Fluorescent Nanobiosensors for Sensing Glucose
Abstract
:1. Introduction
2. Fluorescence-Emitting/Interacting Nanomaterials
2.1. Semiconductor Quantum Dots
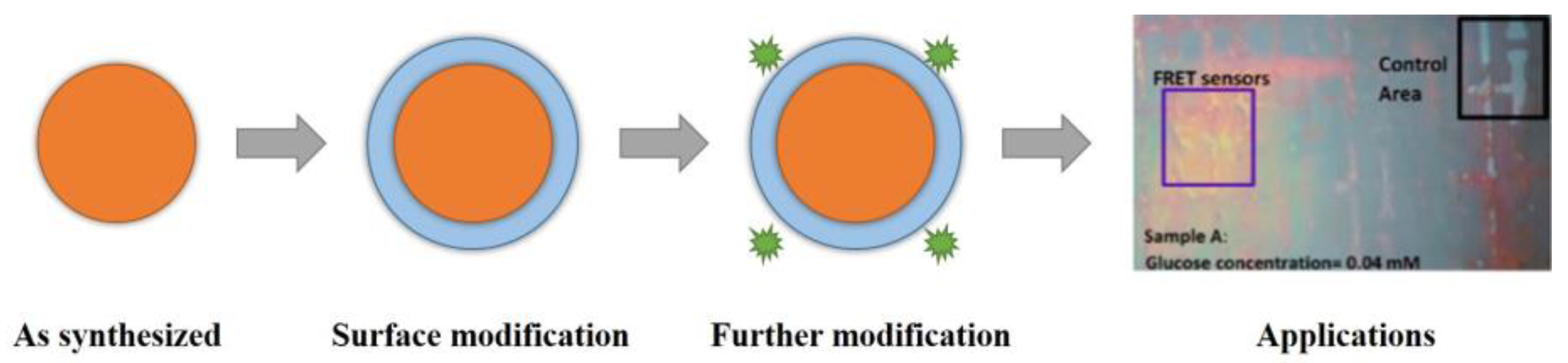
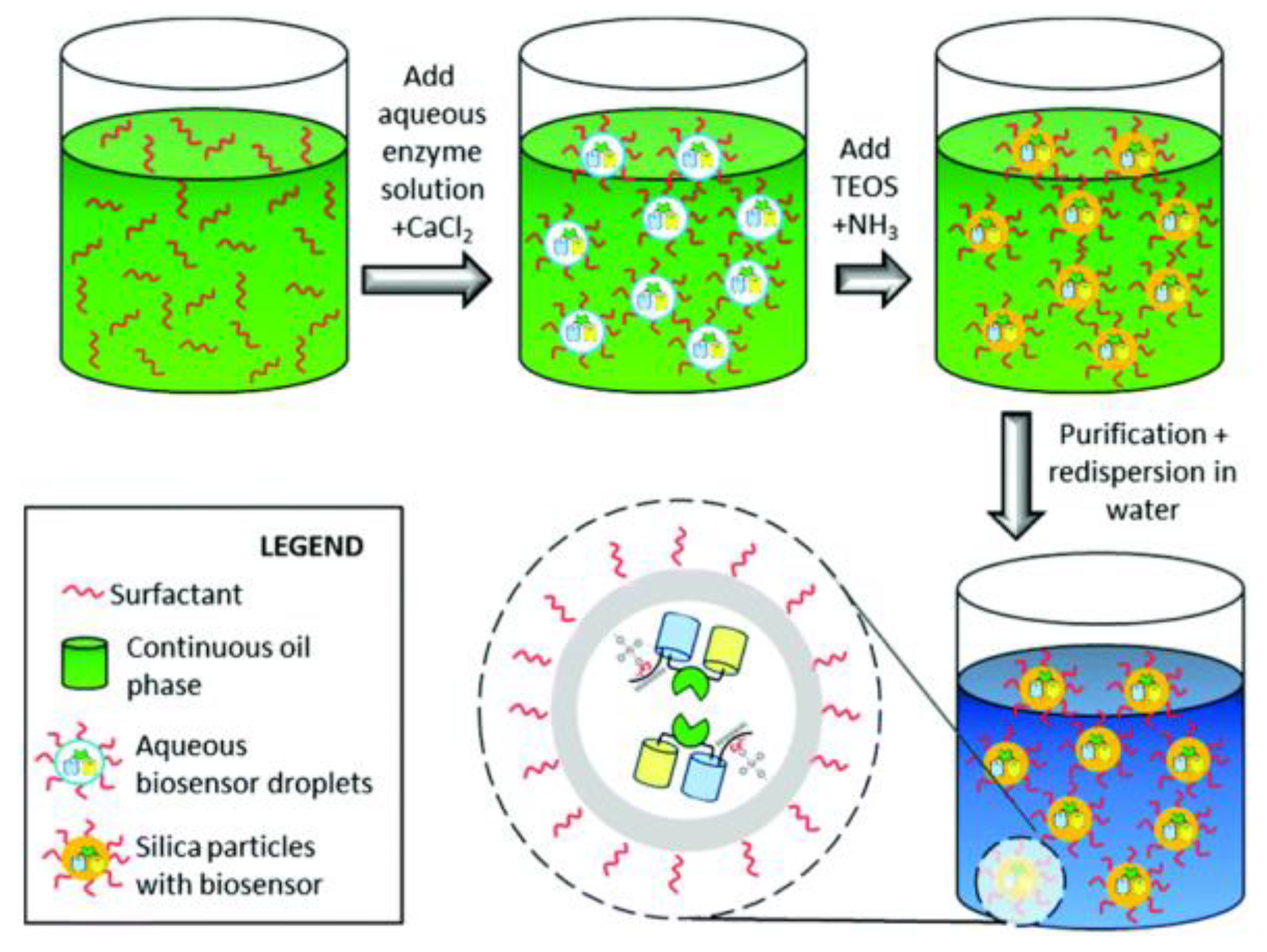
2.2. Fluorescent Silica Nanoparticles
2.3. Upconverting Nanoparticles
2.4. Gold/Silver Nanoparticles/Nanoclusters
2.5. Fluorescent Carbon Nanomaterials
2.6. Graphene Nanomaterials
2.7. Table of Summary
3. Application of Fluorescent Nanomaterials in Glucose Sensing
3.1. Fluorescenct Nanobiosensor Detection Principle
3.2. Glucose Recognition Biomolecules
3.3. Glucose Fluorescent Nanobiosensors
4. Future Prospects and Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Steiner, M.-S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805–4839. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.S.; Toumazou, C.; Cass, A.E.G.; Johnston, D.G. Glucose sensors: A review of current and emerging technology. Diabet. Med. 2009, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. Non-invasive glucose monitoring technology in diabetes management: A review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Zhou, W.; Lu, J.; Lu, C. Luminescent films for chemo- and biosensing. Chem. Soc. Rev. 2015, 44, 6981–7009. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Hussain, F.; Evans, N.D.; Rolinski, O.J.; Birch, D.J.S. Fluorescence-based glucose sensors. Biosens. Bioelectron. 2005, 20, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C. Overview of Fluorescence Glucose Sensing: A Technology with a Bright Future. J. Diabetes Sci. Technol 2012, 6, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, S.; Ghirlanda, G.; Parracino, A.; de Champdoré, M.; Scognamiglio, V.; Staiano, M.; Rossi, M. Fluorescence Biosensors for Continuously Monitoring the Blood Glucose Level of Diabetic Patients. In Glucose Sensing; Geddes, C., Lakowicz, J., Eds.; Springer: New York City, NY, USA, 2006; Volume 11, pp. 117–130. [Google Scholar]
- He, X.-P.; Hu, X.-L.; James, T.D.; Yoon, J.; Tian, H. Multiplexed photoluminescent sensors: Towards improved disease diagnostics. Chem. Soc. Rev. 2017, 46, 6687–6696. [Google Scholar] [CrossRef] [PubMed]
- Björnmalm, M.; Faria, M.; Caruso, F. Increasing the Impact of Materials in and beyond Bio-Nano Science. J. Am. Chem. Soc. 2016, 138, 13449–13456. [Google Scholar] [CrossRef] [PubMed]
- Tel-Vered, R.; Yehezkeli, O.; Willner, I. Biomolecule/Nanomaterial Hybrid Systems for Nanobiotechnology. In Nano-Biotechnology for Biomedical and Diagnostic Research; Zahavy, E., Ordentlich, A., Yitzhaki, S., Shafferman, A., Eds.; Springer: Dordrecht, Netherlands, 2012; pp. 1–16. [Google Scholar]
- Petrovic, N.; Petrovic, M.J.; Sreckovic, S.; Jovanovic, S.; Todorovic, D.; Vulovic, T.S. Nanotechnology in Ophthalmology. In Commercialization of Nanotechnologies–A Case Study Approach; Brabazon, D., Pellicer, E., Zivic, F., Sort, J., Dolors Baró, M., Grujovic, N., Choy, K.-L., Eds.; Springer: Cham, Switzerland, 2018; pp. 275–297. [Google Scholar]
- Parolo, C.; Merkoci, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lieber, C.M. Nano-Bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Heinemann, L.; Ramírez, A.; Zehe, A. Options for the Development of Noninvasive Glucose Monitoring: Is Nanotechnology an Option to Break the Boundaries? J. Diabetes Sci. Technol 2016, 10, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosens. Bioelectron. 2013, 47, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, M.; Ptitsyn, A.; McLamore, E.S.; Claussen, J.C. Nanomaterial-mediated Biosensors for Monitoring Glucose. J. Diabetes Sci. Technol 2014, 8, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.D. Nanoparticles and nanocomposites for fluorescence sensing and imaging. Methods Appli. Fluoresc. 2013, 1, 022001. [Google Scholar]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Anastassiou, C.; O’Hare, D. Biosensor Design and Interfacing. In Body Sensor Networks; Yang, G.-Z., Ed.; Springer: London, UK, 2006; pp. 41–87. [Google Scholar]
- Xu, K.; Purahmad, M.; Brenneman, K.; Meshik, X.; Farid, S.; Poduri, S.; Pratap, P.; Abell, J.; Zhao, Y.; Nichols, B.; et al. Design and Applications of Nanomaterial-Based and Biomolecule-Based Nanodevices and Nanosensors. In Design and Applications of Nanomaterials for Sensors; Seminario, J.M., Ed.; Springer: Dordrecht, Netherlands, 2014; Volume 16, pp. 61–97. [Google Scholar]
- Mahato, K.; Baranwal, A.; Srivastava, A.; Maurya, P.K.; Chandra, P. Smart Materials for Biosensing Applications. In Techno-Societal 2016: Proceedings of the International Conference on Advanced Technologies for Societal Applications; Pawar, P.M., Ronge, B.P., Balasubramaniam, R., Seshabhattar, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 421–431. [Google Scholar]
- Howes, P.D.; Chandrawati, R.; Stevens, M.M. Colloidal nanoparticles as advanced biological sensors. Science 2014, 346, 1247390. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Hötzer, B.; Medintz, I.L.; Hildebrandt, N. Fluorescence in Nanobiotechnology: Sophisticated Fluorophores for Novel Applications. Small 2012, 8, 2297–2326. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, Biology, and Medicine of Fluorescent Nanomaterials and Related Systems: New Insights into Biosensing, Bioimaging, Genomics, Diagnostics, and Therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Villalonga, R.; Gimenez, C.; Sancenon, F.; Marcos, M.D.; Martinez-Manez, R.; Diez, P.; Pingarron, J.M.; Amoros, P. Glucose-triggered release using enzyme-gated mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 6391–6393. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Materials 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Fischer, T.; Singh, A.P.; Singh, T.; Hernández-Ramírez, F.; Prades, D.; Mathur, S. Metal Oxide Nano-architectures and Heterostructures for Chemical Sensors. In Metal Oxide Nanomaterials for Chemical Sensors; Carpenter, M.A., Mathur, S., Kolmakov, A., Eds.; Springer: New York City, NY, USA, 2013; pp. 397–438. [Google Scholar]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Zhang, X.; Wang, J. Biofunctionalization of Nanomaterials. In NanoBiosensing; Springer: New York City, NY, USA, 2011; pp. 1–38. [Google Scholar]
- Yuce, M.; Kurt, H. How to make nanobiosensors: Surface modification and characterisation of nanomaterials for biosensing applications. RSC Adv. 2017, 7, 49386–49403. [Google Scholar] [CrossRef]
- Walper, S.A.; Turner, K.B.; Medintz, I.L. Enzymatic bioconjugation of nanoparticles: Developing specificity and control. Curr. Opin. Biotechnol. 2015, 34, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing Nanoparticles with Biological Molecules: Developing Chemistries that Facilitate Nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljevic, M.; Krizkova, S.; Vaculovicova, M.; Kizek, R.; Adam, V. Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens. Bioelectron. 2015, 74, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Del. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canosa, J.B.; Wu, M.; Susumu, K.; Petryayeva, E.; Jennings, T.L.; Dawson, P.E.; Algar, W.R.; Medintz, I.L. Recent progress in the bioconjugation of quantum dots. Coord. Chem. Rev. 2014, 263–264, 101–137. [Google Scholar] [CrossRef]
- Zhang, T.; Stilwell, J.L.; Gerion, D.; Ding, L.; Elboudwarej, O.; Cooke, P.A.; Gray, J.W.; Alivisatos, A.P.; Chen, F.F. Cellular Effect of High Doses of Silica-Coated Quantum Dot Profiled with High Throughput Gene Expression Analysis and High Content Cellomics Measurements. Nano Lett. 2006, 6, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ye, J.; Tong, L.; Tang, B. A New Route to the Considerable Enhancement of Glucose Oxidase (GOx) Activity: The Simple Assembly of a Complex from CdTe Quantum Dots and GOx, and Its Glucose Sensing. Chem. Eur. J. 2008, 14, 9633–9640. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Spiller, D.G.; Beckett, A.; Prior, I.A.; Sée, V. Highly Stable Dextran-Coated Quantum Dots for Biomolecular Detection and Cellular Imaging. Chem. Mater. 2010, 22, 6361–6369. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, J.-J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst 2013, 138, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Ow, H.; Wiesner, U. Fluorescent core-shell silica nanoparticles: Towards "Lab on a Particle" architectures for nanobiotechnology. Chem. Soc. Rev. 2006, 35, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Han, W.S.; Lee, H.Y.; Jung, S.H.; Lee, S.J.; Jung, J.H. Silica-based chromogenic and fluorogenic hybrid chemosensor materials. Chem. Soc. Rev. 2009, 38, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tu, D.; Zhu, H.; Chen, X. Lanthanide-doped luminescent nanoprobes: Controlled synthesis, optical spectroscopy, and bioapplications. Chem. Soc. Rev. 2013, 42, 6924–6958. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Sun, T.; Chen, B.; Chen, X.; Ai, F.; Zhu, X.; Li, M.; Zhang, W.; Zhu, G.; Wang, F. A General Strategy for Ligand Exchange on Upconversion Nanoparticles. Inorg. Chem. 2017, 56, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Butt, H.-J. Near-Infrared-Sensitive Materials Based on Upconverting Nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F. Upconversion Nanoparticles for Biosensing. In Photon Upconversion Nanomaterials; Springer: Berlin, Germany, 2015; pp. 255–284. [Google Scholar]
- Zheng, W.; Huang, P.; Tu, D.; Ma, E.; Zhu, H.; Chen, X. Lanthanide-doped upconversion nano-bioprobes: Electronic structures, optical properties, and biodetection. Chem. Soc. Rev. 2015, 44, 1379–1415. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Schäfer, H. Upconverting Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S. Perspectives for Upconverting Nanoparticles. ACS Nano 2017, 11, 10644–10653. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Diez, I.; Ras, R.H.A. Fluorescent silver nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nature Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, J.-J.; Xu, K. Fluorescent metal nanoclusters: From synthesis to applications. Trends Anal. Chem. 2014, 58, 90–98. [Google Scholar] [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Del. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Rabti, A.; Raouafi, N.; Merkoçi, A. Bio(Sensing) devices based on ferrocene-functionalized graphene and carbon nanotubes. Carbon 2016, 108, 481–514. [Google Scholar] [CrossRef]
- Du, Y.; Guo, S. Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Minami, K.; Shrestha, L.K. Nanoarchitectonics for carbon-material-based sensors. Analyst 2016, 141, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.-P.; Zhang, G.-J.; Zhang, Z.-L.; Pang, D.-W. Photoluminescent Properties of Carbon Nanodots. In Carbon Nanoparticles and Nanostructures; Yang, N., Jiang, X., Pang, D.-W., Eds.; Springer: Cham, Switzerland, 2016; pp. 239–256. [Google Scholar]
- Dong, Y.; Cai, J.; Chi, Y. Carbon Based Dots and Their Luminescent Properties and Analytical Applications. In Carbon Nanoparticles and Nanostructures; Yang, N., Jiang, X., Pang, D.-W., Eds.; Springer: Cham, Switzerland, 2016; pp. 161–238. [Google Scholar]
- Gerald, U. Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Springer: Berlin, Germany, 2017; pp. 1–31. [Google Scholar]
- Wang, H.; Yi, J.; Yu, Y.; Zhou, S. NIR upconversion fluorescence glucose sensing and glucose-responsive insulin release of carbon dot-immobilized hybrid microgels at physiological pH. Nanoscale 2017, 9, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Li, X.; Zhao, Q.; Liu, B.; Liu, S.; Wang, S. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017, 496, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Liu, Y.; Shu, Q.-W.; Liang, J.-M.; Zhang, F.; Chen, X.-P.; Deng, X.-Y.; Swihart, M.T.; Tan, K.-J. One-Pot Hydrothermal Synthesis of Carbon Dots with Efficient Up- and Down-Converted Photoluminescence for the Sensitive Detection of Morin in a Dual-Readout Assay. Langmuir 2017, 33, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Del. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Joshi, H.; Zhao, Y. Graphene-Based Materials in Biosensing, Bioimaging, and Therapeutics. In Graphene-based Materials in Health and Environment: New Paradigms; Gonçalves, G., Marques, P., Vila, M., Eds.; Springer: Cham, Switzerland, 2016; pp. 35–61. [Google Scholar]
- Eigler, S.; Hirsch, A. Chemistry with Graphene and Graphene Oxide-Challenges for Synthetic Chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef] [PubMed]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors. 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Rao, G. The Glucose Binding Protein as Glucose Sensor. In Glucose Sensing; Geddes., C., Lakowicz, J., Eds.; Springer: New York City, NY, USA, 2006; Volume 11, pp. 323–331. [Google Scholar]
- Veetil, J.V.; Jin, S.; Ye, K. A glucose sensor protein for continuous glucose monitoring. Biosens. Bioelectron. 2010, 26, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C. Engineering periplasmic ligand binding proteins as glucose nanosensors. Nano Reviews 2011, 2, 5743. [Google Scholar] [CrossRef] [PubMed]
- Faccio, G.; Bannwarth, M.B.; Schulenburg, C.; Steffen, V.; Jankowska, D.; Pohl, M.; Rossi, R.M.; Maniura-Weber, K.; Boesel, L.F.; Richter, M. Encapsulation of FRET-based glucose and maltose biosensors to develop functionalized silica nanoparticles. Analyst 2016, 141, 3982–3984. [Google Scholar] [CrossRef] [PubMed]
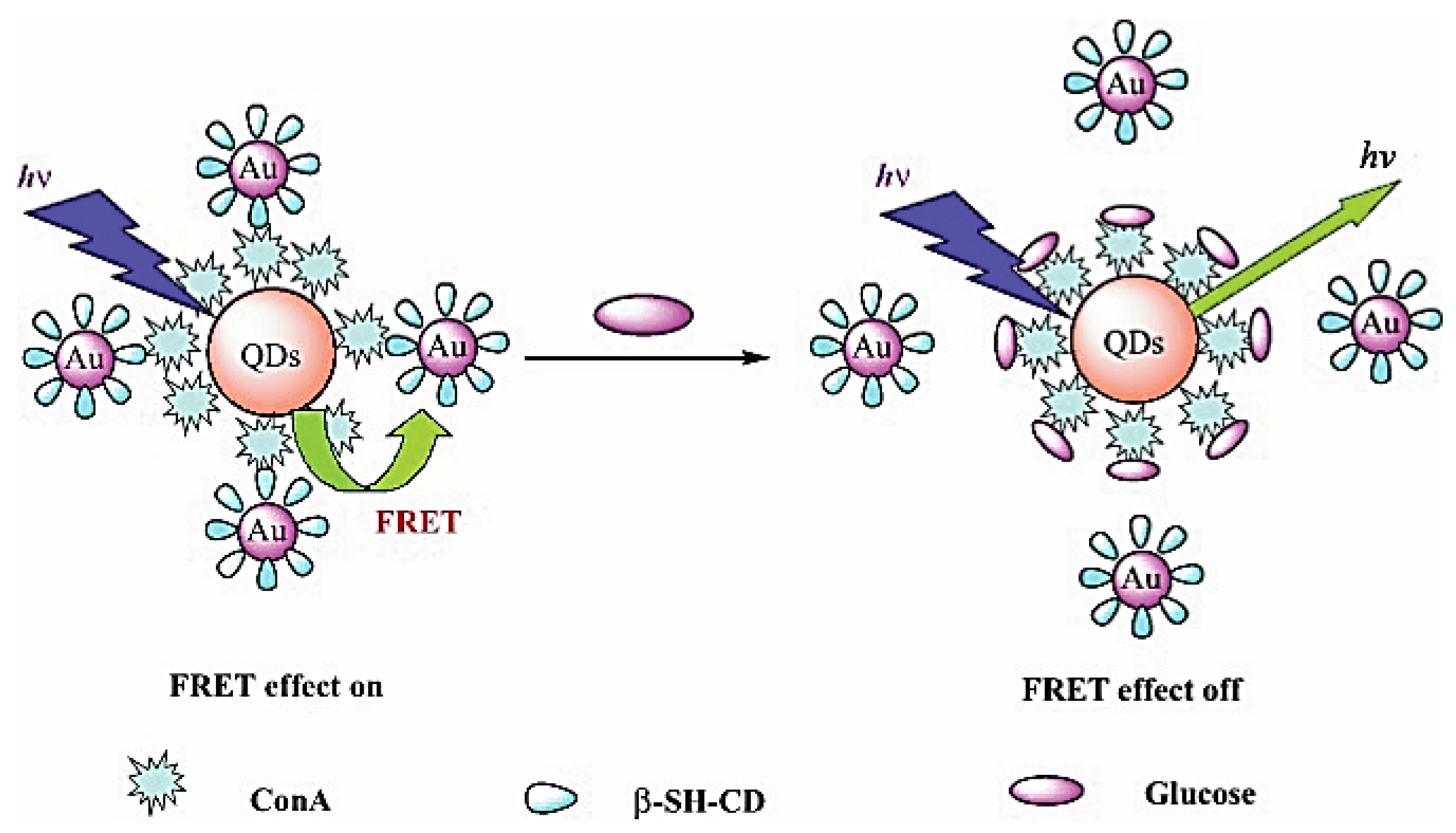
- Tang, B.; Cao, L.; Xu, K.; Zhuo, L.; Ge, J.; Li, Q.; Yu, L. A New Nanobiosensor for Glucose with High Sensitivity and Selectivity in Serum Based on Fluorescence Resonance Energy Transfer (FRET) between CdTe Quantum Dots and Au Nanoparticles. Chem. Eur. J. 2008, 14, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Vaishanav, S.K.; Korram, J.; Nagwanshi, R.; Ghosh, K.K.; Satnami, M.L. Mn2+ doped-CdTe/ZnS modified fluorescence nanosensor for detection of glucose. Sens. Actuators B: Chem. 2017, 245, 196–204. [Google Scholar] [CrossRef]
- Bahshi, L.; Freeman, R.; Gill, R.; Willner, I. Optical Detection of Glucose by Means of Metal Nanoparticles or Semiconductor Quantum Dots. Small 2009, 5, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Zheng, Z.; Yue, X.; Dai, Z.; Liu, S.; Tang, Z. Glucose Biosensor Based on Nanocomposite Films of CdTe Quantum Dots and Glucose Oxidase. Langmuir 2009, 25, 6580–6586. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.D.; Krumholz, D.M.; Sack, R.A.; Morris, C. Tear Glucose Dynamics in Diabetes Mellitus. Curr. Eye Res. 2006, 31, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Chen, L.; Li, J.; Luzak, K. Harnessing a Nanostructured Fluorescence Energy Transfer Sensor for Quick Detection of Extremely Small Amounts of Glucose. J. Diabetes Sci. Technol 2013, 7, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hodge, W.G. Contact Lens Integrated with a Biosensor for the Detection of Glucose and Other Components in Tears. U.S. Patent US8385998B2, 26 February 2013. [Google Scholar]
- Chen, L.; Tse, W.H.; Chen, Y.; McDonald, M.W.; Melling, J.; Zhang, J. Nanostructured biosensor for detecting glucose in tear by applying fluorescence resonance energy transfer quenching mechanism. Biosens. Bioelectron. 2017, 91, 393–399. [Google Scholar] [PubMed]
- Yoon, H.; Ahn, J.-H.; Barone, P.W.; Yum, K.; Sharma, R.; Boghossian, A.A.; Han, J.-H.; Strano, M.S. Periplasmic Binding Proteins as Optical Modulators of Single-Walled Carbon Nanotube Fluorescence: Amplifying a Nanoscale Actuator. Angew. Chem. Int. Ed. 2011, 50, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, L.; Li, A.; Tang, J.; Wang, S.; Xu, S.; Wang, L. Simultaneous detection of hydrogen peroxide and glucose in human serum with upconversion luminescence. Biosens. Bioelectron. 2015, 68, 204–209. [Google Scholar] [PubMed]
- Zhang, C.; Yuan, Y.; Zhang, S.; Wang, Y.; Liu, Z. Biosensing Platform Based on Fluorescence Resonance Energy Transfer from Upconverting Nanocrystals to Graphene Oxide. Angew. Chem. Int. Ed. 2011, 50, 6851–6854. [Google Scholar]
- Dong, J.X.; Gao, Z.F.; Zhang, Y.; Li, B.L.; Zhang, W.; Lei, J.L.; Li, N.B.; Luo, H.Q. The pH-switchable agglomeration and dispersion behavior of fluorescent Ag nanoclusters and its applications in urea and glucose biosensing. NPG Asia Materials 2016, 8, e335. [Google Scholar] [CrossRef]
- Ma, J.-L.; Yin, B.-C.; Wu, X.; Ye, B.-C. Simple and Cost-Effective Glucose Detection Based on Carbon Nanodots Supported on Silver Nanoparticles. Anal. Chem. 2016, 89, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R. Nano Dot Technology Enters Clinical Trials. J. Natl. Cancer Inst. 2011, 103, 1428–1429. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]





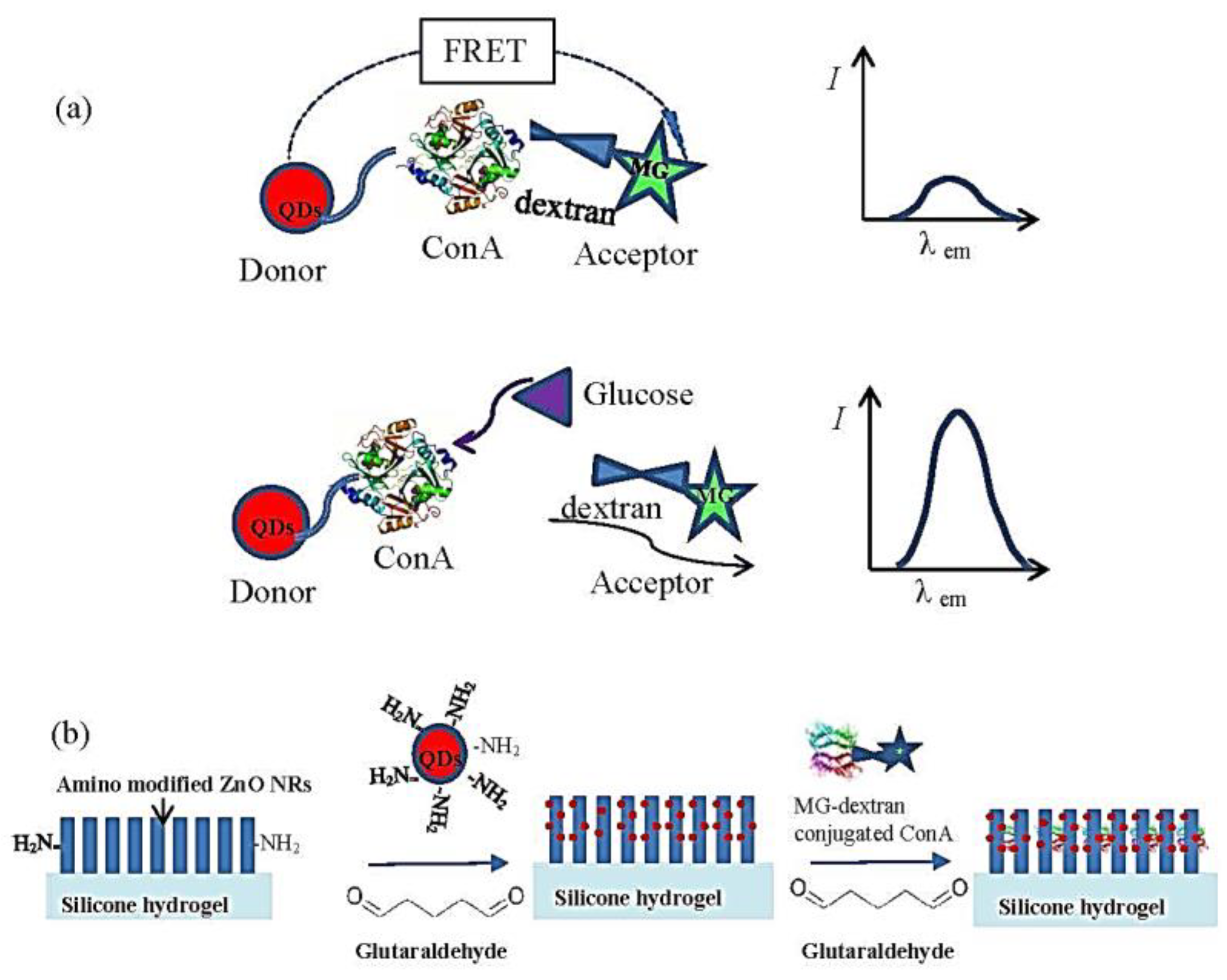
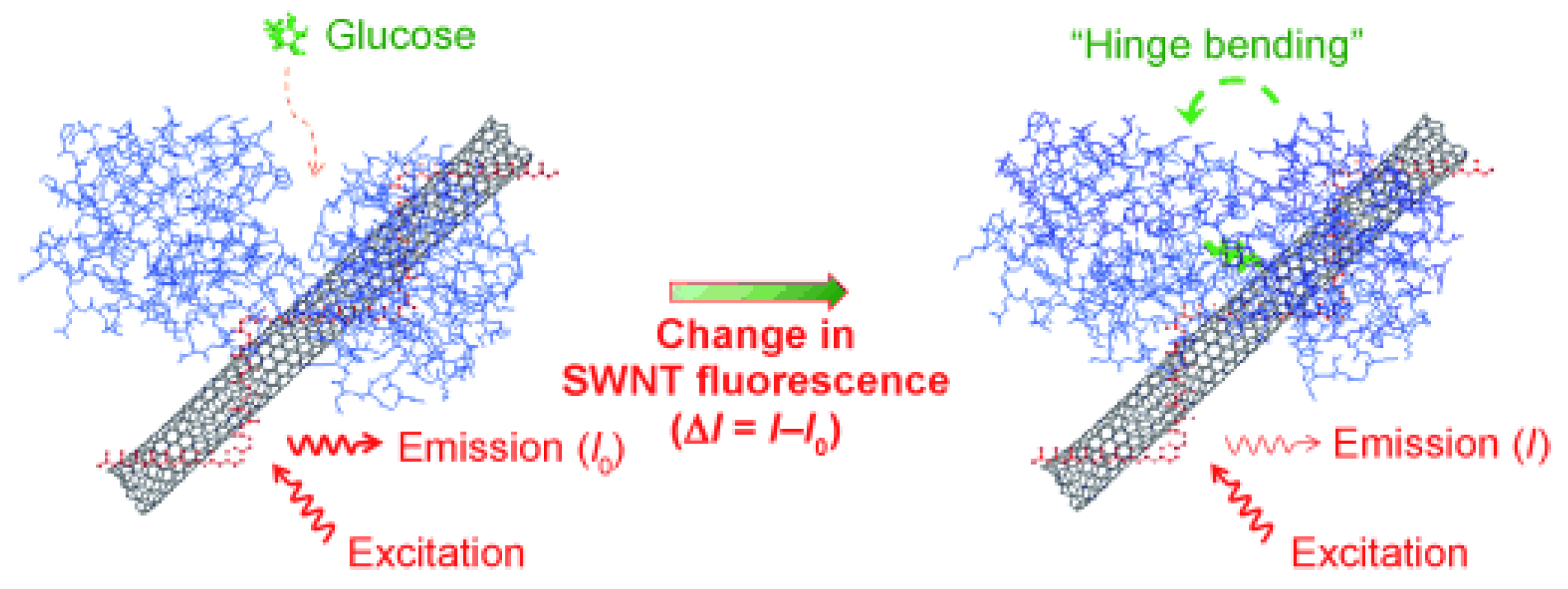
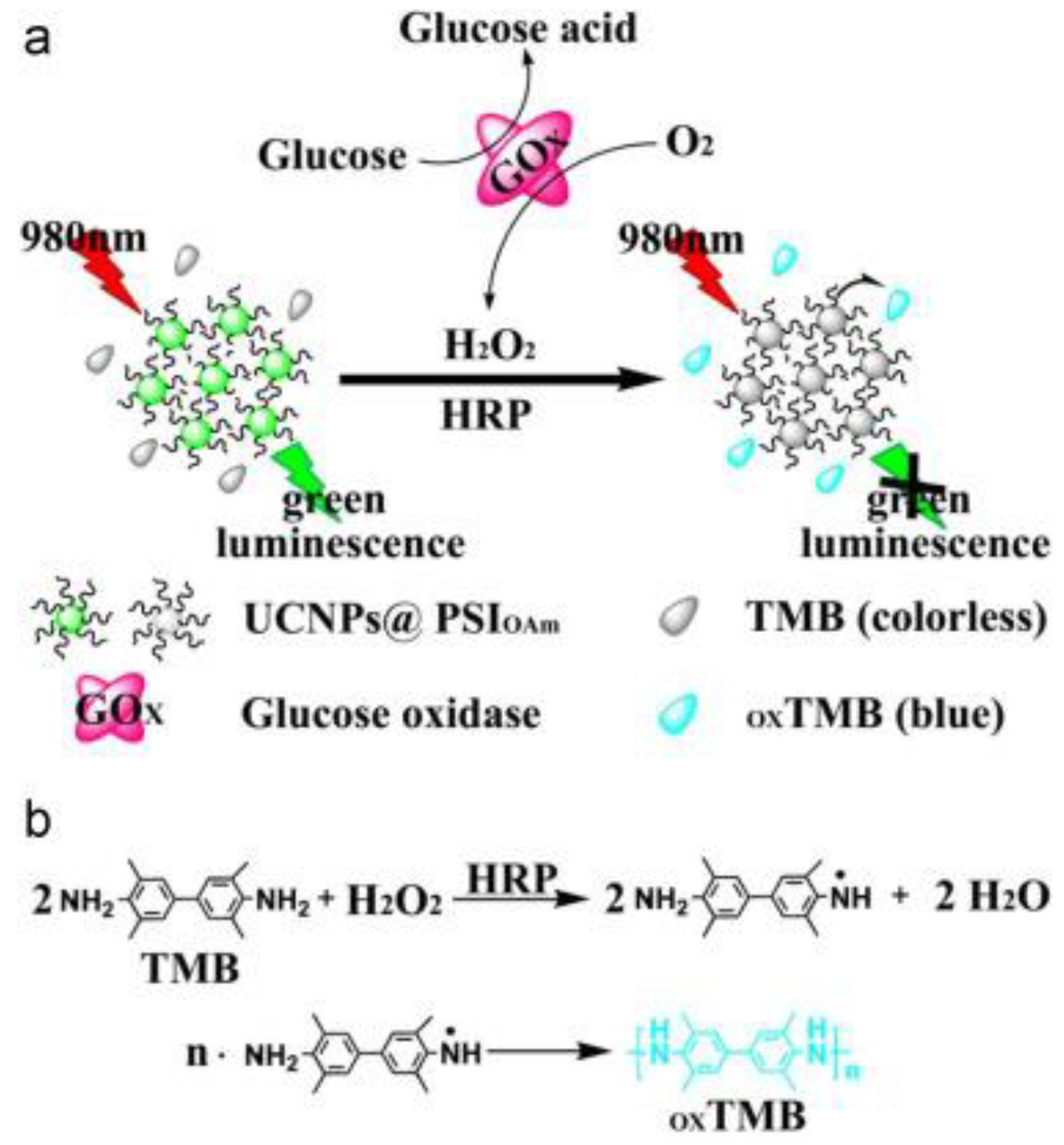
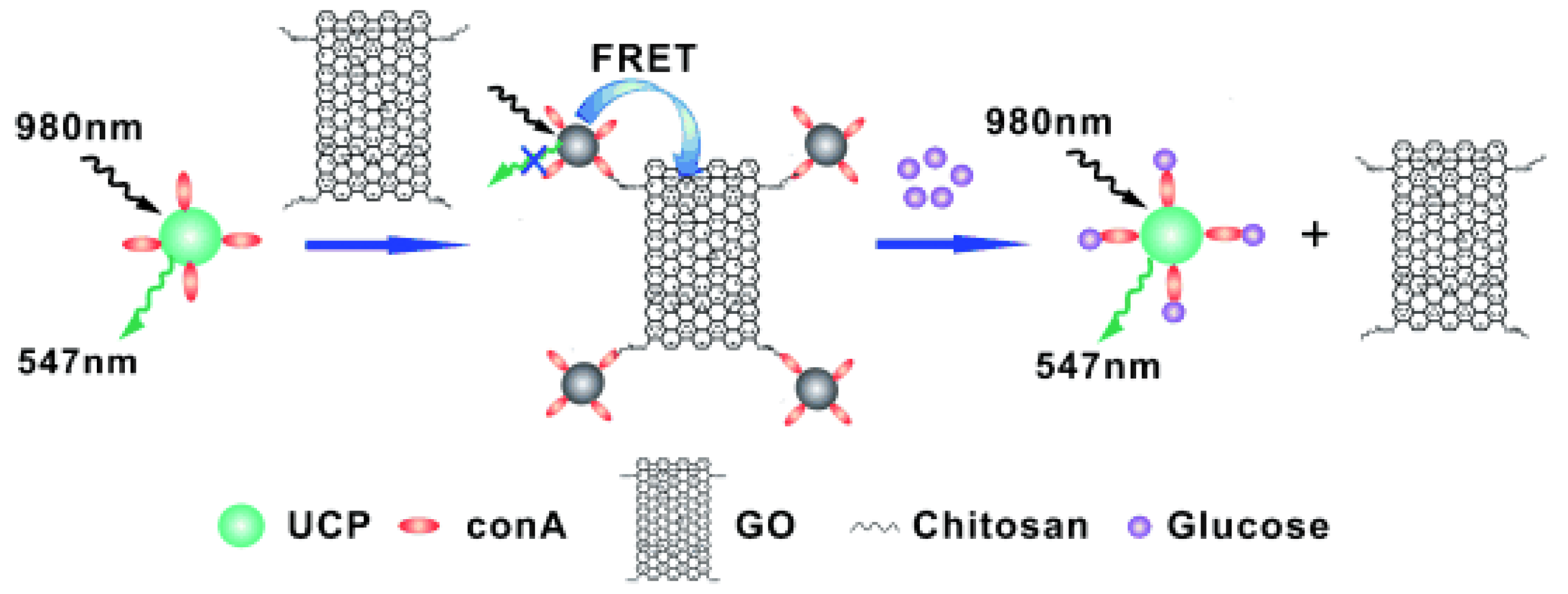
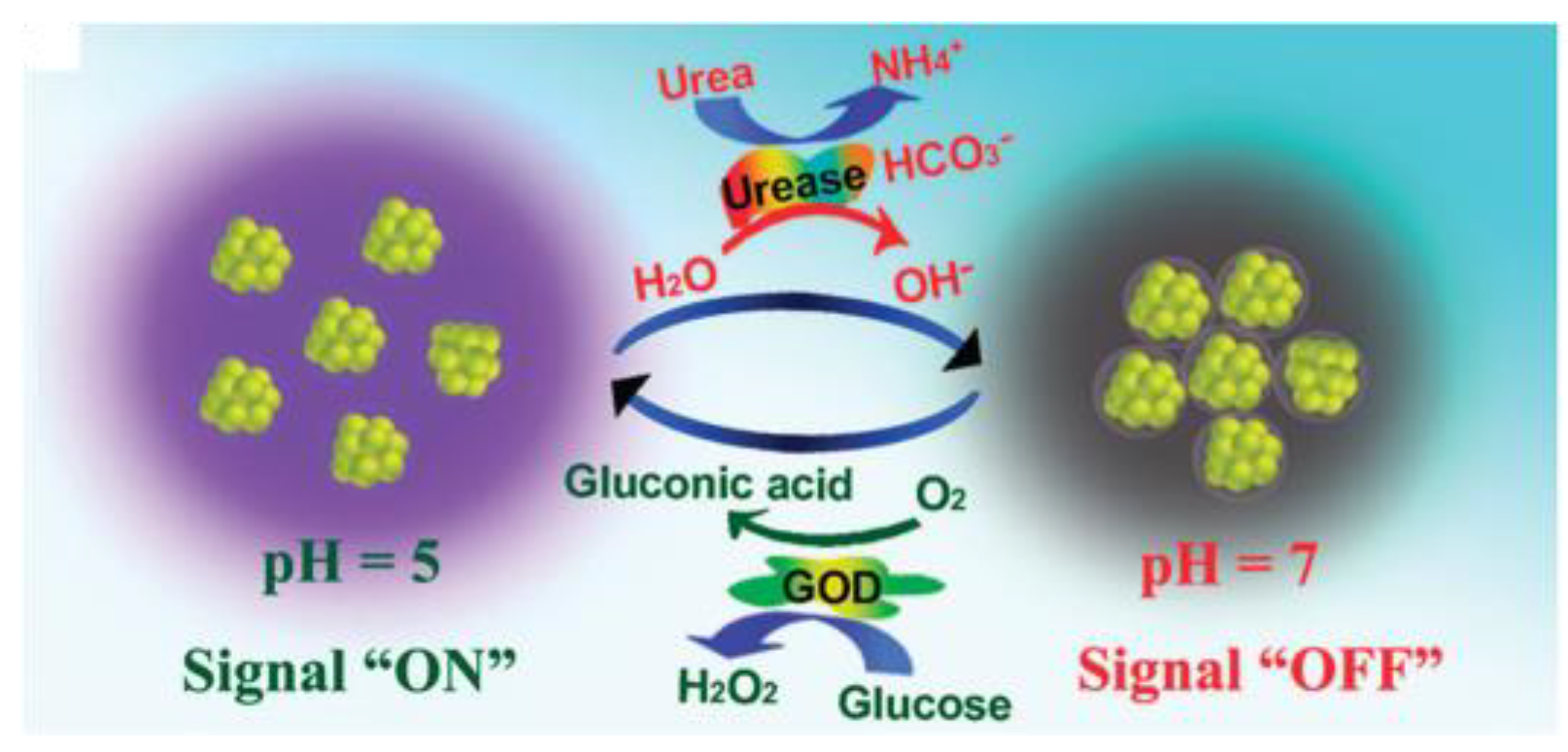
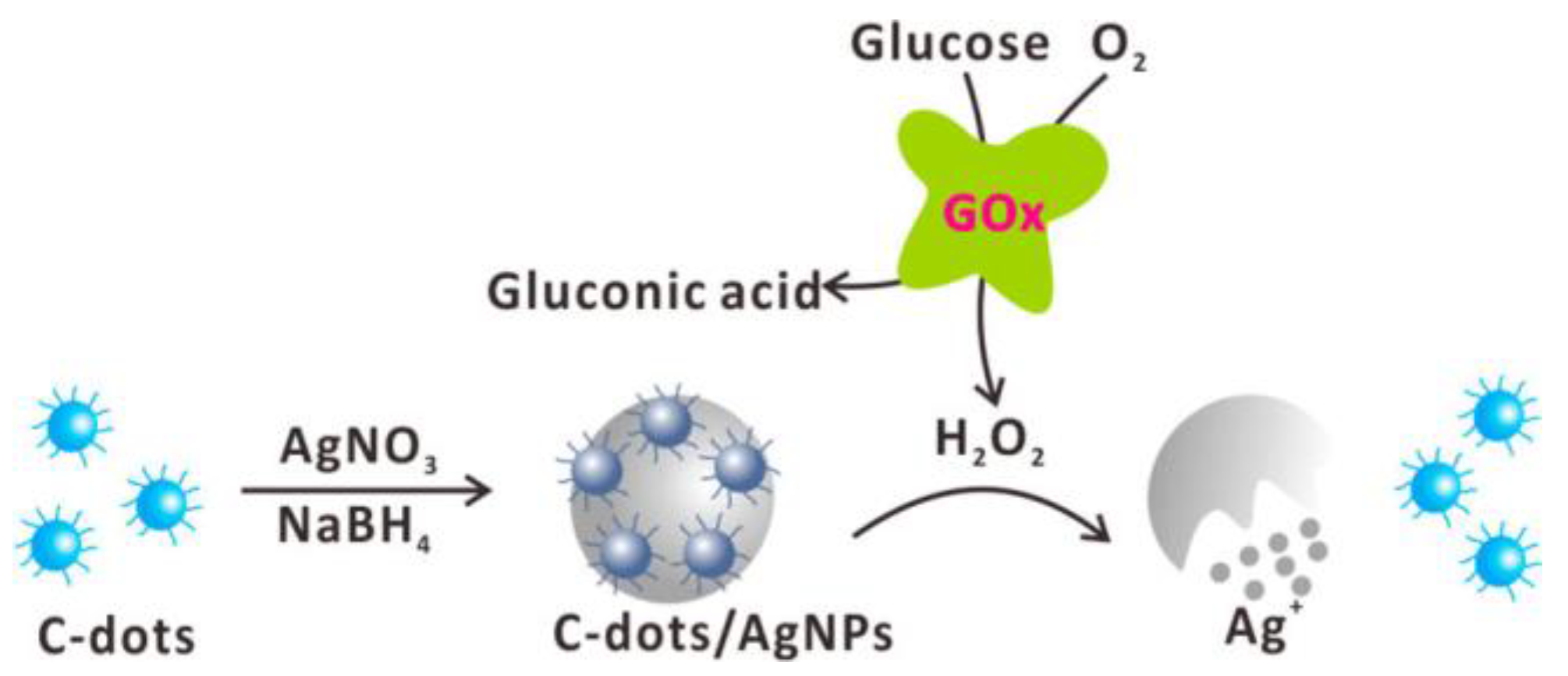
| Fluorescence-Emitting/Interacting Nanomaterials | Pros | Cons |
|---|---|---|
| Semiconductor Quantum Dots [99,100] | Stable and strong fluorescence Multiplex sensing | Toxic elements like Cd UV excitation tissue auto-fluorescence |
| Fluorescent Silica Nanoparticles [101,102,103,104] | Facile surface modification, good carrier for biomedical applications | Matrix may contain unwanted structure directing agents or surfactants |
| Upconverting Nanoparticles [105,106] | 980 nm excitation, low background noise Biocompatible Stable fluorescence | Fluorescence difficult to tune |
| Gold/Silver Nanoparticles/Nanoclusters [107,108,109,110,111] | Stable, bio-inert, nanoparticles with surface plasmonic properties, nanoclusters with fluorescence properties properties | Limited range of absorbance, nanoclusters instability concern |
| Fluorescent Carbon Nanomaterials [112,113,114] | Highly biocompatible Facile preparation by various methods Fluorescence strong/stable enough for biosensing Excitation-dependent emission, tunable by doping with nitrogen, etc. elements Possible upconverting fluorescence | Fluorescence mechanisms not fully understood Reproducibility |
| Graphene Nanomaterials [115,116,117] | Scale production, electrical and optical properties | Reproducibility, size control |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Hwang, E.; Zhang, J. Fluorescent Nanobiosensors for Sensing Glucose. Sensors 2018, 18, 1440. https://doi.org/10.3390/s18051440
Chen L, Hwang E, Zhang J. Fluorescent Nanobiosensors for Sensing Glucose. Sensors. 2018; 18(5):1440. https://doi.org/10.3390/s18051440
Chicago/Turabian StyleChen, Longyi, Eugene Hwang, and Jin Zhang. 2018. "Fluorescent Nanobiosensors for Sensing Glucose" Sensors 18, no. 5: 1440. https://doi.org/10.3390/s18051440





