Real-Time Microscopic Monitoring of Flow, Voltage and Current in the Proton Exchange Membrane Water Electrolyzer
Abstract
:1. Introduction
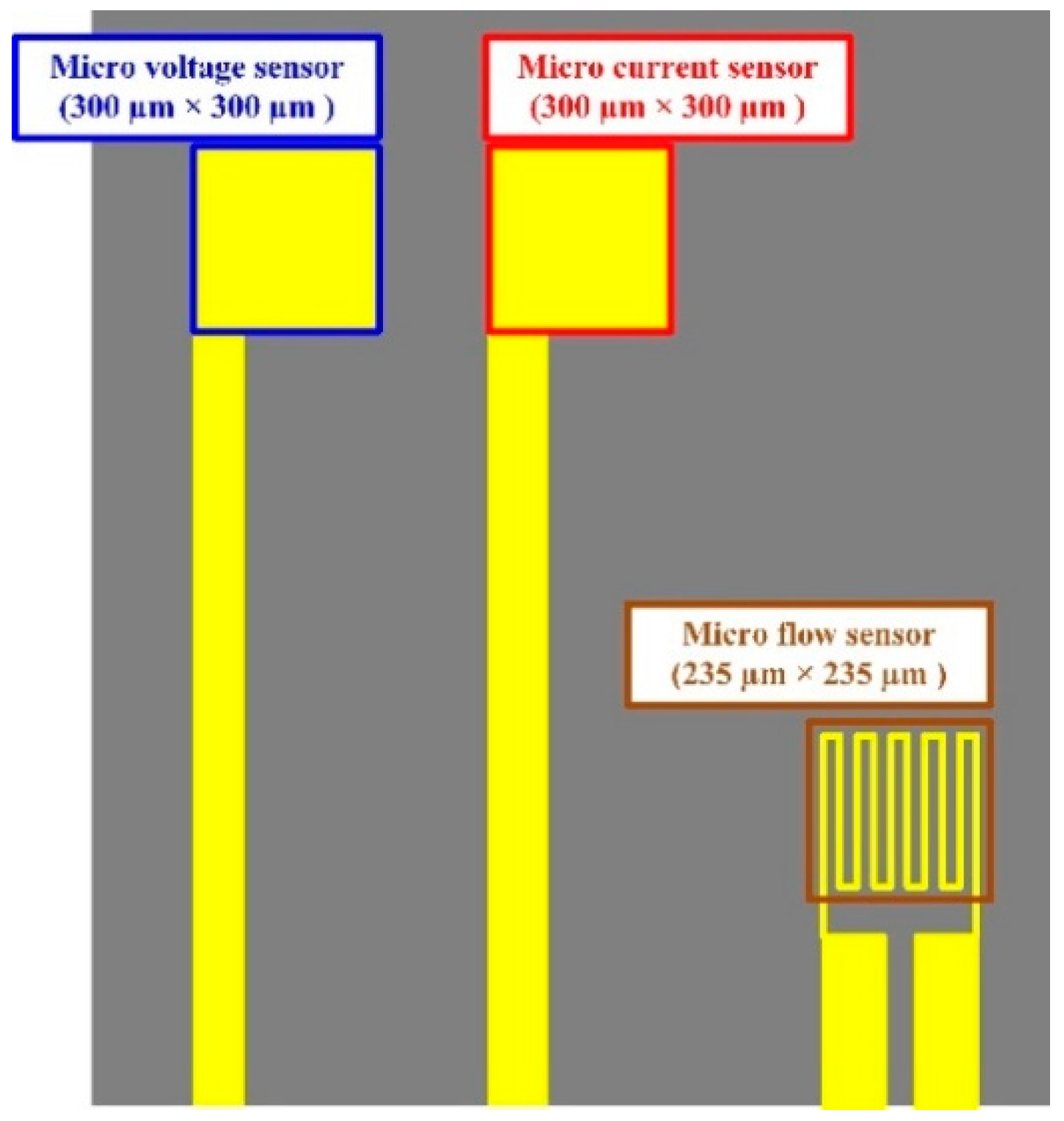
2. Design of a Flexible Integrated Microsensor
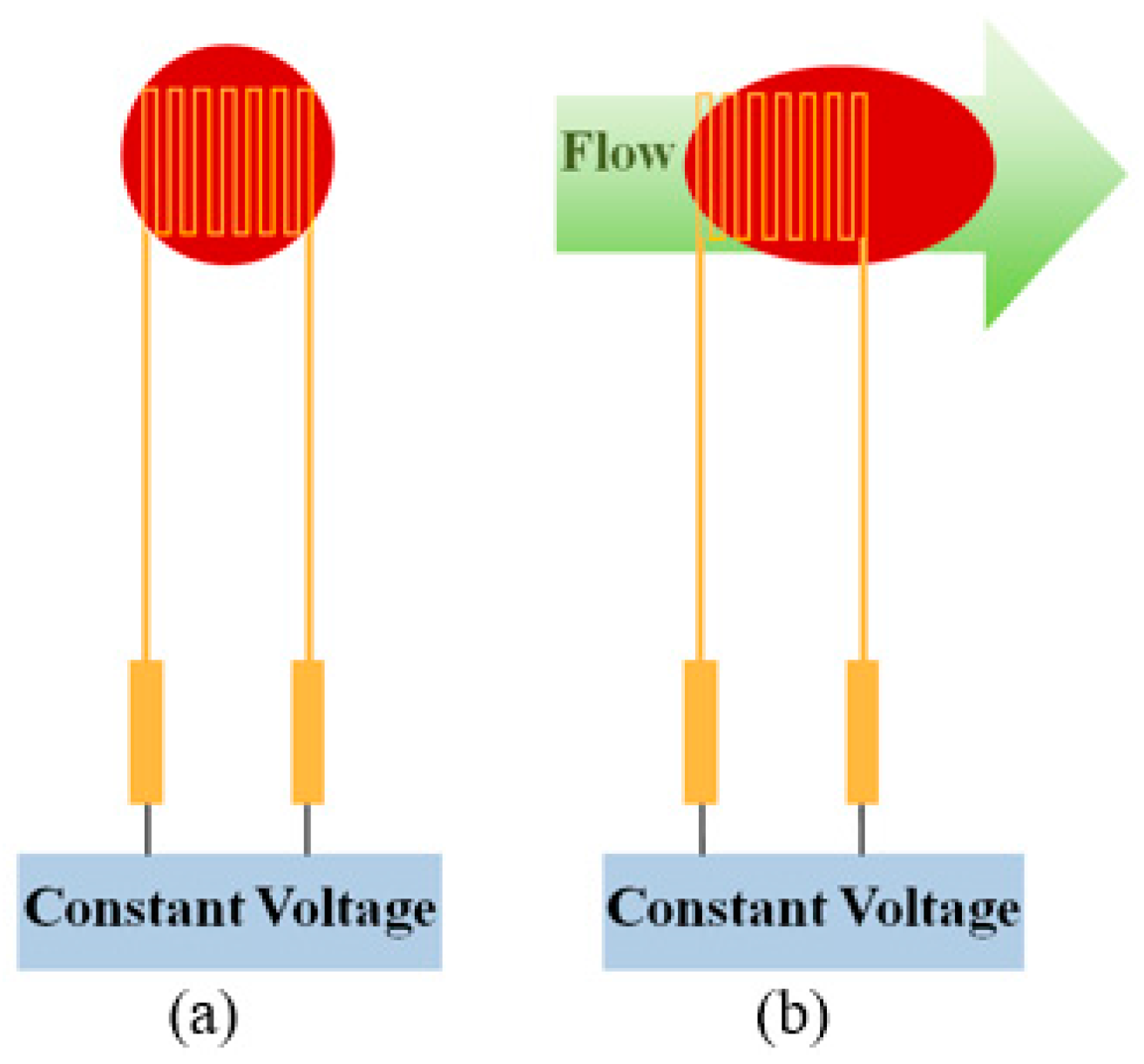
2.1. Micro Flow Sensor
2.2. Micro Voltage Sensor
2.3. Micro Current Sensor
2.4. Integrated Design of a Flexible Integrated Microsensor
3. Integration of the Proton Exchange Membrane Water Electrolyzer and a Flexible Integrated Microsensor
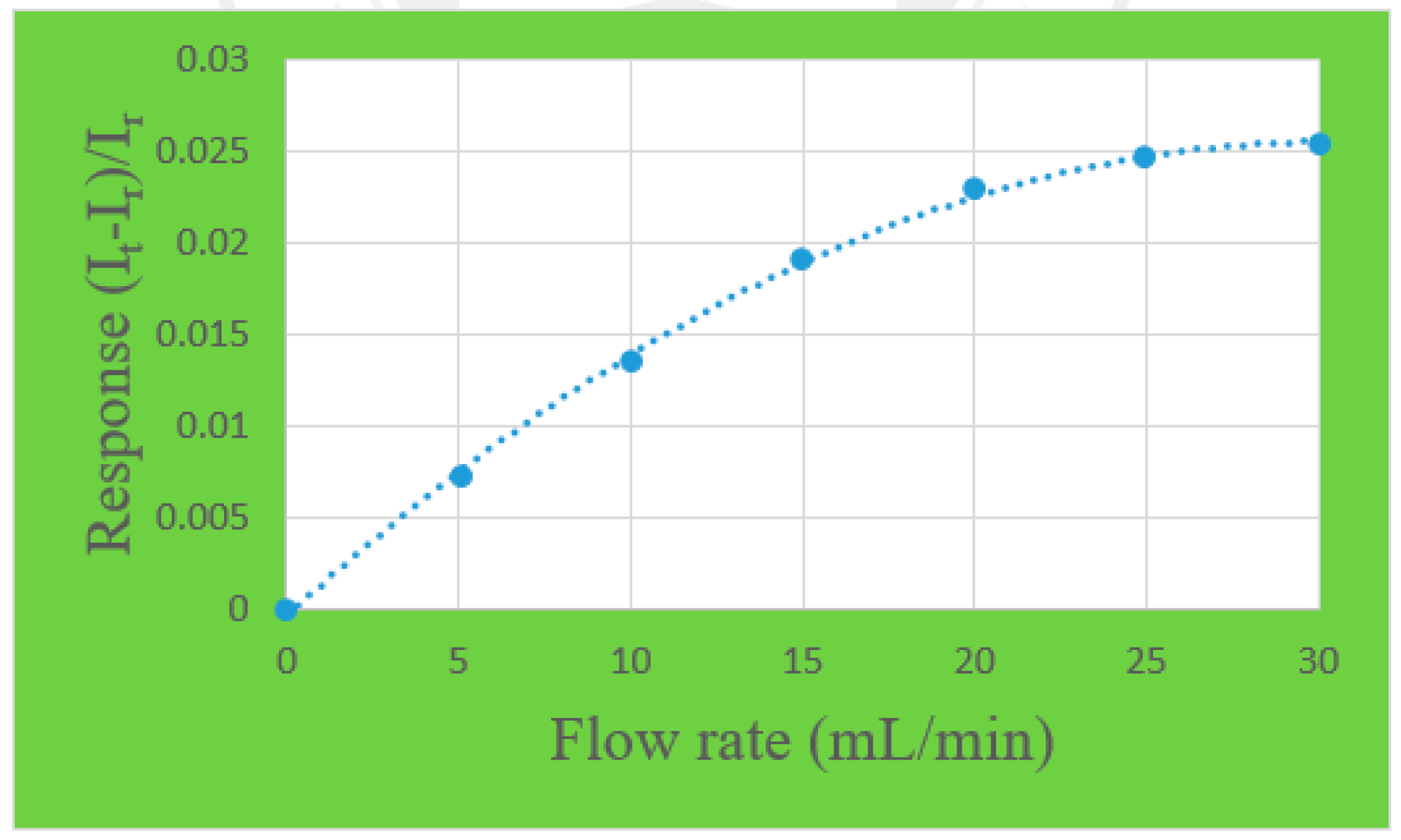
3.1. Correction of Micro Flow Sensor
3.2. Activation of the Proton Exchange Membrane Water Electrolyzer
4. Real-Time Microscopic Monitoring in the Proton Exchange Membrane Water Electrolyzer
4.1. Flow Test
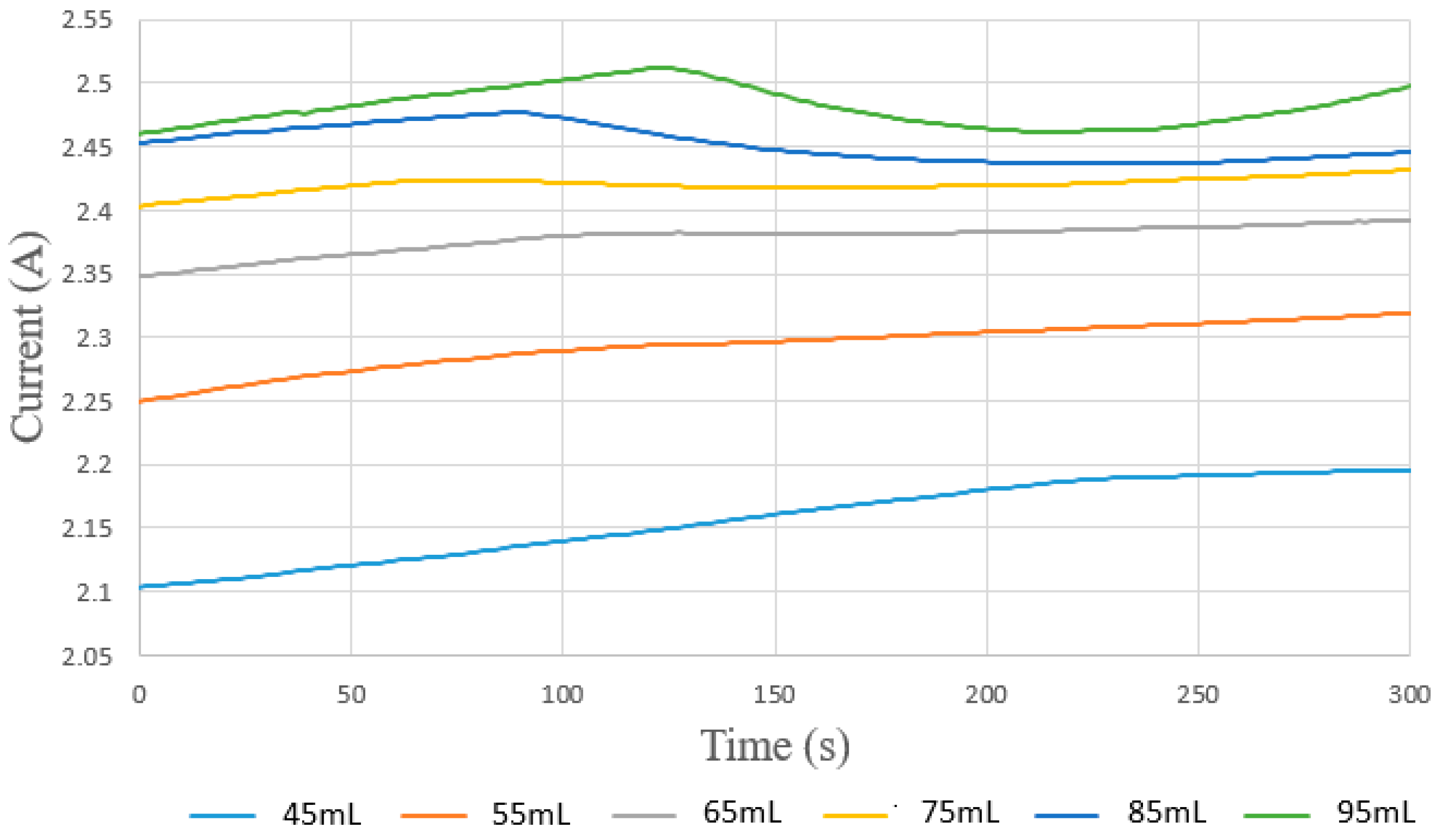
4.2. Local Flow Distribution of the Proton Exchange Membrane Water Electrolyzer
4.3. Local Voltage Distribution of the Proton Exchange Membrane Water Electrolyzer
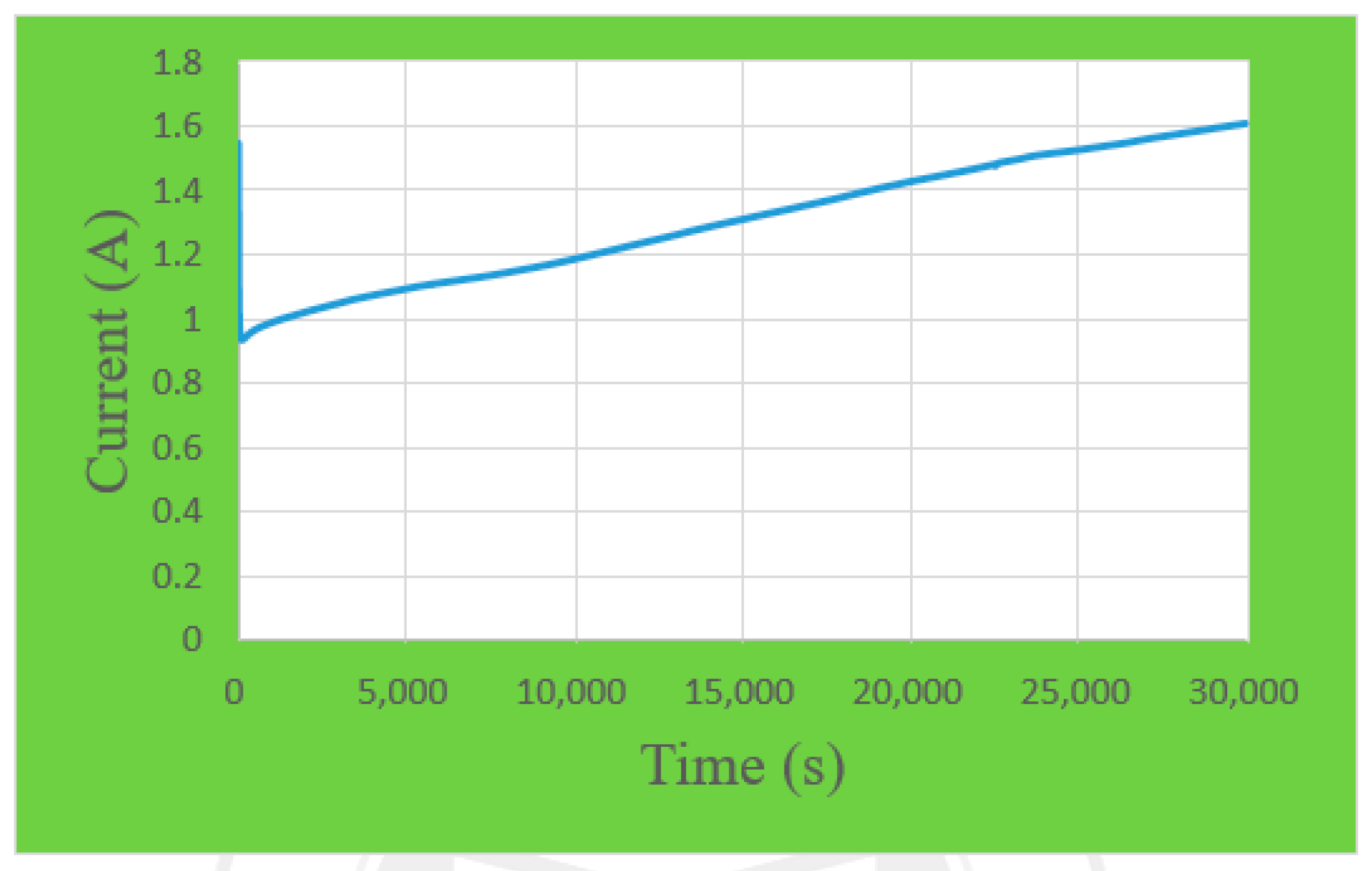
4.4. Local Current Density Distribution of the Proton Exchange Membrane Water Electrolyzer
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvin, G.S. A new sustainable hydrogen clean energy paradigm. Int. J. Hydrog. Energy 2018, 43, 4244–4255. [Google Scholar]
- Irfan, A.G.; Syed, A.M.; Rafiullah, K. Green hydrogen production potential for developing a hydrogen economy in Pakistan. Int. J. Hydrog. Energy 2018, in press. [Google Scholar]
- Kreuter, W.; Hofmann, H. Electrolysis: The important energy transformer in a world of sustainable energy. Int. J. Hydrog. Energy 1998, 23, 661–666. [Google Scholar] [CrossRef]
- Rasten, E.; Hagen, G.; Tunold, R. Electrocatalysis in water electrolysis with solid polymer electrolyte. Electrochim. Acta 2003, 48, 3945–3952. [Google Scholar] [CrossRef]
- Rozain, C.; Millet, P. Electrochemical characterization of polymer electrolyte membrane water electrolysis cells. Electrochim. Acta 2014, 131, 160–167. [Google Scholar] [CrossRef]
- Millet, P.; Nagameni, R.; Grigoriev, S.A.; Mbemba, N.; Brisset, F.; Ranjbari, A.; Etievant, C. PEM water electrolyzers: From electrocatalysis to stack development. Int. J. Hydrog. Energy 2010, 35, 5043–5052. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Millet, P.; Korobtsev, S.V.; Porembskiy, V.I.; Pepic, M.; Etievant, C.; Puyenchet, C.; Fateev, V.N. Hydrogen safety aspects related to high-pressure polymer electrolyte membrane water electrolysis. Int. J. Hydrog. Energy 2009, 34, 5986–5991. [Google Scholar] [CrossRef]
- Selamet, O.F.; Becerikli, F.; Mat, M.D.; Kaplan, Y. Development and testing of a highly efficient proton exchange membrane (PEM) electrolyzer stack. Int. J. Hydrog. Energy 2011, 36, 11480–11487. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Briguglio, N.; Brunaccini, G.; Blasi, A.D.; Stassi, A.; Ornelas, R.; Trifoni, E.; Antonucci, V.; Arico, A.S. An electrochemical study of a PEM stack for water electrolysis. Int. J. Hydrog. Energy 2012, 37, 1939–1946. [Google Scholar] [CrossRef]
- Sung, C.C.; Liu, C.Y. A novel micro protective layer applied on a simplified PEM water electrolyser. Int. J. Hydrog. Energy 2013, 38, 10063–10067. [Google Scholar] [CrossRef]
- Fofana, D.; Natarajan, S.K.; Hamelin, J.; Benard, P. Low platinum, high limiting current density of the PEMFC (proton exchange membrane fuel cell) based on multilayer cathode catalyst approach. Energy 2014, 64, 398–403. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-Y.; Li, S.-C.; Chen, C.-H.; Huang, Y.-T.; Wang, Y.-S. Real-Time Microscopic Monitoring of Flow, Voltage and Current in the Proton Exchange Membrane Water Electrolyzer. Sensors 2018, 18, 867. https://doi.org/10.3390/s18030867
Lee C-Y, Li S-C, Chen C-H, Huang Y-T, Wang Y-S. Real-Time Microscopic Monitoring of Flow, Voltage and Current in the Proton Exchange Membrane Water Electrolyzer. Sensors. 2018; 18(3):867. https://doi.org/10.3390/s18030867
Chicago/Turabian StyleLee, Chi-Yuan, Shih-Chun Li, Chia-Hung Chen, Yen-Ting Huang, and Yu-Syuan Wang. 2018. "Real-Time Microscopic Monitoring of Flow, Voltage and Current in the Proton Exchange Membrane Water Electrolyzer" Sensors 18, no. 3: 867. https://doi.org/10.3390/s18030867



