Design and Investigation of Optical Properties of N-(Rhodamine-B)-Lactam-Ethylenediamine (RhB-EDA) Fluorescent Probe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Starting Materials
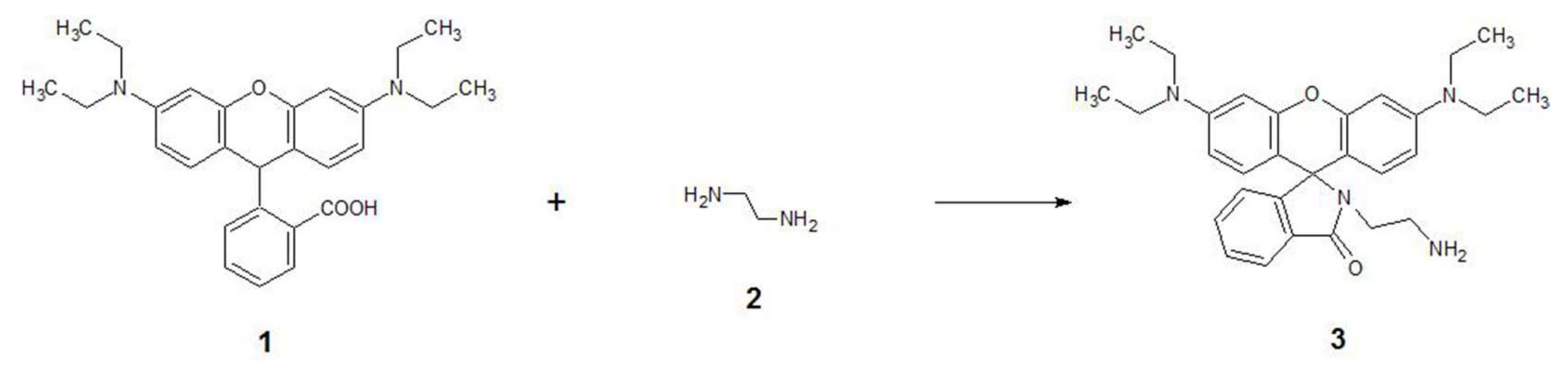
2.2. Synthetic Procedure and Characterisation Data for RhB-EDA
2.3. Solutions
2.4. Instrumentation
2.5. Fluorescence Quantum Yield
3. Results
3.1. Design and Synthesis of RhB-EDA
3.2. Spectral Properties of Synthesised RhB-EDA Fluorescent Probe
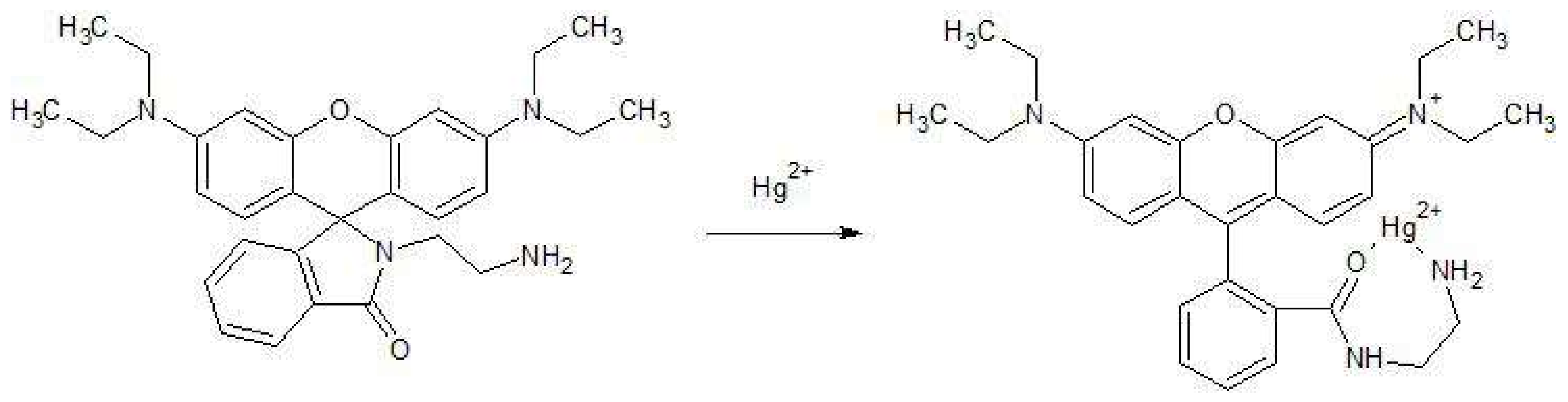
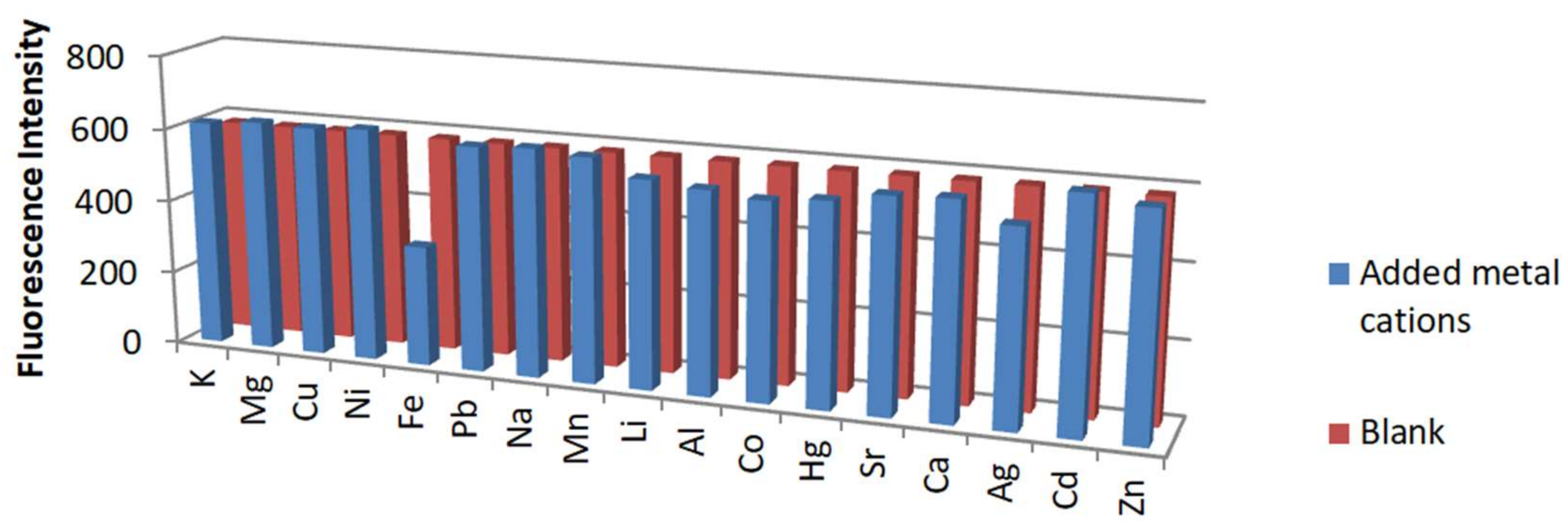
3.3. Response of RhB-EDA Fluorescent Probe to Various Metals
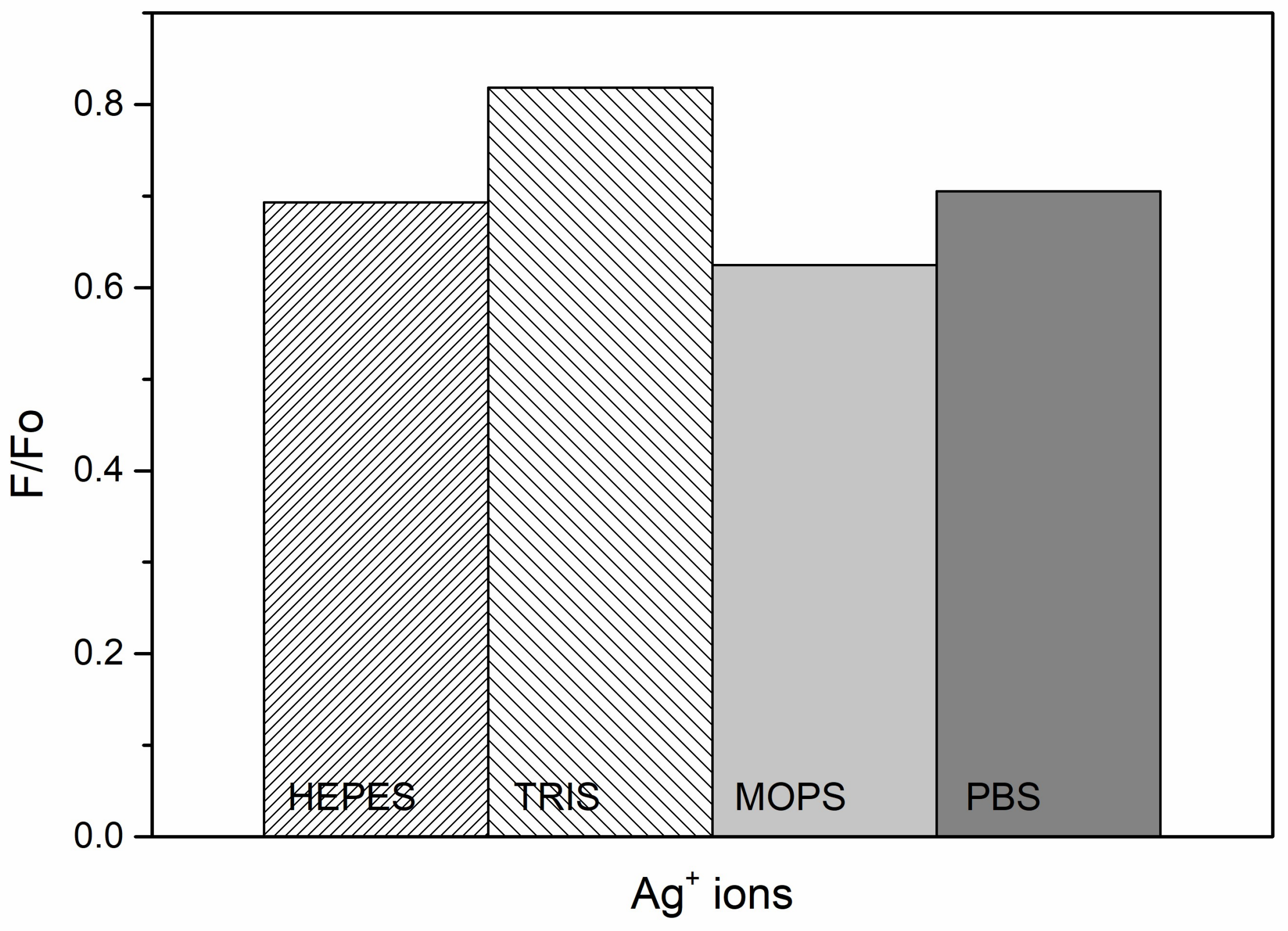
3.4. Effect of Buffers
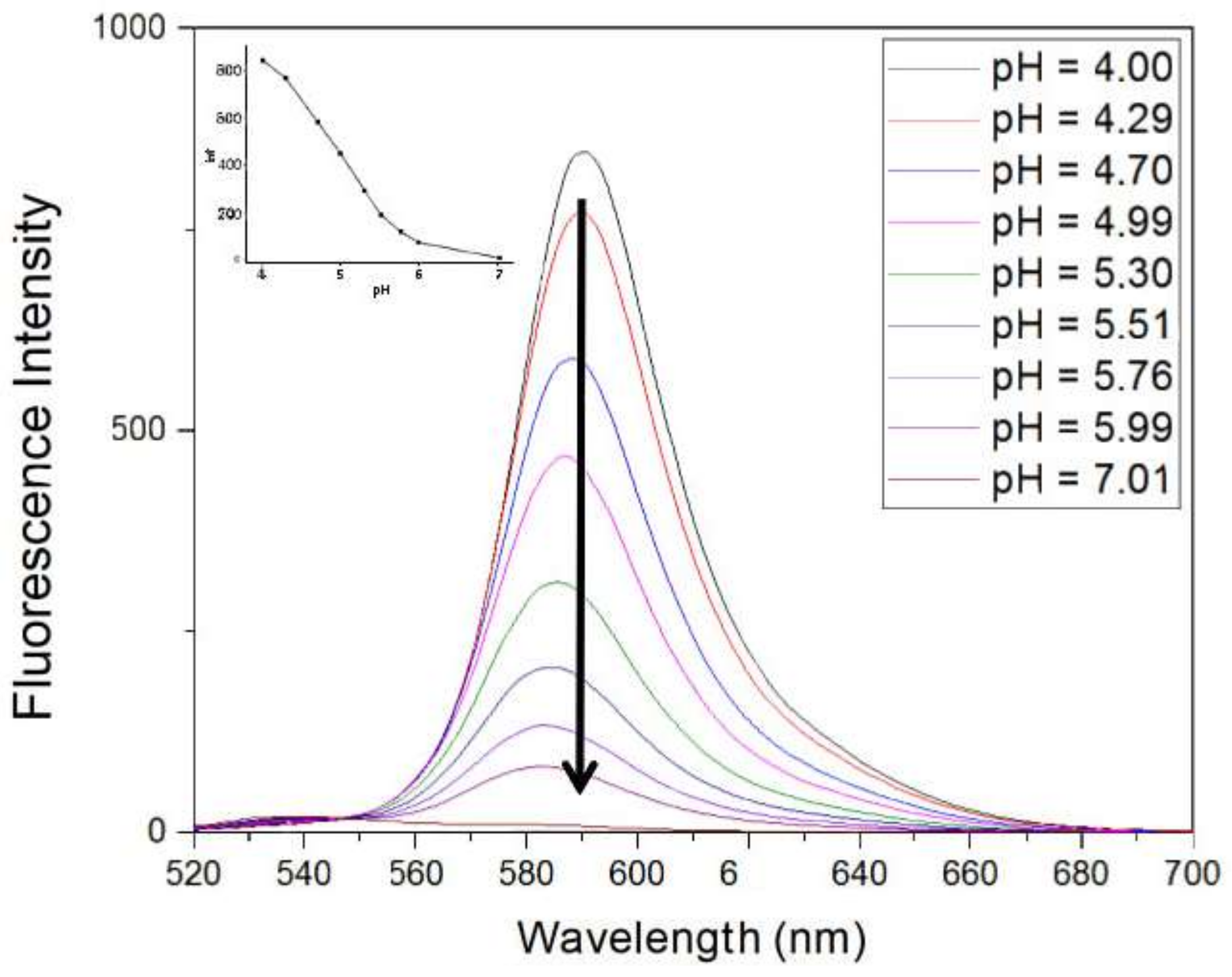
3.5. Effect of Various pHs of RhB-EDA Fluorescence in the Presence of Ag+ Ions
3.6. Sensitivity of RhB-EDA to Ag+
3.7. Comparison with Developed Probe-Based Sensors Intended for on-Site Detection of Ag+
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Lv, Y.; Zhu, L.; Liu, H.; Wu, Y.; Chen, Z.; Fu, H.; Tian, Z. Single-fluorophore-based fluorescent probes enable dual-channel detection of Ag+ and Hg2+ with high selectivity and sensitivity. Anal. Chim. Acta 2014, 839, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Arulraj, A.D.; Devasenathipathy, R.; Chen, S.-M.; Vasantha, V.S.; Wang, S.-F. Highly selective and sensitive fluorescent chemosensor for femtomolar detection of silver ion in aqueous medium. Sens. Bio-Sens. Res. 2015, 6, 19–24. [Google Scholar] [CrossRef]
- Jones, P.; Beere, H.G. Ion chromatography determination of trace silver ions using hydrophilic resins with chemiluminescence detection. Anal. Proc. Incl. Anal. Commun. 1995, 32, 169–171. [Google Scholar] [CrossRef]
- Kazemi, E.; Haji Shabani, A.M.; Dadfarnia, S. Synthesis and characterization of a nanomagnetic ion imprinted polymer for selective extraction of silver ions from aqueous samples. Microchim. Acta 2015, 182, 1025–1033. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Fayazi, M.; Taher, M.A.; Jalalinejad, A. Application of magnetic nanocomposite modified with a thiourea based ligand for the preconcentration and trace detection of silver(I) ions by electrothermal atomic absorption spectrometry. Chem. Eng. J. 2016, 290, 53–62. [Google Scholar] [CrossRef]
- Ndung’u, K.; Ranville, M.A.; Franks, R.P.; Flegal, A.R. On-line determination of silver in natural waters by inductively-coupled plasma mass spectrometry: Influence of organic matter. Mar. Chem. 2006, 98, 109–120. [Google Scholar] [CrossRef]
- Shamsipur, M.; Reza Hashemi, O.; Salavati-Niasari, M. Selective flotation-separation and inductively coupled plasma-atomic emission spectrometric determination of ultra trace amounts of silver ion using bis(2-mercaptoanil)acetylacetone. Sep. Sci. Technol. 2007, 42, 567–578. [Google Scholar] [CrossRef]
- Zhiani, R.; Ghanei-Motlag, M.; Razavipanah, I. Selective voltammetric sensor for nanomolar detection of silver ions using carbon paste electrode modified with novel nanosized Ag(I)-imprinted polymer. J. Mol. Liq. 2016, 219, 554–560. [Google Scholar] [CrossRef]
- Kaur, K.; Saini, R.; Kumar, A.; Luxami, V.; Kaur, N.; Singh, P.; Kumar, S. Chemodosimeters: An approach for detection and estimation of biologically and medically relevant metal ions, anions and thiols. Coord. Chem. Rev. 2012, 256, 1992–2028. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, L.; Yan, W.; He, C.; Cao, X.; Duan, C. Conformational switching fluorescent chemodosimeter for the selective detection of silver(I) ions. New J. Chem. 2009, 33, 1478–1481. [Google Scholar] [CrossRef]
- Chatterjee, A.; Santra, M.; Won, N.; Kim, S.; Kim, J.K.; Kim, S.B.; Ahn, K.H. Selective fluorogenic and chromogenic probe for detection of silver ions and silver nanoparticles in aqueous media. J. Am. Chem. Soc. 2009, 131, 2040–2041. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Shen, C.; Qian, Y.; Liu, J.; Xi, F.; Dong, X. Facile synthesis of sulfur-doped graphene quantum dots as fluorescent sensing probes for Ag+ ions detection. Sens. Actuators B Chem. 2017, 242, 231–237. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, Q.; Li, S.; Fu, S.; Chai, F.; Wang, C.; Qu, F. One pot synthesis of highly fluorescent N doped C-dots and used as fluorescent probe detection for Hg2+ and Ag+ in aqueous solution. Sens. Actuators B Chem. 2016, 243, 244–253. [Google Scholar] [CrossRef]
- Xiang, G.; Wang, L.; Cui, W.; An, X.; Zhou, L.; Li, L.; Cao, D. 2-pyridine-1h-benzo[d]imidazole based conjugated polymers: A selective fluorescent chemosensor for Ni2+ or Ag+ depending on the molecular linkage sites. Sens. Actuators B Chem. 2014, 196, 495–503. [Google Scholar] [CrossRef]
- Qi, L.; Yan, Z.; Huo, Y.; Hai, X.-M.; Zhang, Z.-Q. MnO2 nanosheet-assisted ligand-DNA interaction-based fluorescence polarization biosensor for the detection of Ag+ ions. Biosens. Bioelectron. 2017, 87, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-T.; Wu, G.-Y.; Qu, W.-J.; Li, Q.; Lou, J.-C.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. A colorimetric and reversible fluorescent chemosensor for Ag+ in aqueous solution and its application in implication logic gate. Sens. Actuators B Chem. 2017, 239, 671–678. [Google Scholar] [CrossRef]
- Chawla, H.M.; Gupta, T. New chromogenic bis(isatin hydrazonyl)calix[4]arenes for dual recognition of fluoride and silver ions. Tetrahedron Lett. 2013, 54, 1794–1797. [Google Scholar] [CrossRef]
- Wang, H.-H.; Xue, L.; Qian, Y.-Y.; Jiang, H. Novel ratiometric fluorescent sensor for silver ions. Org. Lett. 2010, 12, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Han, K.; Wang, B.; Luo, G.; Wang, P.; Chen, M.; Tang, Y. Electrochemical detection of aqueous Ag+ based on Ag+-assisted ligation reaction. Sci. Rep. 2015, 5, 9161. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, X.; Fei, R.; Hu, Y. Sensitive and selective detection of Ag+ in aqueous solutions using Fe3O4@Au nanoparticles as smart electrochemical nanosensors. Talanta 2013, 116, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, C.; Lin, Q.; Fu, N. A fluorescent probe for the dual-channel detection of Hg2+/Ag+ and its Hg2+-based complex for detection of mercapto biomolecules with a tunable measuring range. Sens. Actuators B Chem. 2012, 173, 874–881. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thangavelu, K.; Chen, S.-M.; Velusamy, V.; Chang, M.-H.; Chen, T.-W.; Al-Hemaid, F.M.A.; Ali, M.A.; Ramaraj, S.K. Synthesis and characterization of polypyrrole decorated graphene/β-cyclodextrin composite for low level electrochemical detection of mercury (II) in water. Sens. Actuators B Chem. 2017, 243, 888–894. [Google Scholar] [CrossRef]
- Palanisamy, S.; Madhu, R.; Chen, S.-M.; Ramaraj, S.K. A highly sensitive and selective electrochemical determination of Hg(II) based on an electrochemically activated graphite modified screen-printed carbon electrode. Anal. Methods 2014, 6, 8368–8373. [Google Scholar] [CrossRef]
- Amanulla, B.; Palanisamy, S.; Chen, S.-M.; Chiu, T.-W.; Velusamy, V.; Hall, J.M.; Chen, T.-W.; Ramaraj, S.K. Selective colorimetric detection of nitrite in water using chitosan stabilized gold nanoparticles decorated reduced graphene oxide. Sci. Rep. 2017, 7, 14182. [Google Scholar] [CrossRef] [PubMed]
- Kaewtong, C.; Noiseephum, J.; Uppa, Y.; Morakot, N.; Morakot, N.; Wanno, B.; Tuntulani, T.; Pulpoka, B. A reversible Emfret rhodamine-based chemosensor for carboxylate anions using a ditopic receptor strategy. New J. Chem. 2010, 34, 1104–1108. [Google Scholar] [CrossRef]
- He, G.J.; Zhang, X.L.; He, C.; Zhao, X.W.; Duan, C.Y. Ratiometric fluorescence chemosensors for copper(II) and mercury(II) based on fret systems. Tetrahedron 2010, 66, 9762–9768. [Google Scholar] [CrossRef]
- Soh, J.H.; Swamy, K.M.K.; Kim, S.K.; Kim, S.; Lee, S.H.; Yoon, J. Rhodamine urea derivatives as fluorescent chemosensors for Hg2+. Tetrahedron Lett. 2007, 48, 5966–5969. [Google Scholar] [CrossRef]
- Lei, Y.L.; Su, Y.Q.; Huo, J.C. Photophysical property of rhodamine-cored poly(amidoamine) dendrimers: Simultaneous effect of spirolactam ring-opening and pet process on sensing trivalent chromium ion. J. Lumin. 2011, 131, 2521–2527. [Google Scholar] [CrossRef]
- Sorsak, E.; Valh, J.V.; Urek, S.K.; Lobnik, A. Application of pamam dendrimers in optical sensing. Analyst 2015, 140, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Wurth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Bhorge, Y.R.; Chou, T.-L.; Chen, Y.-Z.; Yen, Y.-P. New coumarin-based dual chromogenic probe: Naked eye detection of copper and silver ions. Sens. Actuators B Chem. 2015, 220, 1139–1144. [Google Scholar] [CrossRef]
- Geddes, C.D. Metal-enhanced fluorescence. Phys. Chem. Chem. Phys. 2013, 15, 19537. [Google Scholar] [CrossRef] [PubMed]
- Lobnik, A.; Wolfbeis, O.S. Probing the polarity of sol-gels and ormosils via the absorption of nile red. J. Sol-Gel Sci. Technol. 2001, 20, 303–311. [Google Scholar] [CrossRef]
- Xue, Z.W.; Chen, M.L.; Chen, J.M.; Han, J.H.; Han, S.F. A rhodamine-benzimidazole based sensor for selective imaging of acidic pH. RSC Adv. 2014, 4, 374–378. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, Y.; Li, J.-Y.; Rui, Q.-Q.; Yao, C. Naphthalimide–rhodamine based chemosensors for colorimetric and fluorescent sensing Hg2+ through different signaling mechanisms in corresponding solvent systems. Sens. Actuators B Chem. 2015, 215, 350–359. [Google Scholar] [CrossRef]
- Lee, S.; Rao, B.A.; Son, Y.-A. A highly selective fluorescent chemosensor for Hg2+ based on a squaraine–bis(rhodamine-b) derivative: Part II. Sens. Actuators B Chem. 2015, 210, 519–532. [Google Scholar] [CrossRef]
- Xie, P.H.; Guo, F.Q.; Xia, R.R.; Wang, Y.; Yao, D.H.; Yang, G.Y.; Xie, L.X. A rhodamine-dansyl conjugate as a fret based sensor for Fe3+ in the red spectral region. J. Lumin. 2014, 145, 849–854. [Google Scholar] [CrossRef]
- Lobnik, A.; Turel, M.; Urek, S.K.; Kosak, A. Nanostructured materials use in sensors: Their benefits and drawbacks. Carbon Oxide Nanostruct. 2010, 5, 307–354. [Google Scholar]
- Oehme, I.; Wolfbeis, O.S. Optical sensors for determination of heavy metal ions. Microchim. Acta 1997, 126, 177–192. [Google Scholar] [CrossRef]
- Chhatwal, M.; Kumar, A.; Singh, V.; Gupta, R.D.; Awasthi, S.K. Addressing of multiple-metal ions on a single platform. Coord. Chem. Rev. 2015, 292, 30–55. [Google Scholar] [CrossRef]
- Yang, Y.K.; Yook, K.J.; Tae, J. A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J. Am. Chem. Soc. 2005, 127, 16760–16761. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Hwang, I.C.; Kim, K.S.; Kim, J.S. Rhodamine-based Hg2+-selective chemodosimeter in aqueous solution: Fluorescent off-on. Org. Lett. 2007, 9, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Turel, M.; Duerkop, A.; Yegorova, A.; Karasyov, A.; Scripinets, Y.; Lobnik, A. Microtiterplate phosphate assay based on luminescence quenching of a terbium complex amenable to decay time detection. Anal. Chim. Acta 2010, 675, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Saari, L.A.; Seitz, W.R. Ph sensor based on immobilized fluoresceinamine. Anal. Chem. 1982, 54, 821–823. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Duan, Y. An improved optical pH sensor based on polyaniline. Sens. Actuators B Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]







| K+ | Mg2+ | Cu2+ | Ni2+ | Fe2+ | Pb2+ | Na+ | Mn2+ | Li+ | Al3+ | Co2+ | Hg2+ | Sr2+ | Ca2+ | Ag+ | Cd2+ | Zn2+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∆If (RhB-EDA) (%) | 0.51 | −0.61 | −7.69 | −1.68 | +0.08 | +4.96 | −0.56 | −3.82 | −2.75 | −5.21 | −2.59 | +11.29 | +1.26 | −0.16 | −75.05 | +0.53 | −2.21 |
| ∆If (RhB) (%) | +11.07 | +13.32 | +12.57 | +14.05 | −41.30 | +9.96 | +11.30 | +9.35 | +1.13 | −1.31 | −3.99 | −1.60 | +3.06 | +4.10 | −5.69 | +11.43 | +7.36 |
| Indicator | Tested Interference | Solutions | LOD | Remark | [Ref.] |
|---|---|---|---|---|---|
| RhB-EDA | K+, Mg2+, Cu2+, Ni2+, Fe2+, Pb2+, Na+, Mn2+, Li+, Al3+, Co2+, Hg2+, Sr2+, Ca2+, Cd2+, Zn2+ | 0.1 µM | our work | ||
| H2L∙2Br | Cu2+, Ni2+, Fe2+, Pb2+, Mn2+, Co2+, Hg2+, Cr3+, Zn2+ | Acetonitrile/dichloromethane (1:1) | Not given | F, Low number of tested interference, 100% organic solution | [11] |
| Rhodamine B derivate | Mg2+, Cu+, Cu2+, Ba2+, Ni2+, Fe3+, Pd2+, Mn2+, Co2+, Hg2+, Hg+, Ca2+, Cd2+, Cr3+, Zn2+ | 20% ethanolic water | 0.144 µM | F, Rhodamine B derivate, less number of tested interference, it is not reversible | [12] |
| S-GQDs | K+, Mg2+, Cu2+, Ni2+, Fe2+, Pb2+, Na+, Al3+, Co2+, Ca2+, Cd2+ Zn2+ | HEPES buffer solution | 30 nM | F, Not tested on Hg+ as well know interference for Ag+ ot tested | [13] |
| NCDs | Mg2+, Cu2+, Ni2+, Fe2+, Fe3+, Ba2+, Mn2+, Pb2+, Cr6+, Cr3+, Hg2+, Ca2+, Cd2+, Zn2+ | 100% water solution | 1 nM | F, No selectivity: same response to Ag+ and Hg2+, not tested to different pH | [14] |
| Polymer (ethyl 2-(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl) acetate units) | Ba2+, Mg2+, Cr3+, Ni2+, Fe2+, Na+, Mn2+, Li+, Al3+, Co3+, Hg2+, Sn2+, Ca2+, Cd2+, Zn2+ | THF solution | Not given | F, 100% organic solution | [15] |
| MnO2 nanosheets + ligand-DNA | Ba2+, Mg2+, Fe3+, Mn2+, Li+, Al3+, Cu2+, Hg2+, Ca2+, Cd2+, Zn2+ | Ammonium acetate-buffered saline solution | 9.1 nM | [16] | |
| Phenazine derivate | Fe3+, Hg2+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+, Mg2+ | DMSO/H2O (6:4) HEPES buffer (pH = 7.2) | 0.325 µM | A, F, Long and complicated synthesis | [17] |
| bis(isatin hydrazonyl) calix[4]arene based dual receptors | Cu2+, Ni2+, Fe2+, Mn2+, Co2+, Hg2+, Cd2+, Zn2+ | THF solution | 3.98 µM | A, 100% organic solution | [18] |
| FQ-crown | K+, Mg2+, Cu2+, Ni2+, Fe3+, Pb2+, Mn2+, Co2+, Hg2+, Cr3+, Ca2 +, Cd2+, Zn2+ | EtOH | Not given | F, All tested interference slightly interfere | [19] |
| DNA probe | Cu2+, Hg2+, Ca2+, Al3+, Na+, K+, Pb2+, Cd2+, Zn2+ | 10 mM Tris-HCl with 50 mM [Ru(NH3)6]3+ (pH 7.4) | 0.1 nM | E, complicated preparation of probe | [20] |
| Fe3O4@Au | Mg2+, Cu2+, Fe3+, Co2+, Hg2+, Ca2+, Al3+, Pb2+, Cd2+, Zn2+ | Water, pH > 12 | 59 nM | E, interference with high concentration of Hg2+, measuring in media with high pH | [21] |
| Squaraine dye | K+, Mg2+, Cu2+, Ni2+, Fe3+, Pb2+, Na+, Mn2+, Li+, Al3+, Co2+, Hg2+, Ca2+, Cd2+, Ba2+, Zn2+ | EtOH/H20 (50:50, v/v) | 40 µM | F, Long and complicated synthesis, No selectivity: similar response to Ag+ and Hg2+ | [22] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soršak, E.; Volmajer Valh, J.; Korent Urek, Š.; Lobnik, A. Design and Investigation of Optical Properties of N-(Rhodamine-B)-Lactam-Ethylenediamine (RhB-EDA) Fluorescent Probe. Sensors 2018, 18, 1201. https://doi.org/10.3390/s18041201
Soršak E, Volmajer Valh J, Korent Urek Š, Lobnik A. Design and Investigation of Optical Properties of N-(Rhodamine-B)-Lactam-Ethylenediamine (RhB-EDA) Fluorescent Probe. Sensors. 2018; 18(4):1201. https://doi.org/10.3390/s18041201
Chicago/Turabian StyleSoršak, Eva, Julija Volmajer Valh, Špela Korent Urek, and Aleksandra Lobnik. 2018. "Design and Investigation of Optical Properties of N-(Rhodamine-B)-Lactam-Ethylenediamine (RhB-EDA) Fluorescent Probe" Sensors 18, no. 4: 1201. https://doi.org/10.3390/s18041201




