Development of Implantable Wireless Sensor Nodes for Animal Husbandry and MedTech Innovation
Abstract
:1. Introduction
2. Design, Assembly and Package
2.1. Wireless Sensor Nodes
2.2. Wireless Power Transmission System
3. Results and Discussion
3.1. Temperature Varitions of Laboratory Mice
3.2. Monitoring of Wagyu for Animal Husbandry
3.3. Positioning for Surgical Navigation
4. Conclusions
Acknowledgments
Ethical Statement
Author Contributions
Conflicts of Interest
References
- Fryer, T.B. Implantable Biotelemetry System—A Report. Technology Utilization Division; National Aeronautics and Space Administration: Washingyon, DC, USA, 1971.
- Flammini, F.; Gaglione, A.; Ottello, F.; Pappalardo, A.; Pragliola, C.; Tedesco, A. Towards wireless sensor networks for railway infrastructure monitoring. In Proceedings of the Electrical Systems for Aircraft, Railway and Ship Propulsion (ESARS), Bologna, Italy, 19–21 October 2010. [Google Scholar]
- Minoli, D.; Sohraby, K.; Occhiogrosso, B. IoT considerations, requirements, and architectures for smart buildings—Energy optimization and next-generation building management systems. IEEE Internet Things J. 2017, 4, 269–283. [Google Scholar] [CrossRef]
- Gómez, J.; Oviedo, B.; Zhuma, E. Patient monitoring system based on internet of things. Procedia Comput. Sci. 2016, 83, 90–97. [Google Scholar] [CrossRef]
- Sghaier, N.; Mellouk, A.; Augustin, B.; Amirat, Y.; Marty, J.; Khoussa, M.E.A.; Abid, A.; Zitouni, R. Wireless sensor networks for medical care services. In Proceedings of the 7th International Wireless Communications and Mobile Computing Conference (IWCMC), Istanbul, Turkey, 4–8 July 2011. [Google Scholar]
- Cousin, M.; Castillo-Hi, T.; Snyder, G.H. Devices and Diseases: How the IoT is Transforming Medtech; Deloitte University Press: Warsaw, Poland, 2015. [Google Scholar]
- Dohr, A.; Modre-Opsrian, R.; Drobics, M. The Internet of Things for Ambient Assisted Living. In Proceedings of the 7th International Conference on Information Technology: New Generations, Las Vegas, NV, USA, 12–14 April 2010. [Google Scholar]
- Chen, X.; Brox, D.; Assadsangabi, B.; Takahata, K. Intelligent telemetric stent for wireless monitoring of intravascular pressure and its in vivo testing. Biomed. Microdevices 2014, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Nattapon, C.; Wen, H.K.; Darrin, J.Y. A wireless and batteryless 10-bit implantable blood pressure sensing microsystem with adaptive RF powering for real-time laboratory mice monitoring. IEEE J. Solid-State Circuits 2009, 44, 3631–3644. [Google Scholar] [CrossRef]
- Steve, M.; Iryna, M.; Hui, Z.; Wen, K.; Margot, S.D. Wireless implantable pressure monitor for conditional bladder neuromodulation. In Proceedings of the 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS), Altanta, GA, USA, 22–24 October 2015. [Google Scholar]
- Pardue, M.T.; Walker, T.A.; Faulkner, A.E.; Kim, M.K.; Bonner, C.M.; Mclean, G.Y. Implantation of mouse eyes with a subretinal microphotodiode array. Adv. Exp. Med. Biol. 2010, 613, 377–382. [Google Scholar] [CrossRef]
- Lu, J.; Okada, H.; Itoh, T.; Harada, T.; Maeda, R. Towards the world smallest wireless sensor nodes with ultralow power consumption. IEEE Sens. J. 2014, 14, 2035–2041. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, L.; Matsumoto, S.; Hiroshima, H.; Serizawa, K.; Hayase, M.; Gotoh, T. Miniaturization and packaging of implantable wireless sensor nodes for animals monitoring. In Proceedings of the 2016 IEEE Sensors Conference, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Lu, J.; Zhang, L.; Arakawa, M.; Harada, T. Wireless power supply for all-in-one LTCC packaged sensor nodes applicable to infrastructure monitoring. IEEJ Trans. Sens. Micromach. 2017, 139, 267–271. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, J.; Okada, H.; Nogami, H.; Itoh, T.; Arai, S. Low-power highly sensitive pH sensor with μ dots protective sructures for monitoring rumen in cows in real-time. IEEE Sens. J. 2017, 17, 7281–7289. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V. Implantable devices: Issues and challenges. Electronics 2013, 2, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Saito, K.; Takahashi, M.; Ito, K. Performances of an implanted cavity slot antenna embedded in the human arm. IEEE Trans. Antennas Propag. 2009, 57, 894–899. [Google Scholar] [CrossRef]
- Islam, M.S.; Esselle, K.P.; Bull, D.; Pilowsky, P.M. Converting a wireless biotelemetry system to an implantable system through antenna redesign. IEEE Trans. Microw. Tech. 2014, 62, 1890–1897. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, L.; Yamashita, T.; Takei, R.; Makimoto, N.; Kobayashi, T. A Silicon Disk with Sandwiched Piezoelectric Springs for Ultra-Low Frequency Energy Harvesting. In Proceedings of the 15th International Conference on Micro and Nanotechnology for Power Generation and Energy Conversion Applications (PowerMEMS 2015), Boston, MA, USA, 1–4 December 2015. [Google Scholar]
- Shi, Q.; Wang, T.; Lee, C. MEMS based broadband pieszoelectric ultrasonic energy harvester (PUEH) for enabling self-powered implantable biomedical devices. Sci. Rep. 2016, 6, 24946. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.; Blaauw, D.; Phillips, J.D. Subcutaneous Photovoltaic Infrared Energy Harvesting for Bio-implantable Devices. IEEE Trans. Electron Devices 2017, 64, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Kibret, B.; Teshome, A.K.; Lai, D.T.H. Human body as antenna and its effects on human body communications. Prog. Electromagn. Res. 2014, 148, 193–207. [Google Scholar] [CrossRef]
- Bradford, E.M.; Miller, M.L.; Prasad, V.; Nieman, M.L.; Gawenis, L.R.; Berryman, M.; Lorenz, J.N.; Tso, P.; Shull, G.E. Clic5 mutant mice are resistant to diet-induced obesity and exhibit gastric hemorrhaging and increased susceptibility to torpor. Am. J. Physoil. Regul. Integr. Comp. Physiol. 2010, 298, R1531–R1542. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Yeh, F.M.; Ghavami, M. Miniaturized implantable boardband antenna for biotelemery communications. Microw. Opt. Technol. Lett. 2008, 50, 2407–2409. [Google Scholar] [CrossRef]
- Gotoh, T.; Maeda, M.; Hirano, O.; Nishiki, M.; Fujita, T.; Shibata, T.; Takayama, Y.; Yokoo, K.; Nishidoi, T.; Urabe, H.; et al. Challenges of Application of ICT in Cattle Management: Remote Management System for Cattle Grazing in Mountainous Areas of Japan Using a Smartphone. In Smart Sensors and Systems; Kyung, C.M., Yasuura, H., Liu, Y., Lin, Y.L., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Kunert, W.; Storz, P.; Kirschniak, A. For 3D laparoscopy: A step toward advanced surgical navigation: How to get maximum benefit from 3D vision. Surg. Endos. 2013, 27, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, R.; Oshiro, Y.; Nakayama, K.; Ohkohchi, N. Impact of three-dimensional surgical simulation on pancreatic surgery. Gastrointest. Tumors 2017, 4. [Google Scholar] [CrossRef]

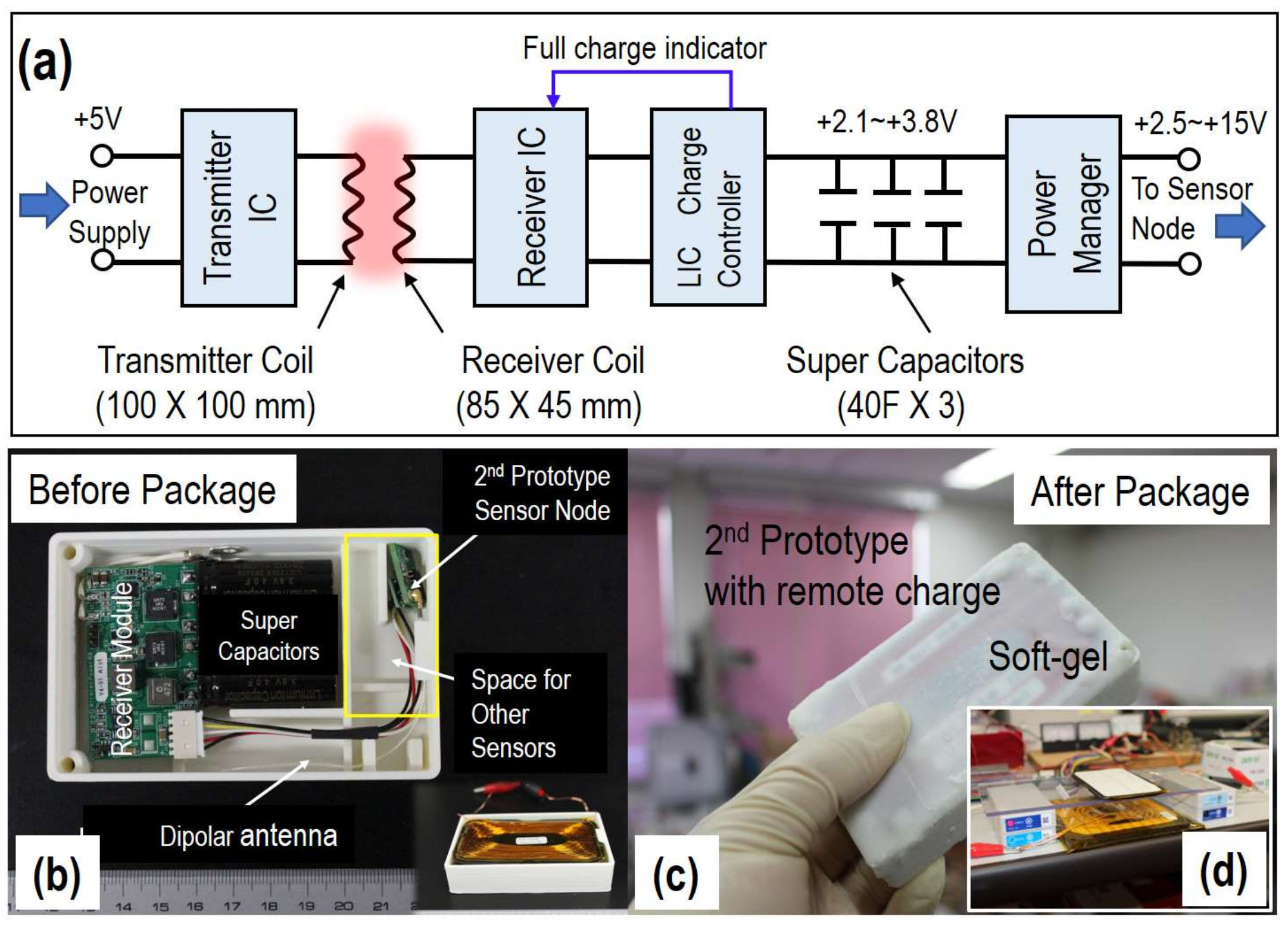
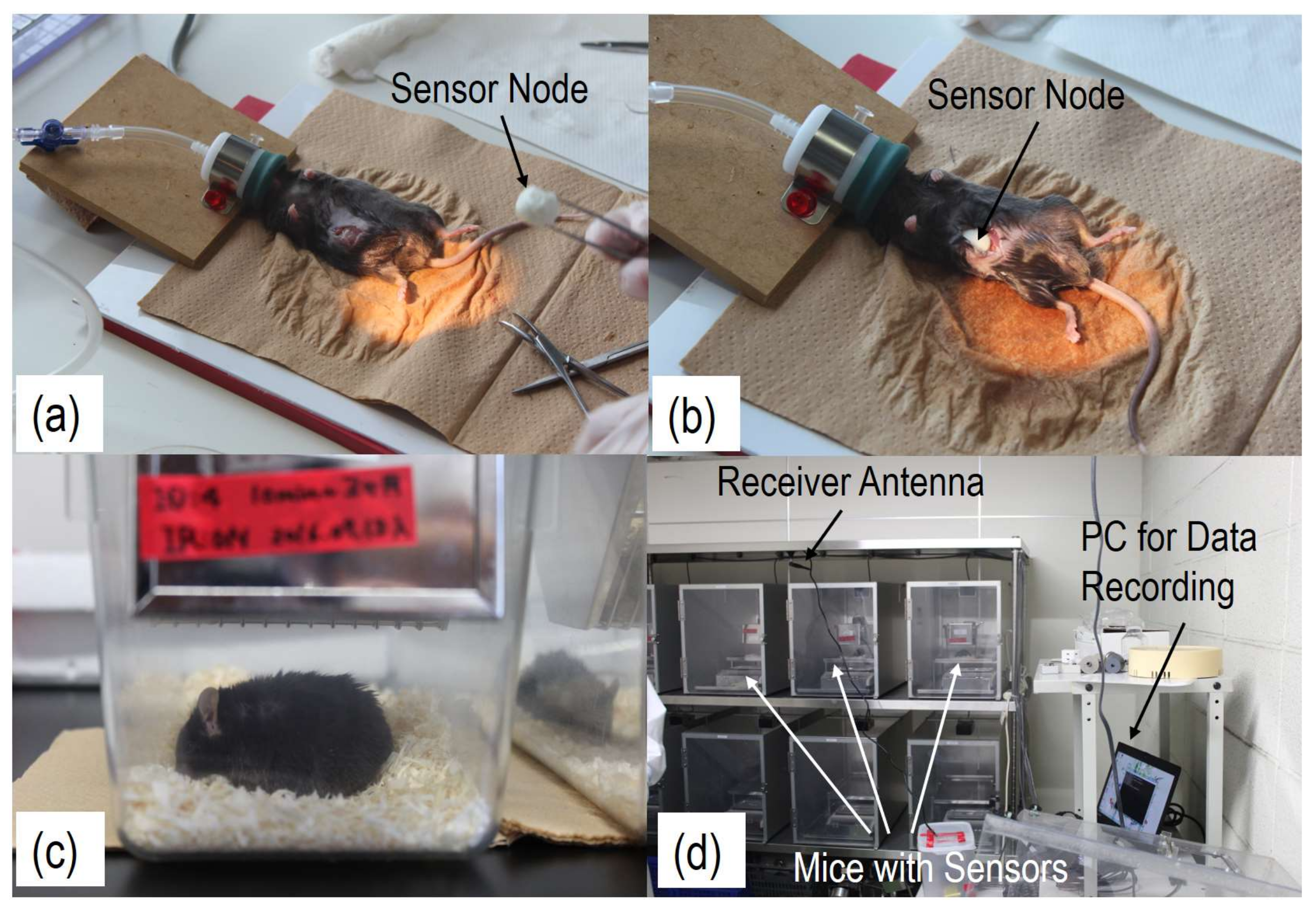
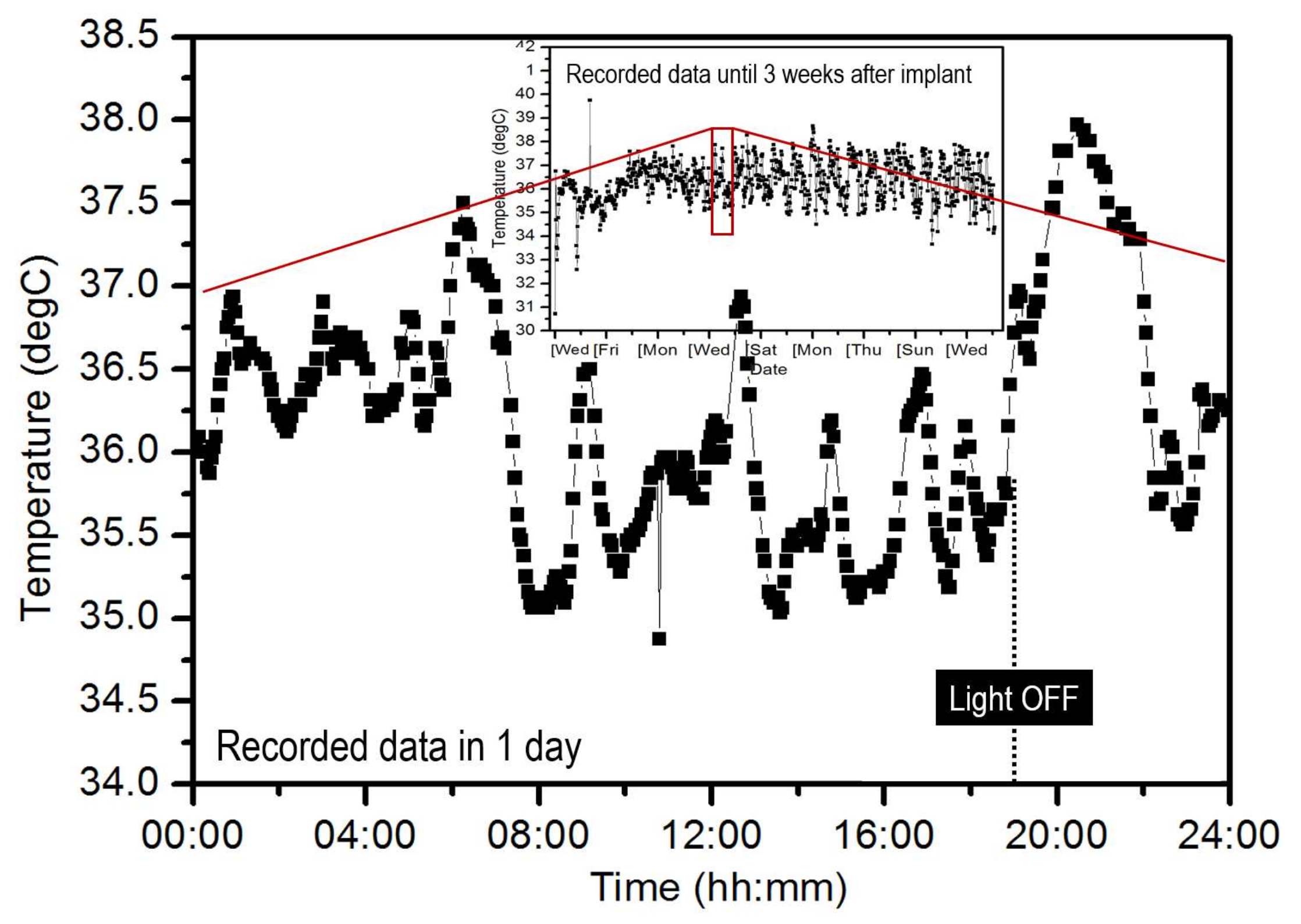


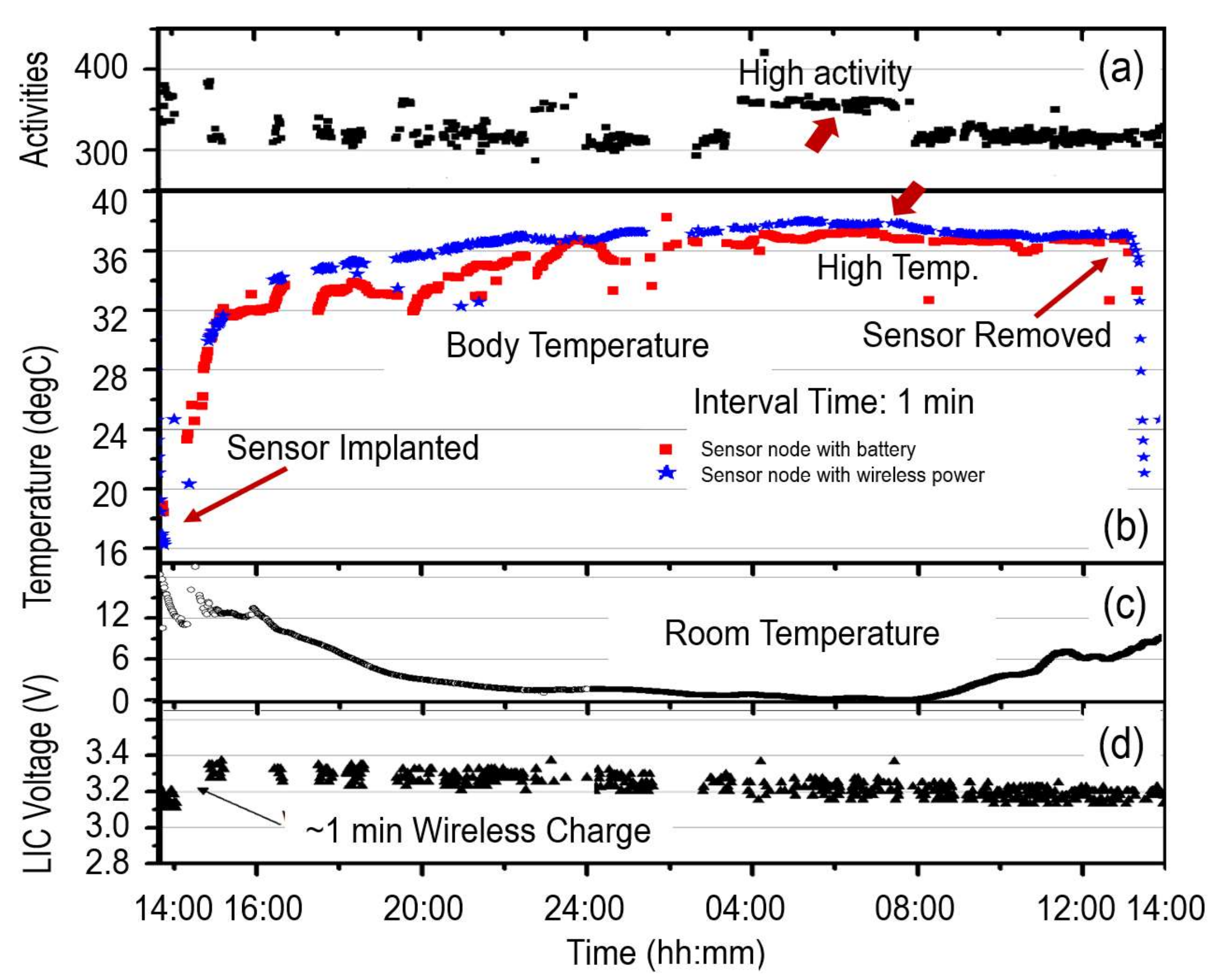


| Sensor Node | Interval Time | Battery | Meas. Lifetime | Cal. Power Consumption |
|---|---|---|---|---|
| 1st Prototype Temperature | 10 min | SW612 | 3 weeks | 24 μW |
| 10 min | CR1025 | 16 weeks | ||
| 2nd Prototype Temperature Accelerometer/Voltage Hybrid RFID | 1 min | CR1632 | 12 weeks | 600 μW |
| 10 min | CR1632 | 31 weeks | 230 μW | |
| 1 min | Remote-charge | 600 μW | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Zhang, L.; Zhang, D.; Matsumoto, S.; Hiroshima, H.; Maeda, R.; Sato, M.; Toyoda, A.; Gotoh, T.; Ohkohchi, N. Development of Implantable Wireless Sensor Nodes for Animal Husbandry and MedTech Innovation. Sensors 2018, 18, 979. https://doi.org/10.3390/s18040979
Lu J, Zhang L, Zhang D, Matsumoto S, Hiroshima H, Maeda R, Sato M, Toyoda A, Gotoh T, Ohkohchi N. Development of Implantable Wireless Sensor Nodes for Animal Husbandry and MedTech Innovation. Sensors. 2018; 18(4):979. https://doi.org/10.3390/s18040979
Chicago/Turabian StyleLu, Jian, Lan Zhang, Dapeng Zhang, Sohei Matsumoto, Hiroshi Hiroshima, Ryutaro Maeda, Mizuho Sato, Atsushi Toyoda, Takafumi Gotoh, and Nobuhiro Ohkohchi. 2018. "Development of Implantable Wireless Sensor Nodes for Animal Husbandry and MedTech Innovation" Sensors 18, no. 4: 979. https://doi.org/10.3390/s18040979




