Latent Factors Limiting the Performance of sEMG-Interfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Short-Term Training
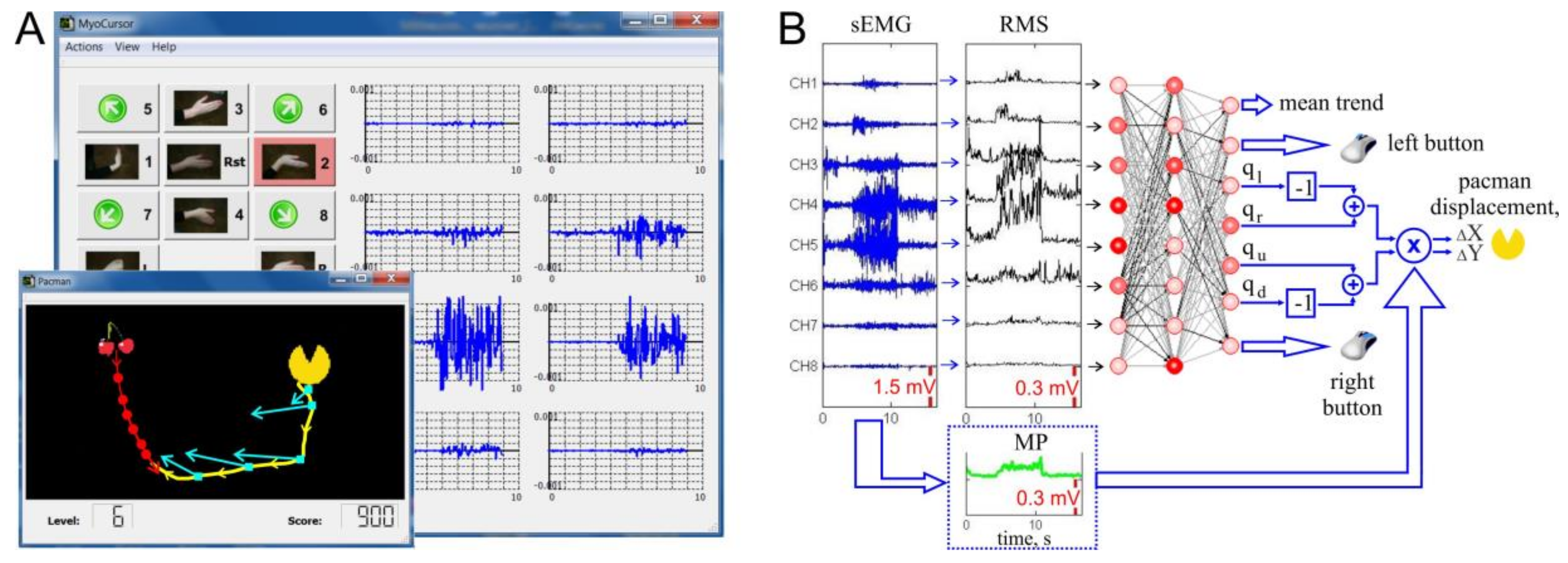
2.2. sEMG-Interface, “Pacman” Game, and Synthetic Tests
2.2.1. Gaming Environment
2.2.2. Synthetic Tests
2.3. Real-Time Processing of sEMG
2.3.1. ANN Approach
2.3.2. LDA Approach
2.4. Proportional Control
2.5. Performance of sEMG Interface
2.6. Assessment of Factors Influencing Performance of sEMG-Interface in Synthetic Tests
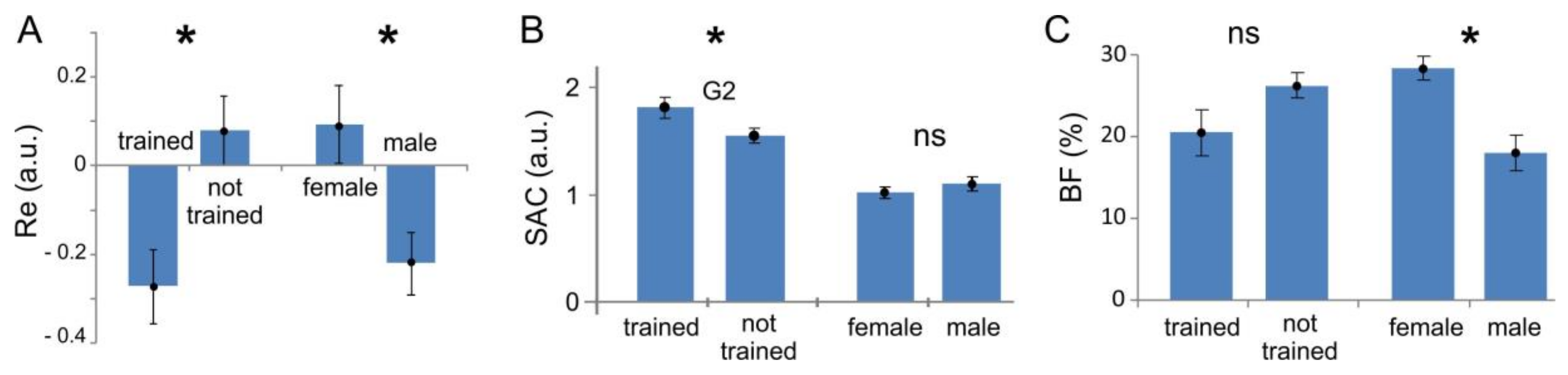
2.6.1. Body Fat (BF) Index
2.6.2. Synergist-Antagonist Coefficient (SAC)
2.7. Quantification of Interface Performance in Gaming Environment
3. Results
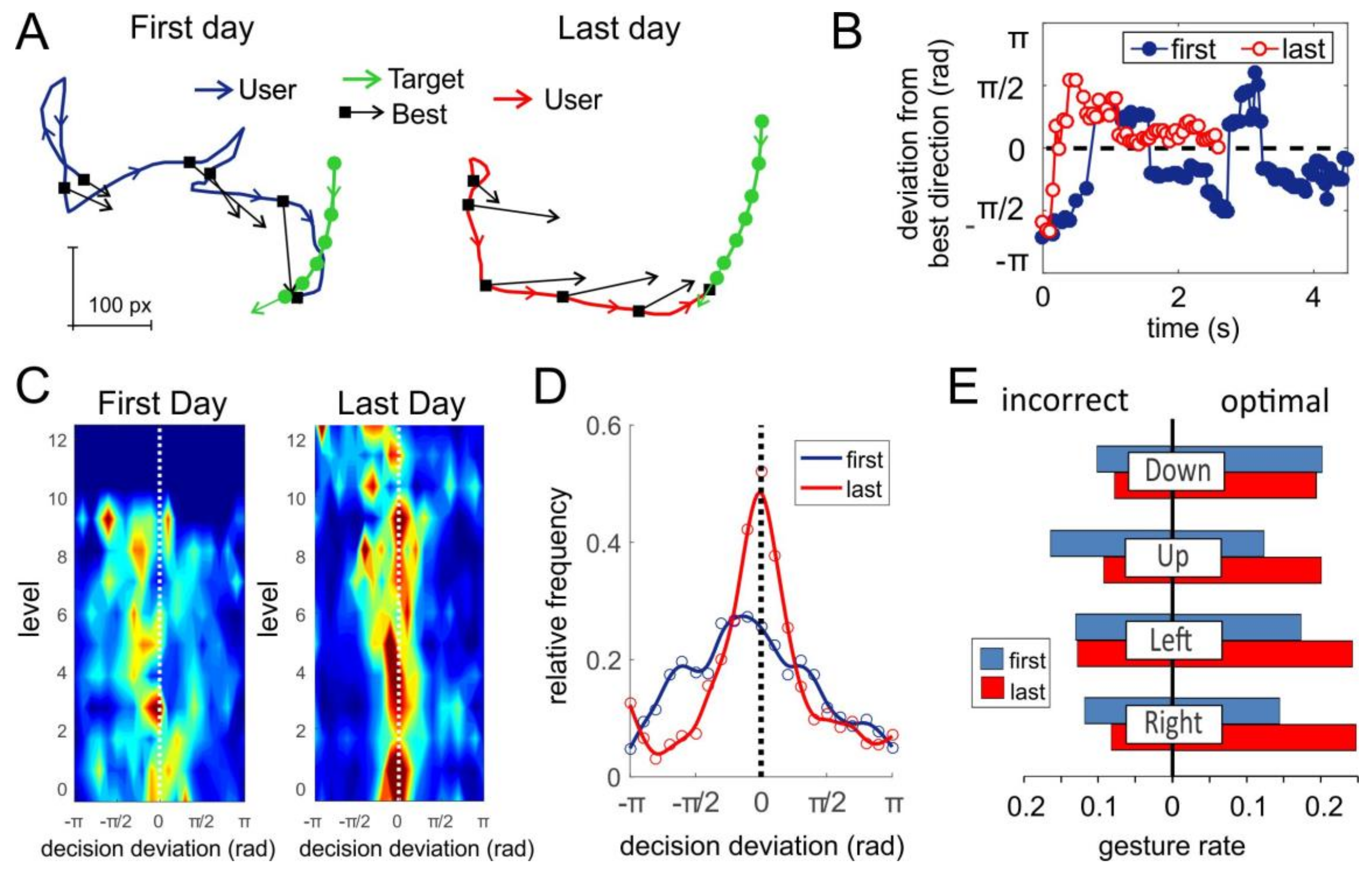
3.1. General Performance of sEMG-Interface and Short-Term Training
3.2. Synergist–Antagonist Coefficient (SAC)
- G1 (“left”): , ;
- G2 (“right”): , ;
- G3 (“up”): , ;
- G4 (“down”): , .
3.3. Similar Means and High Variance of sEMG Performance for Different Classifiers
3.4. Latent Factors Influencing sEMG Performance
3.5. Short-Term Training in Gaming Environment
4. Conclusions and Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Basmajian, J.V.; De Luca, C.J. Muscles Alive: Their Functions Revealed by Electromyography; Williams & Wilkins: Baltimore, MD, USA, 1985. [Google Scholar]
- Winter, D.A. Electromyogram recording, processing, and normalization: Procedures and considerations. J. Hum. Muscle Perform 1991, 1, 5–15. [Google Scholar]
- Bishop, M.D.; Pathare, N. Considerations for the use of surface electromyography. Phys. Theor. Korea 2004, 11, 61–69. [Google Scholar]
- Pullman, S.L.; Goodin, D.S.; Marquinez, A.I.; Tabbal, S.; Rubin, M. Clinical utility of surface EMG. Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2000, 55, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wakeling, J.M. Spectral properties of the surface EMG can characterize motor unit recruitment strategies. J. Appl. Physiol. 2008, 105, 1676–1677. [Google Scholar] [PubMed]
- Gopura, R.A.R.C.; Kiguchi, K.; Li, Y. SUEFUL-7: A 7DOF upper-limb exoskeleton robot with muscle-model-oriented EMG-based control. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, St. Louis, MO, USA, 10–15 October 2009; pp. 1126–1131. [Google Scholar]
- Kiguchi, K.; Hayashi, Y. An EMG-based control for an upper-limb power-assist exoskeleton robot. IEEE Trans. Syst. Man Cybern. Part B Cybern. 2012, 42, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- MyoTM Gesture Control Armband—Wearable Technology by Thalmic Labs. Available online: www.myo.com (accessed on 26 May 2016).
- Lobov, S.A.; Mironov, V.I.; Kastalskiy, I.A.; Kazantsev, V.B. A spiking neural network in sEMG feature extraction. Sensors 2015, 15, 27894–27904. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Ramadas, R.; Karmakar, S. Muscle computer interface: A review. In ICoRD’13, Lect. Notes Mechan. Eng.; Chakrabarti, A., Prakash, R.V., Eds.; Springer: New Delhi, India, 2013; pp. 411–421. [Google Scholar]
- Roche, A.D.; Rehbaum, H.; Farina, D.; Aszmann, O.C. Prosthetic myoelectric control strategies: A clinical perspective. Curr. Surg. Rep. 2014, 2, 44. [Google Scholar] [CrossRef]
- Hahne, J.M.; Biessmann, F.; Jiang, N.; Rehbaum, H.; Farina, D.; Meinecke, F.C.; Muller, K.-R.; Parra, L.C. Linear and nonlinear regression techniques for simultaneous and proportional myoelectric control. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.E.; Kinnard, C.R.; Ferris, D.P. Locomotor adaptation to a soleus EMG-controlled antagonistic exoskeleton. J. Neurophysiol. 2013, 109, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.I.; Lobov, S.A.; Kastalskiy, I.A.; Kazantsev, V.B. Myoelectric control system of lower limb exoskeleton for re-training motion deficiencies. Lect. Notes Comput. Sci. 2015, 9492, 428–435. [Google Scholar]
- Peerdeman, B.; Boere, D.; Witteveen, H.J.B.; Hermens, H.; Stramigioli, S.; Rietman, J.S.; Veltnik, P.H.; Misra, S. Myoelectric forearm prostheses: State of the art from a user-centered perspective. J. Rehabil. Res. Dev. 2011, 48, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.U.; Moon, I.; Mun, M.S. A real-time EMG pattern recognition system based on linear-nonlinear feature projection for a multifunction myoelectric hand. IEEE Trans. Biomed. Eng. 2006, 53, 2232–2239. [Google Scholar] [PubMed]
- Chan, B.S.; Sia, C.L.; Wong, F.; Chin, R.; Dargham, J.A.; Siang, Y.S. Analysis of surface electromyography for on-off control. Adv. Mater. Res. 2013, 701, 435–439. [Google Scholar] [CrossRef]
- Englehart, K.; Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Fevotte, C.; Doncarli, C.; Merletti, R. Blind separation of linear instantaneous mixtures of nonstationary surface myoelectric signals. IEEE Trans. Biomed. Eng. 2004, 51, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Englehart, K.B.; Hudgins, B.; Chan, A.D. A Gaussian mixture model based classification scheme for myoelectric control of powered upper limb prostheses. IEEE Trans. Biomed. Eng. 2005, 52, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, D.T.; Parker, P.A.; Englehart, K.B.; Rogers, D.R. Fatigue estimation with a multivariable myoelectric mapping function. IEEE Trans. Biomed. Eng. 2006, 53, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, K.; Imada, Y.; Liyanage, M. EMG-based neuro-fuzzy control of a 4DOF upper-limb power-assist exoskeleton. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 3040–3043. [Google Scholar]
- Shenoy, P.; Miller, K.J.; Crawford, B.; Rao, R.P. Online electromyographic control of a robotic prosthesis. IEEE Trans. Biomed. Eng. 2008, 55, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, T.; Jiang, N.; Farina, D. Influence of the training set on the accuracy of surface EMG classification in dynamic contractions for the control of multifunction prostheses. J. Neuroeng. Rehabil. 2011, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Fougner, A.; Stavdahl, O.; Kyberd, P.J.; Losier, Y.G.; Parker, P.A. Control of upper limb prostheses: Terminology and proportional myoelectric control—A review. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Wurth, S.M.; Hargrove, L.J. A real-time comparison between direct control, sequential pattern recognition control and simultaneous pattern recognition control using a Fitts’ law style assessment procedure. J. Neuroeng. Rehabil. 2014, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Earley, E.J.; Hargrove, L.J.; Kuiken, T.A. Dual window pattern recognition classifier for improved partial-hand prosthesis control. Front. Neurosci. 2016, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Hahne, J.M.; Farina, D.; Jiang, N.; Liebetanz, D. A novel percutaneous electrode implant for improving robustness in advanced myoelectric control. Front. Neurosci. 2016, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Vest-Nielsen, J.L.; Muceli, S.; Farina, D. EMG-based simultaneous and proportional estimation of wrist/hand kinematics in uni-lateral trans-radial amputees. J. Neuroeng. Rehabil. 2012, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, L.; Englehart, K.; Hudgins, B. A comparison of surface and intramuscular myoelectric signal classification. IEEE Trans. Biomed. Eng. 2007, 54, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Merletti, R.; Indino, B.; Graven-Nielsen, T. Surface EMG crosstalk evaluated from experimental recordings and simulated signals. Reflections on crosstalk interpretation, quantification and reduction. Methods Arch. 2004, 43, 30–35. [Google Scholar]
- Mann, P.S. Introductory Statistics; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning internal representations by error propagation. In Parallel Distributed Processing; California Univ.: San Diego, CA, USA, 1985; pp. 318–362. [Google Scholar]
- Huang, H.; Zhou, P.; Li, G.; Kuiken, T.A. An analysis of EMG electrode configuration for targeted muscle reinnervation based neural machine interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lobov, S.A.; Mironov, V.I.; Kastalskiy, I.A.; Kazantsev, V.B. Combined use of command-proportional control of external robotic devices based on electromyography signals. Mod. Technol. Med. 2015, 7, 30–38. [Google Scholar] [CrossRef][Green Version]
- Lobov, S.; Krilova, N.; Kastalskiy, I.; Kazantsev, V.; Makarov, V.A. Human-computer interface based on electromyography command-proportional control. In Proceedings of the 4th International Congress on Neurotechnology, Electronics and Informatics, Porto, Portugal, 7–8 November 2016; pp. 57–64. [Google Scholar]
- Sammut, C.; Webb, G.I. Encyclopedia of Machine Learning; Springer: New York, NY, USA, 2010; pp. 81–89. [Google Scholar]
- Moussaid, M.; Helbing, D.; Theraulaz, G. How simple rules determine pedestrian behaviour and crowd disasters. Proc. Natl. Acad. Sci. USA 2011, 108, 6884–6888. [Google Scholar] [CrossRef] [PubMed]
- Villacorta-Atienza, J.A.; Calvo, C.; Makarov, V.A. Prediction-for-CompAction: Navigation in social environments using generalized cognitive maps. Biol. Cybern. 2015, 109, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; Villacorta-Atienza, J.A.; Mironov, V.I.; Gallego, V.; Makarov, V.A. Waves in isotropic totalistic cellular automata: Application to real-time robot navigation. Adv. Complex Syst. 2016, 19, 1650012. [Google Scholar] [CrossRef]
- Makarov, V.A.; Makarova, J.; Herreras, O. Disentanglement of local field potential sources by independent component analysis. J. Comput. Neurosci. 2010, 29, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Herreras, O.; Makarova, J.; Makarov, V.A. New uses of LFPs: Pathway-specific threads obtained through spatial discrimination. Neuroscience 2015, 310, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Martin-Vazquez, G.; Makarova, J.; Makarov, V.A.; Herreras, O. The right hippocampus leads the bilateral integration of gamma-parsed lateralized information. eLife 2016, 5, e16658. [Google Scholar] [CrossRef] [PubMed]
- Kurenkov, A.L.; Kuzenkova, L.M.; Bursagova, B.I.; Petrova, S.A.; Klochkova, O.A.; Nikitin, S.S.; Artemenko, A.R.; Mamadyarov, A.M. An electromyographic study on the development of optimal tactics of botulinum toxin type a injections in children with spastic forms of cerebral palsy. Zhurnal Nevrologii i Psihiatrii 2013, 113, 53–60. [Google Scholar]
- Matsubara, T.; Morimoto, J. Bilinear modeling of EMG signals to extract user-independent features for multiuser myoelectric interface. IEEE Trans. Biomed. Eng. 2013, 60, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Tyukin, I.; Gorban, A.N.; Calvo, C.; Makarova, J.; Makarov, V.A. High-dimensional brain: A tool for encoding and rapid learning of memories by single neurons. Bull. Math. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Cherapkina, L. The neurofeedback successfulness of sportsmen. J. Hum. Sport Exerc. 2012, 7, S116–S127. [Google Scholar] [CrossRef]
- Bianco, V.; Berchicci, M.; Perri, R.L.; Quinzi, F.; Di Russo, F. Exercise-related cognitive effects on sensory-motor control in athletes and drummers compared to non-athletes and other musicians. Neuroscience 2017, 360, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Liburkina, S.P.; Vasilyev, A.N.; Yakovlev, L.V.; Gordleeva, S.Y.; Kaplan, A.Y. Motor imagery based brain computer interface with vibrotactile interaction. Zhurnal vysshey nervnoy deyatel’nosti im. I.P. Pavlova 2017, 67, 414–429. [Google Scholar]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobov, S.; Krilova, N.; Kastalskiy, I.; Kazantsev, V.; Makarov, V.A. Latent Factors Limiting the Performance of sEMG-Interfaces. Sensors 2018, 18, 1122. https://doi.org/10.3390/s18041122
Lobov S, Krilova N, Kastalskiy I, Kazantsev V, Makarov VA. Latent Factors Limiting the Performance of sEMG-Interfaces. Sensors. 2018; 18(4):1122. https://doi.org/10.3390/s18041122
Chicago/Turabian StyleLobov, Sergey, Nadia Krilova, Innokentiy Kastalskiy, Victor Kazantsev, and Valeri A. Makarov. 2018. "Latent Factors Limiting the Performance of sEMG-Interfaces" Sensors 18, no. 4: 1122. https://doi.org/10.3390/s18041122
APA StyleLobov, S., Krilova, N., Kastalskiy, I., Kazantsev, V., & Makarov, V. A. (2018). Latent Factors Limiting the Performance of sEMG-Interfaces. Sensors, 18(4), 1122. https://doi.org/10.3390/s18041122






