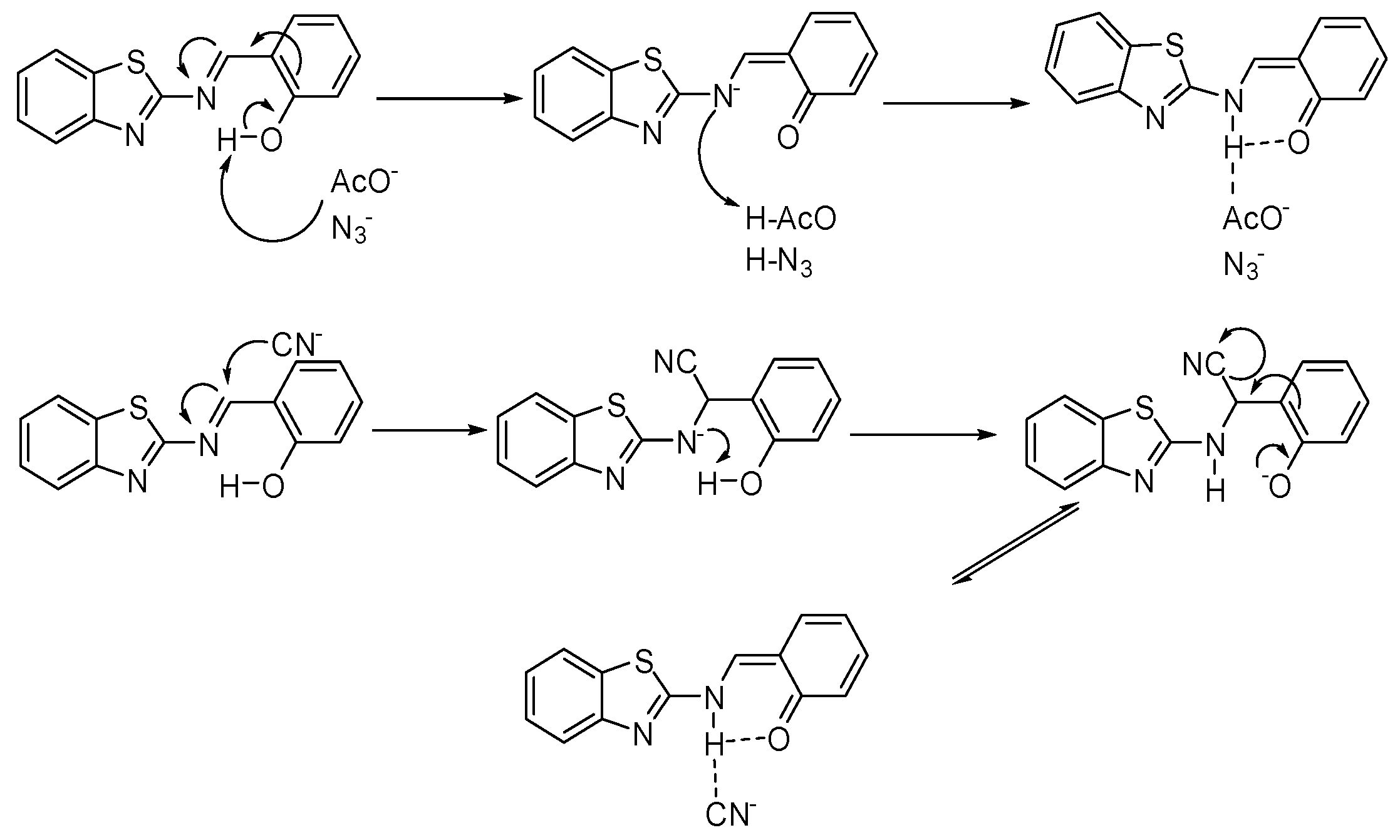
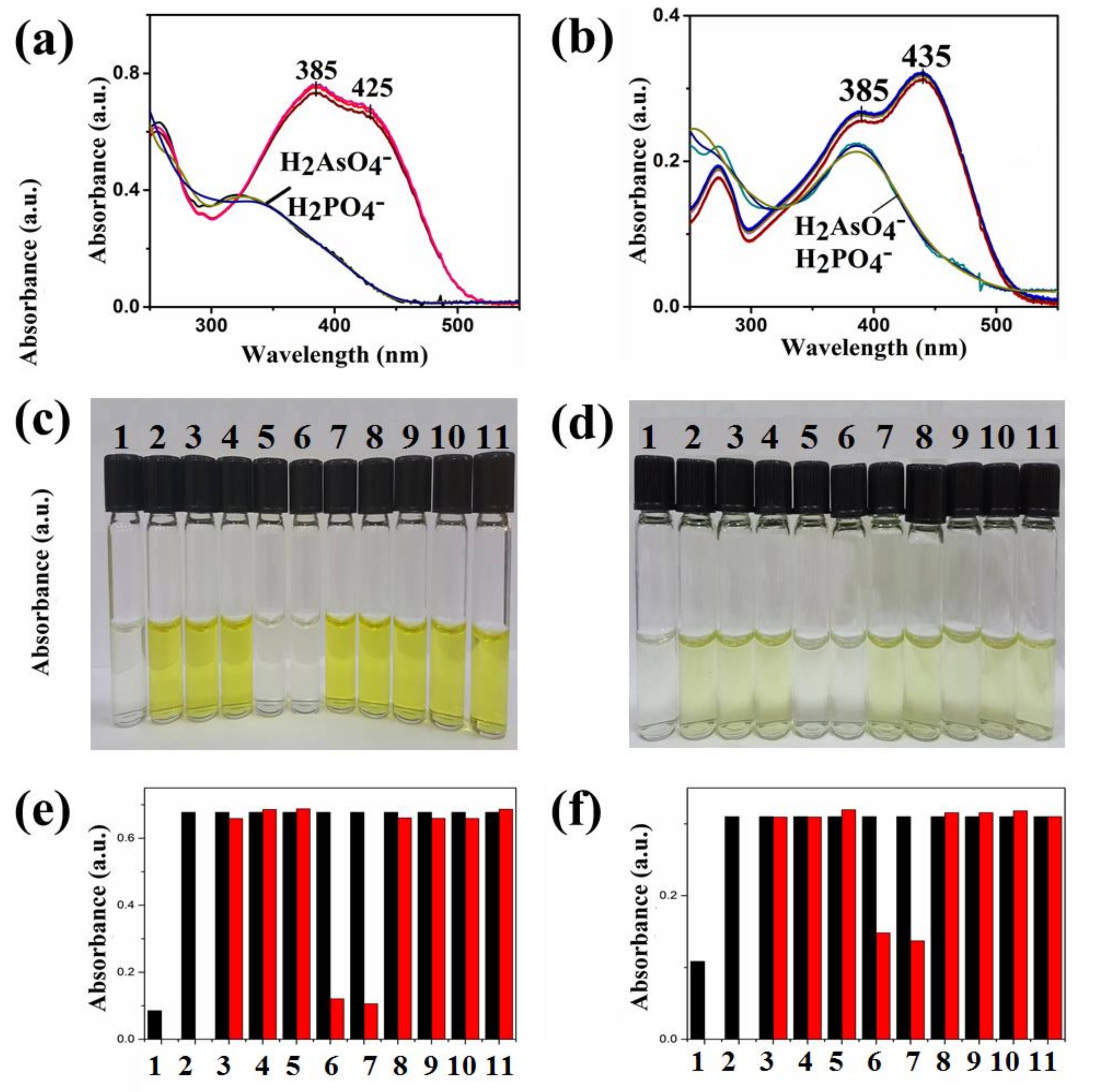
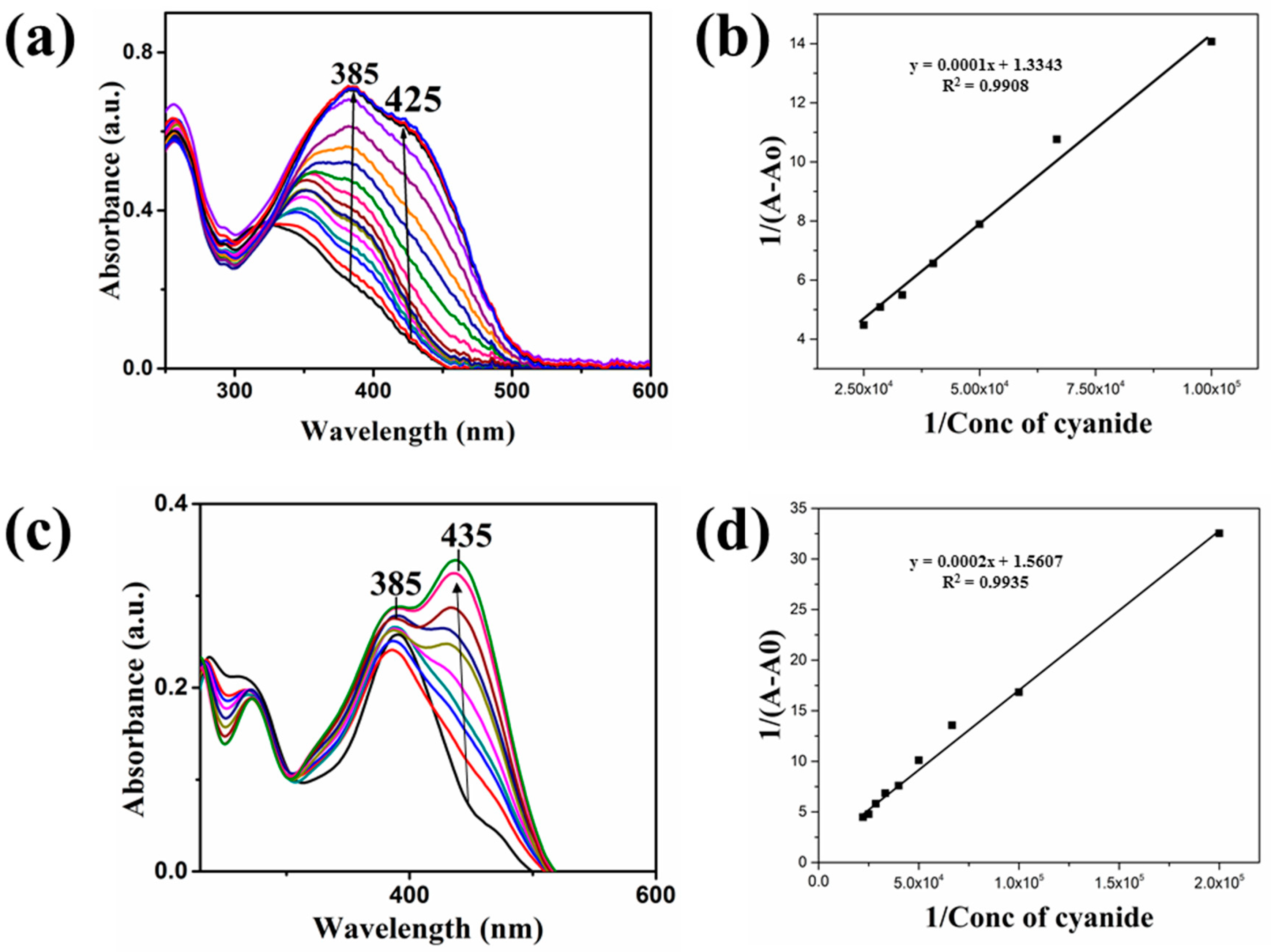

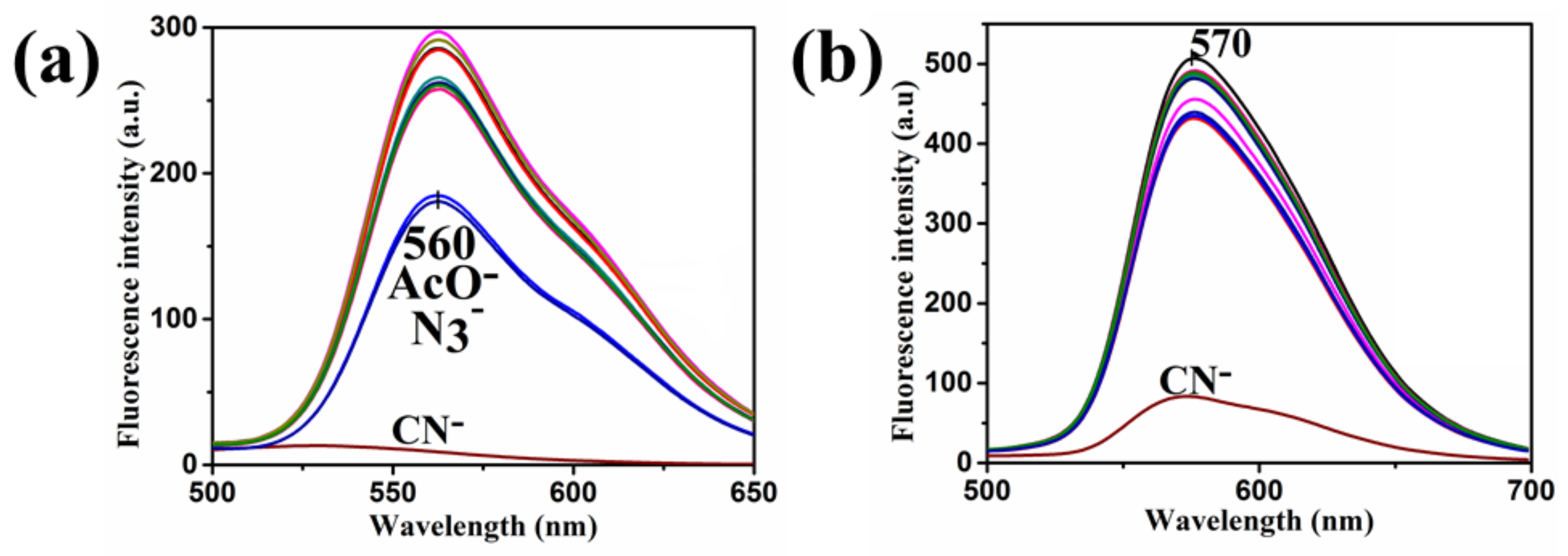
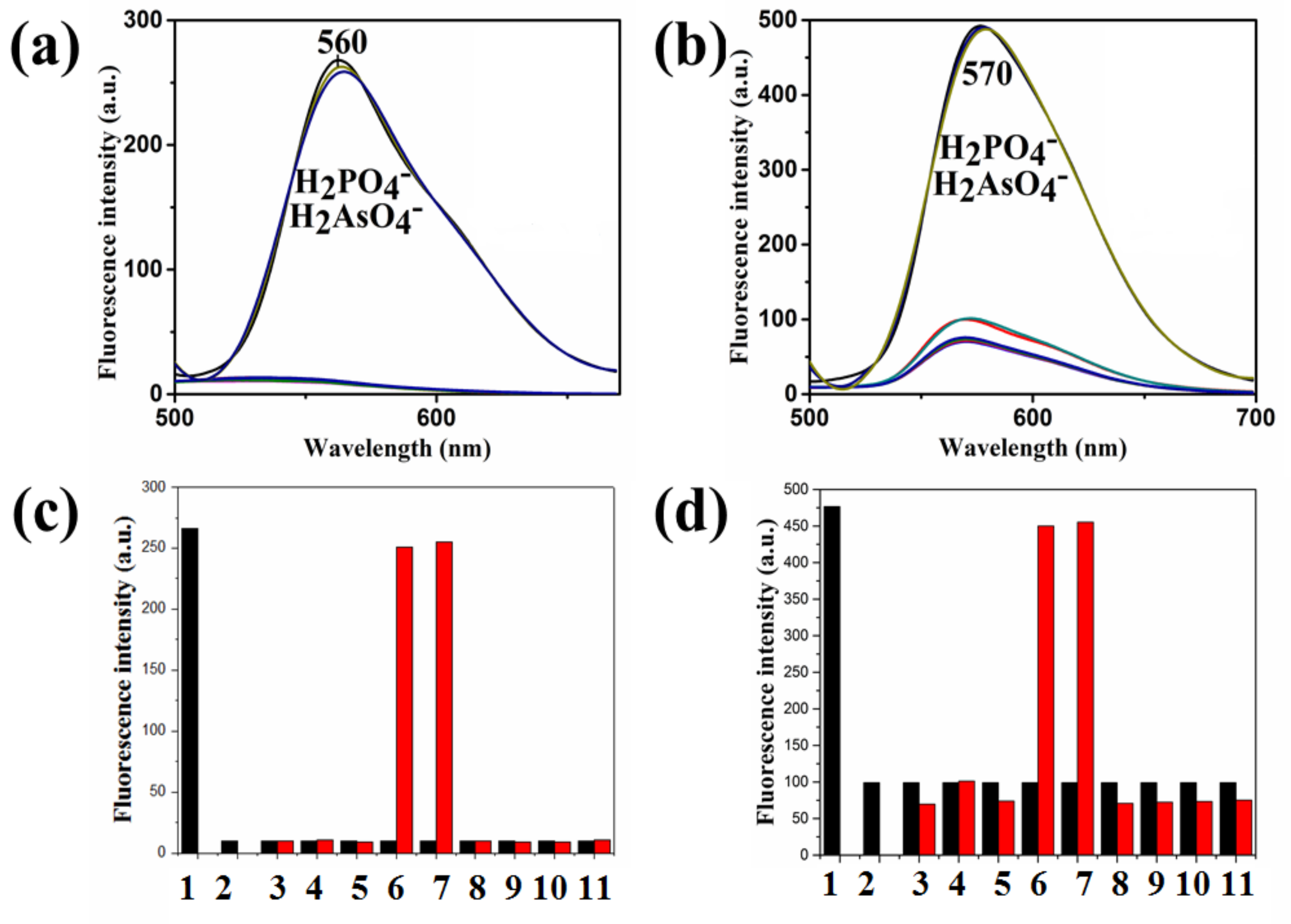
| Compound | Ka (M−1) | LDL (M) | Isosbestic Point |
|---|---|---|---|
| 1 | 13,343 | 1 × 10−6 | 325 nm |
| 2 | 7803 | 1.35 × 10−6 | 305 nm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsafy, A.G.; Al-Easa, H.S.; Hijji, Y.M. Substituted 2-Aminobenzothiazoles Salicylidenes Synthesis and Characterization as Cyanide Sensors in Aqueous Medium. Sensors 2018, 18, 2219. https://doi.org/10.3390/s18072219
Elsafy AG, Al-Easa HS, Hijji YM. Substituted 2-Aminobenzothiazoles Salicylidenes Synthesis and Characterization as Cyanide Sensors in Aqueous Medium. Sensors. 2018; 18(7):2219. https://doi.org/10.3390/s18072219
Chicago/Turabian StyleElsafy, Anas G., Hala Sultan Al-Easa, and Yousef M. Hijji. 2018. "Substituted 2-Aminobenzothiazoles Salicylidenes Synthesis and Characterization as Cyanide Sensors in Aqueous Medium" Sensors 18, no. 7: 2219. https://doi.org/10.3390/s18072219






