Highly Sensitive Acetone Gas Sensor Based on g-C3N4 Decorated MgFe2O4 Porous Microspheres Composites
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the MgFe2O4/g-C3N4 Composites
2.2. Sample Characterization
2.3. Gas Sensing Property Test
3. Results and Discussion
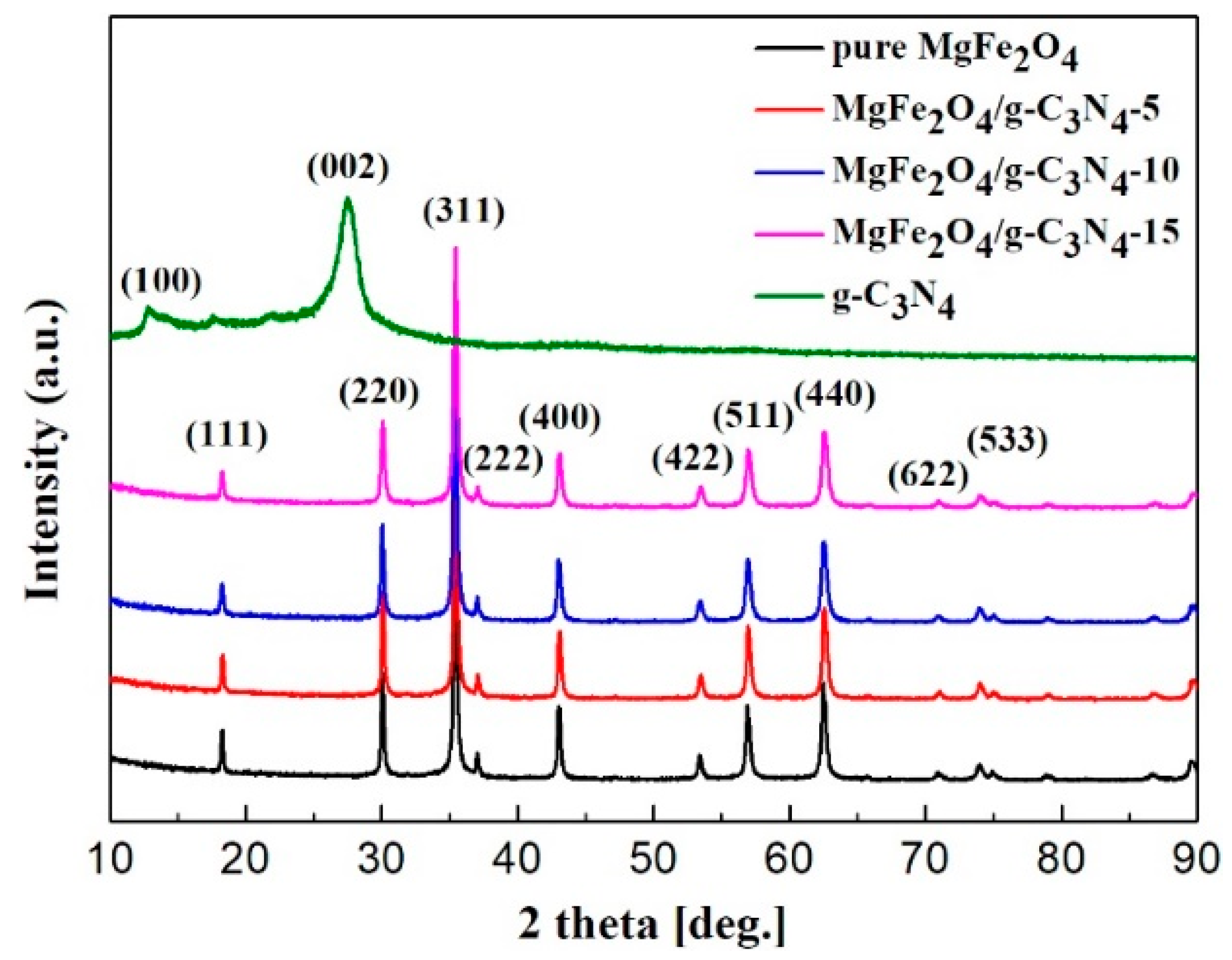
3.1. Sample Characterization
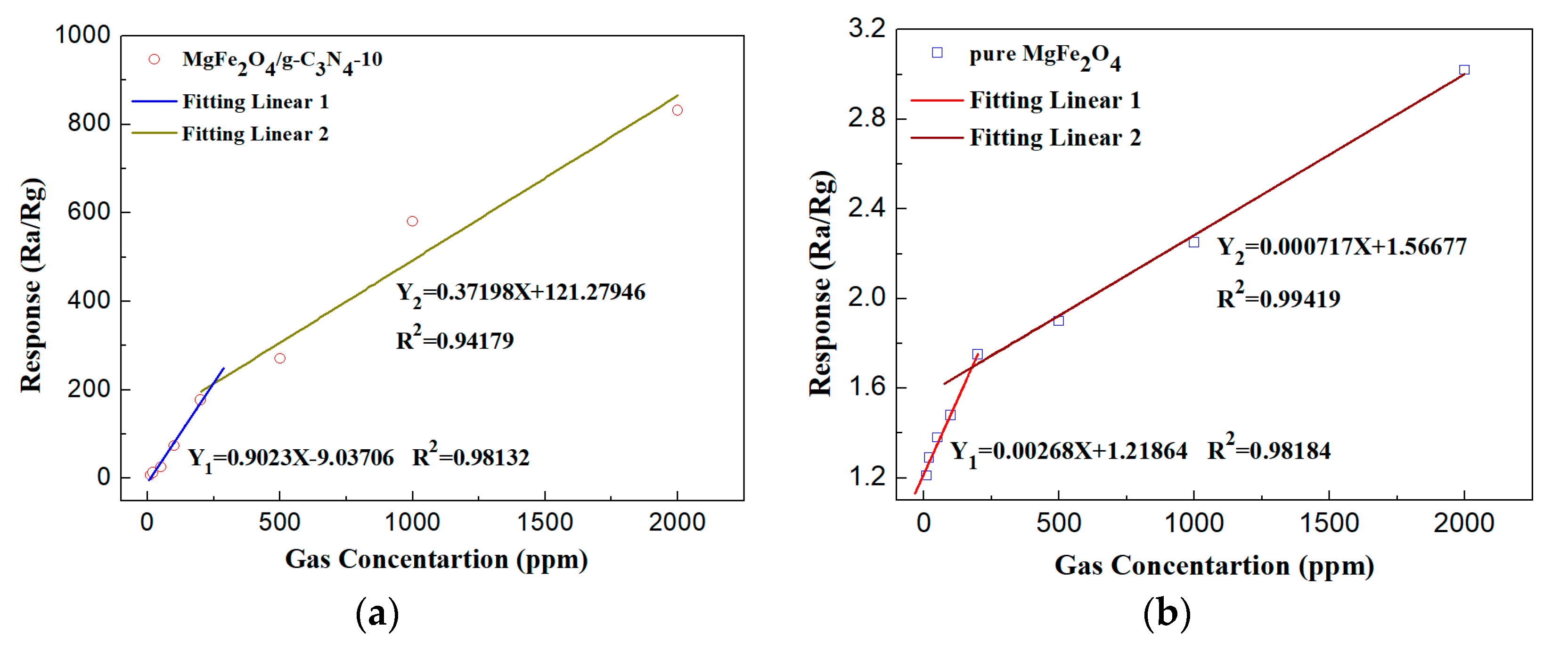
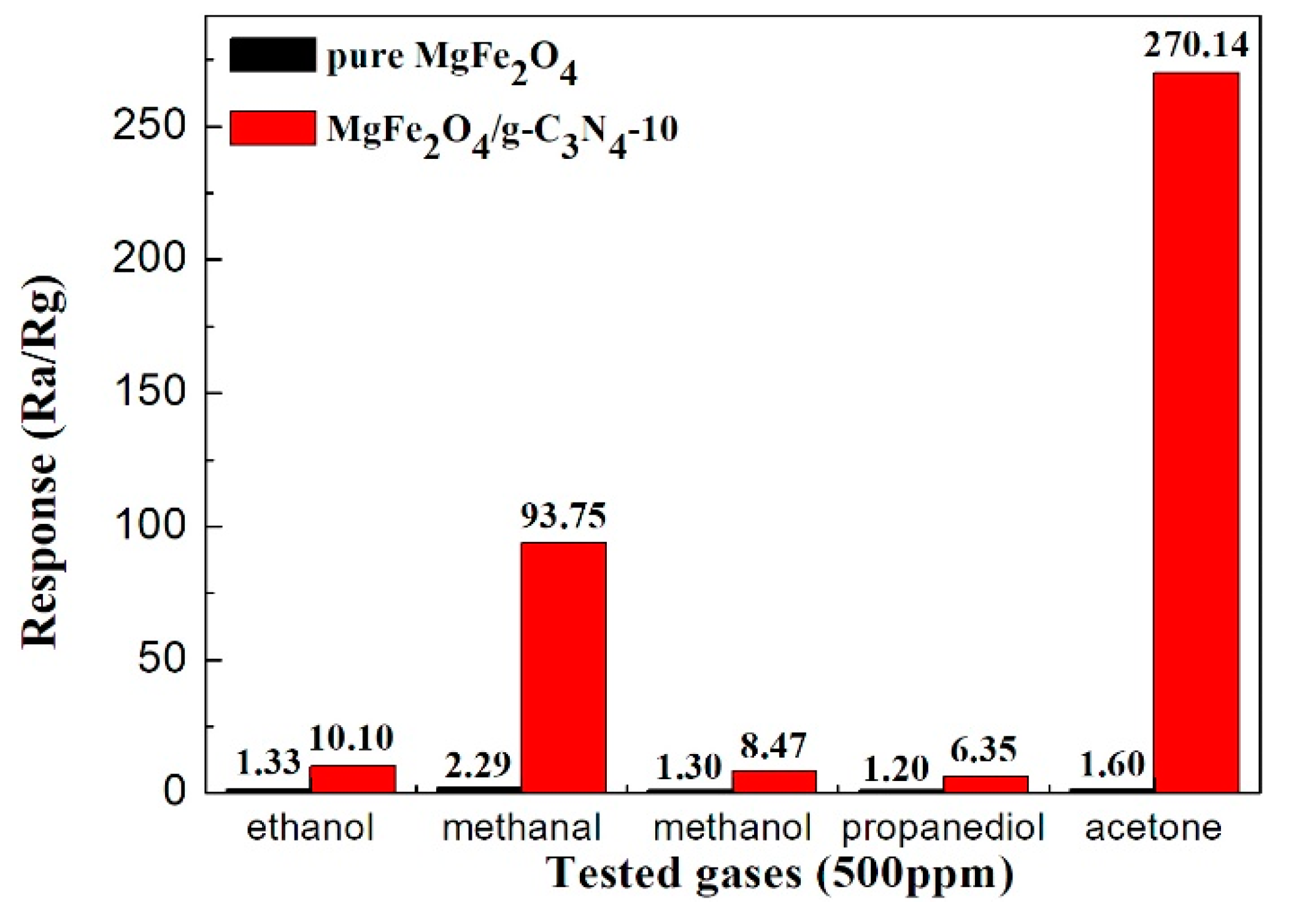
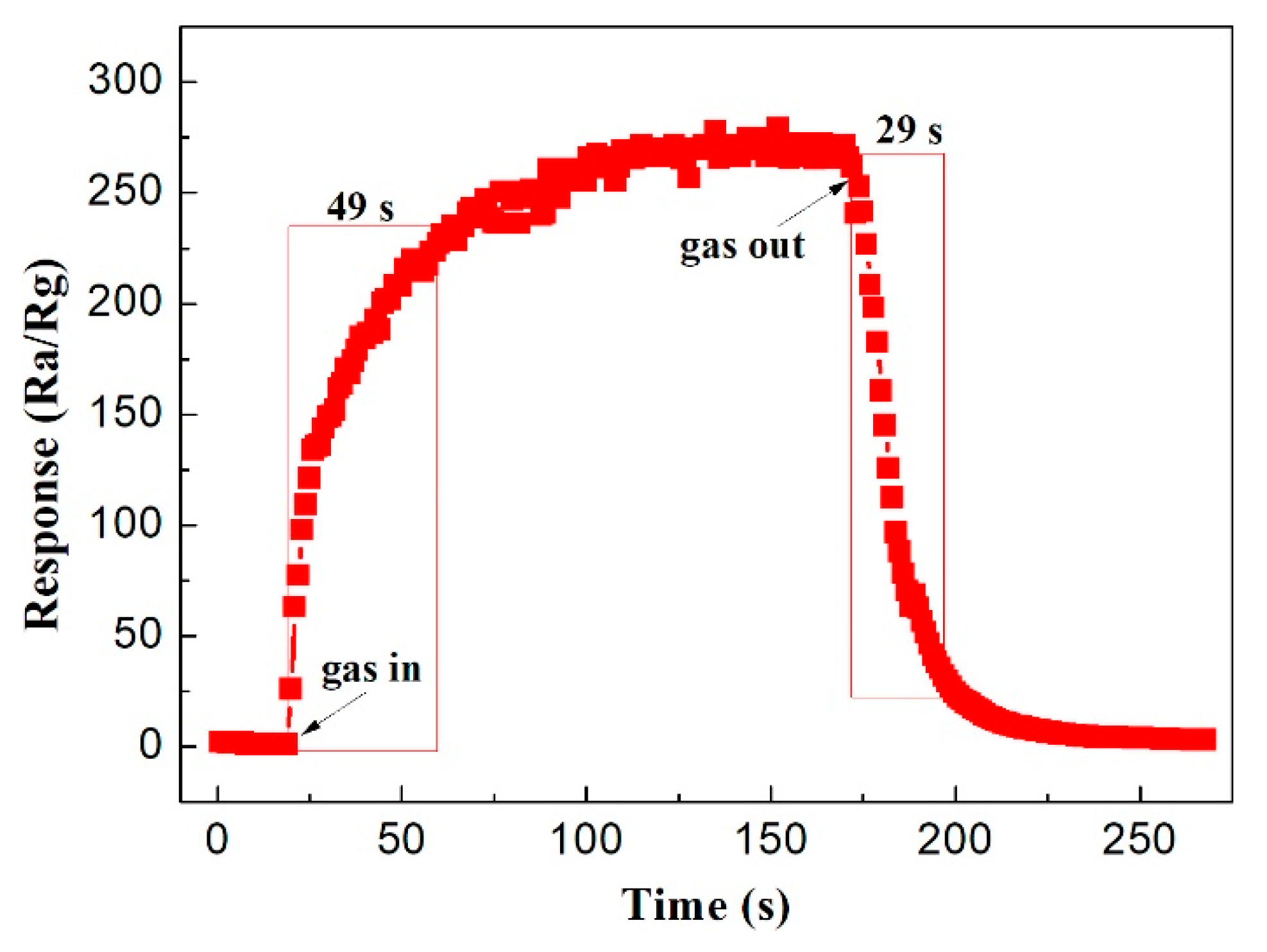
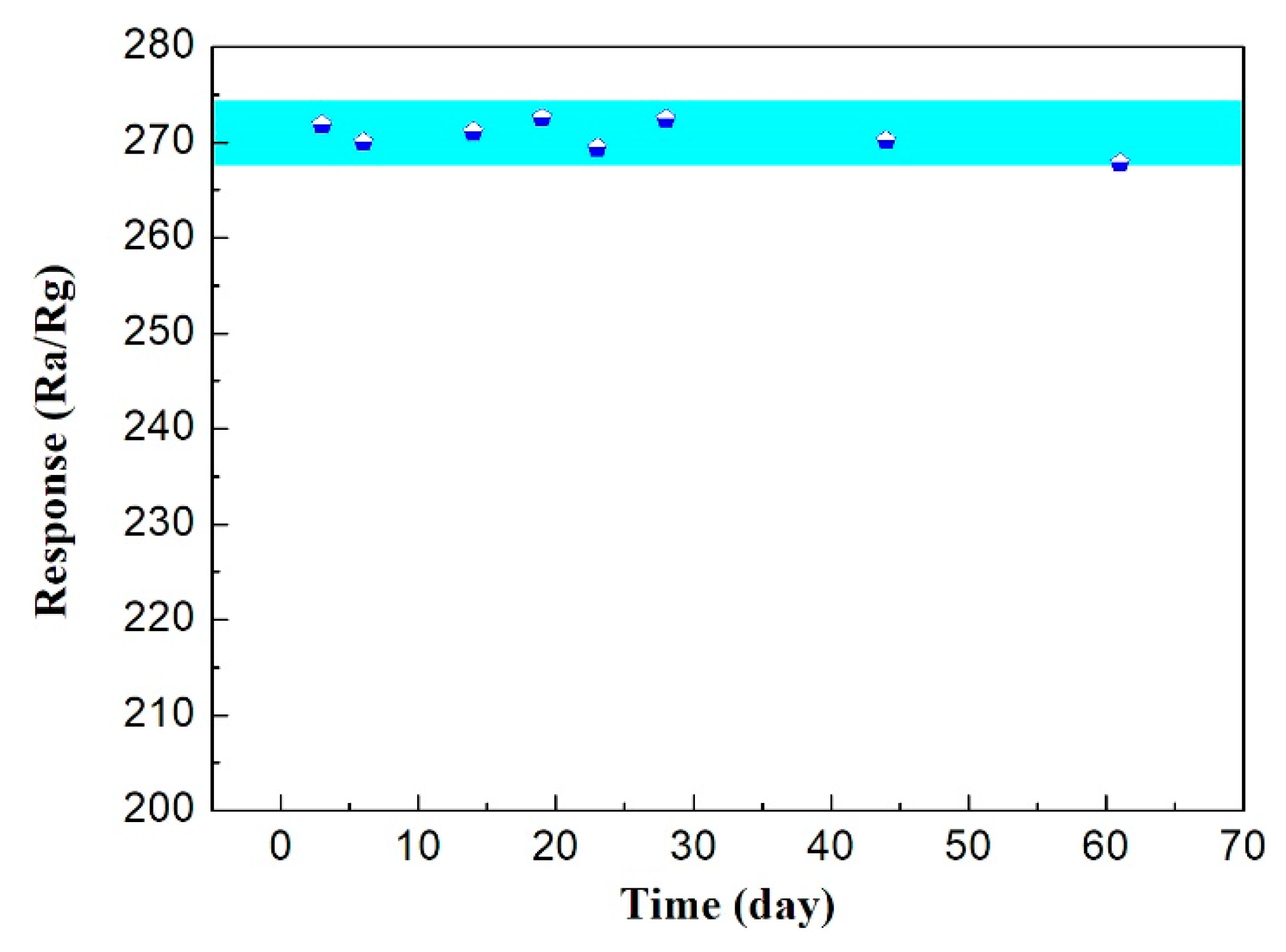
3.2. Gas Sensing Property
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Ma, W.; Jiang, F.; Cao, E.-S.; Sun, K.-M.; Cheng, L.; Song, X.-Z. Prussian blue analogue derived porous NiFe2O4 nanotubes for low-concentration acetone sensing at low working temperature. Chem. Eng. J. 2018, 338, 504–512. [Google Scholar] [CrossRef]
- Lourenço, C.; Turner, C. Breath analysis in disease diagnosis: Methodological considerations and applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef] [PubMed]
- Ueta, I.; Saito, Y.; Hosoe, M.; Okamoto, M.; Ohkita, H.; Shirai, S.; Tamura, H.; Jinno, K. Breath acetone analysis with miniaturized sample preparation device: In-needle preconcentration and subsequent determination by gas chromatography–mass spectroscopy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, N.; Sun, G.; Zhang, B.; Ma, G.; Jin, H.; Wang, Y.; Cao, J.; Zhang, Z. Facile synthesis of ZnFe2O4/α-Fe2O3 porous microrods with enhanced tea-sensing performance. J. Alloys Compd. 2018, 737, 255–262. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, G.; Li, Y.; Zhang, B.; Lin, L.; Wang, Y.; Cao, J.; Zhang, Z. Continuously improved gas-sensing performance of SnO2/Zn2SnO4 porous cubes by structure evolution and further NIO decoration. Sens. Actuators B 2018, 255, 2936–2943. [Google Scholar] [CrossRef]
- Ma, R.-J.; Li, G.-D.; Zou, X.; Gao, R.; Chen, H.; Zhao, X. Bimetallic Pt–Au nanocatalysts decorated In2O3 nests composed of ultrathin nanosheets for Type 1 diabetes diagnosis. Sens. Actuators B 2018, 270, 247–255. [Google Scholar]
- Sachdeva, S.; Agarwal, A.; Agarwal, R. Tungsten Oxide Thin Film Characterizations for Acetone Gas Detection. MAPAN 2018, 33, 57–62. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.; Lin, Y.; Sai, L.; Chang, Q.; Shi, W.; Chen, Q. Fully gravure-printed WO3/Pt-decorated rGO nanosheets composite film for detection of acetone. Sens. Actuators B 2018, 255, 1482–1490. [Google Scholar] [CrossRef]
- Qin, L.; Xu, J.; Dong, X.; Pan, Q.; Cheng, Z.; Xiang, Q.; Li, F. The template-free synthesis of square-shaped SnO2 nanowires: The temperature effect and acetone gas sensors. Nanotechnology 2008, 19, 185705. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, Y.; Xu, K.; Hou, X.; Lv, Y. Sensitive and selective acetone sensor based on its cataluminescence from nano-La2O3 surface. Sens. Actuators B 2008, 132, 243–249. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Wang, L.; Zhang, T. Metal−Organic Frameworks-Derived Hierarchical Co3O4 Structures as Efficient Sensing Materials for Acetone Detection. ACS Appl. Mater. Interfaces 2018, 10, 9765–9773. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-M.; Zhang, L.-X.; Zhu, M.-Y.; Ji, G.-J.; Zhao, L.-X.; Yin, J.; Bie, L.-J. Acetone sensing of ZnO nanosheets synthesized using room-temperature precipitation. Sens. Actuators B 2017, 249, 611–623. [Google Scholar] [CrossRef]
- Cheng, Y.; He, Y.; Li, S.; Wang, Y.; Zhao, Y.; Li, Y.; Li, H.; Liu, L. Ultra-sensitive and selective acetone gas sensor with fast response at low temperature based on Cu-doped α-Fe2O3 porous nanotubes. J. Mater. Sci. Mater. Electron. 2018, 29, 11178–11186. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of spinel. J. Am. Ceram. Soc. 2010, 82, 3279–3292. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Liu, Z.-M.; Yang, Y.; Yang, H.-F.; Shen, G.-L.; Yu, R.-Q. Simple synthesis of MgFe2O4 nanoparticles as gas sensing materials. Sens. Actuators B 2005, 107, 600–604. [Google Scholar] [CrossRef]
- Hankare, P.P.; Jadhav, S.D.; Sankpal, U.B.; Patil, R.P.; Sasikala, R.; Mulla, I.S. Gas sensing properties of magnesium ferrite prepared by co-precipitation method. J. Alloys Compd. 2009, 488, 270–272. [Google Scholar] [CrossRef]
- Godbole, R.; Rao, P.; Bhagwat, S. Magnesium ferrite nanoparticles: A rapid gas sensor for alcohol. Mater. Res. Express 2017, 4, 025032. [Google Scholar] [CrossRef]
- Patil, J.; Nadargi, D.; Mulla, I.S.; Suryavanshi, S.S. Spinel MgFe2O4 thick films: A colloidal approach for developing gas sensors. Mater. Lett. 2018, 213, 27–30. [Google Scholar] [CrossRef]
- Lu, J.; Jia, N.; Cheng, L.; Liang, K.; Huang, J.; Li, J. Rgo/CoTiO3 nanocomposite with enhanced gas sensing performance at low working temperature. J. Alloys Compd. 2018, 739, 227–234. [Google Scholar] [CrossRef]
- Hassan, M.; Wang, Z.H.; Huang, W.R.; Li, M.Q.; Liu, J.W.; Chen, J.F. Ultrathin tungsten oxide nanowires/reduced graphene oxide composites for toluene sensing. Sensors 2017, 17, 2245. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, N.; Sun, G.; Zhang, B.; Lin, L.; Jin, H.; Wang, Y.; Bala, H.; Cao, J.; Zhang, Z. In situ decoration of Zn2SnO4 nanoparticles on reduced graphene oxide for high performance ethanol sensor. Ceram. Int. 2018, 44, 6836–6842. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Hu, Y.; Li, L.; Zhang, L.; Lv, Y. Dielectric barrier discharge plasma-assisted fabrication of g-C3N4-Mn3O4 composite for high-performance cataluminescence H2S gas sensor. Sens. Actuators B 2017, 239, 1177–1184. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Sun, G.; Jia, T.; Jia, L.; Zhang, F.; Lin, L.; Zhang, B.; Cao, J.; Zhang, Z. Carbon nitride decorated ball-flower like Co3O4 hybrid composite: Hydrothermal synthesis and ethanol gas sensing application. Nanomaterials 2018, 8, E132. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, S.; Xu, G.; Peng, Y.; Gong, L.; Li, X.; Li, Y.; Lin, Y.; Chen, G. Highly photoactive heterojunction based on g-C3N4 nanosheets decorated with dendritic zinc(ii) phthalocyanine through axial coordination and its ultrasensitive enzyme-free sensing of choline. RSC Adv. 2014, 4, 58226–58230. [Google Scholar] [CrossRef]
- Shao, L.; Jiang, D.; Xiao, P.; Zhu, L.; Meng, S.; Chen, M. Enhancement of g-C3N4 nanosheets photocatalysis by synergistic interaction of ZnS microsphere and rGO inducing multistep charge transfer. Appl. Catal. B 2016, 198, 200–210. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, H.; Zhang, B.; Gong, Y.; Wang, X.; Sun, G.; Bala, H.; Zhang, Z. Synthesis of g-C3N4 nanosheet modified SnO2 composites with improved performance for ethanol gas sensing. RSC Adv. 2017, 7, 25504–25511. [Google Scholar] [CrossRef]
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Yang, Z.; Ge, M.; Zhang, Y. One-step synthesis of 2D C3N4-tin oxide gas sensors for enhanced acetone vapor detection. Sens. Actuators B 2017, 253, 641–651. [Google Scholar] [CrossRef]
- Cao, J.; Gong, Y.; Wang, Y.; Zhang, B.; Zhang, H.; Sun, G.; Bala, H.; Zhang, Z. Cocoon-like ZnO decorated graphitic carbon nitride nanocomposite: Hydrothermal synthesis and ethanol gas sensing application. Mater. Lett. 2017, 198, 76–80. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, B.; Gong, Y.; Zhang, H.; Sun, G.; Bala, H.; Zhang, Z. Calcination method synthesis of SnO2/g-C3N4 composites for a high-performance ethanol gas sensing application. Nanomaterials 2017, 7, E98. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhao, Y.; Xu, K.; Tong, Y.; Zhao, F. Preparation and comparison of catalytic performance for nano MgFe2O4, go-loaded MgFe2O4 and go-coated MgFe2O4 nanocomposites. Ceram. Int. 2016, 42, 18844–18850. [Google Scholar] [CrossRef]
- Ao, Z.M.; Peeters, F.M. High-capacity hydrogen storage in al-adsorbed graphene. Phys. Rev. B 2010, 81, 2498–2502. [Google Scholar] [CrossRef]
- Tshabalala, Z.; Shingange, K.; Dhonge, B.; Ntwaeaborwa, O.; Mhlongo, G.; Motaung, D. Fabrication of ultra-high sensitive and selective CH4, room temperature gas sensing of TiO2 nanorods: Detailed study on the annealing temperature. Sens. Actuators B 2017, 238, 402–419. [Google Scholar] [CrossRef]
- Liao, L.; Lu, H.B.; Li, J.C.; He, H.; Wang, D.F.; And, D.J.F.; Liu, C.; Zhang, W.F. Size dependence of gas sensitivity of ZnO nanorods. J. Phys. Chem. C 2007, 111, 1900–1903. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhowmick, T.; Majumder, S. Multi-layered zinc oxide-graphene composite thin films for selective nitrogen dioxide sensing. J. Appl. Phys. 2018, 123, 084501. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, Y.; Zhang, Z.; Cao, J. Highly Sensitive Acetone Gas Sensor Based on g-C3N4 Decorated MgFe2O4 Porous Microspheres Composites. Sensors 2018, 18, 2211. https://doi.org/10.3390/s18072211
Zhang R, Wang Y, Zhang Z, Cao J. Highly Sensitive Acetone Gas Sensor Based on g-C3N4 Decorated MgFe2O4 Porous Microspheres Composites. Sensors. 2018; 18(7):2211. https://doi.org/10.3390/s18072211
Chicago/Turabian StyleZhang, Run, Yan Wang, Zhanying Zhang, and Jianliang Cao. 2018. "Highly Sensitive Acetone Gas Sensor Based on g-C3N4 Decorated MgFe2O4 Porous Microspheres Composites" Sensors 18, no. 7: 2211. https://doi.org/10.3390/s18072211




