A Miniaturized Colorimeter with a Novel Design and High Precision for Photometric Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical Principle
2.2. Design of the Colorimeter
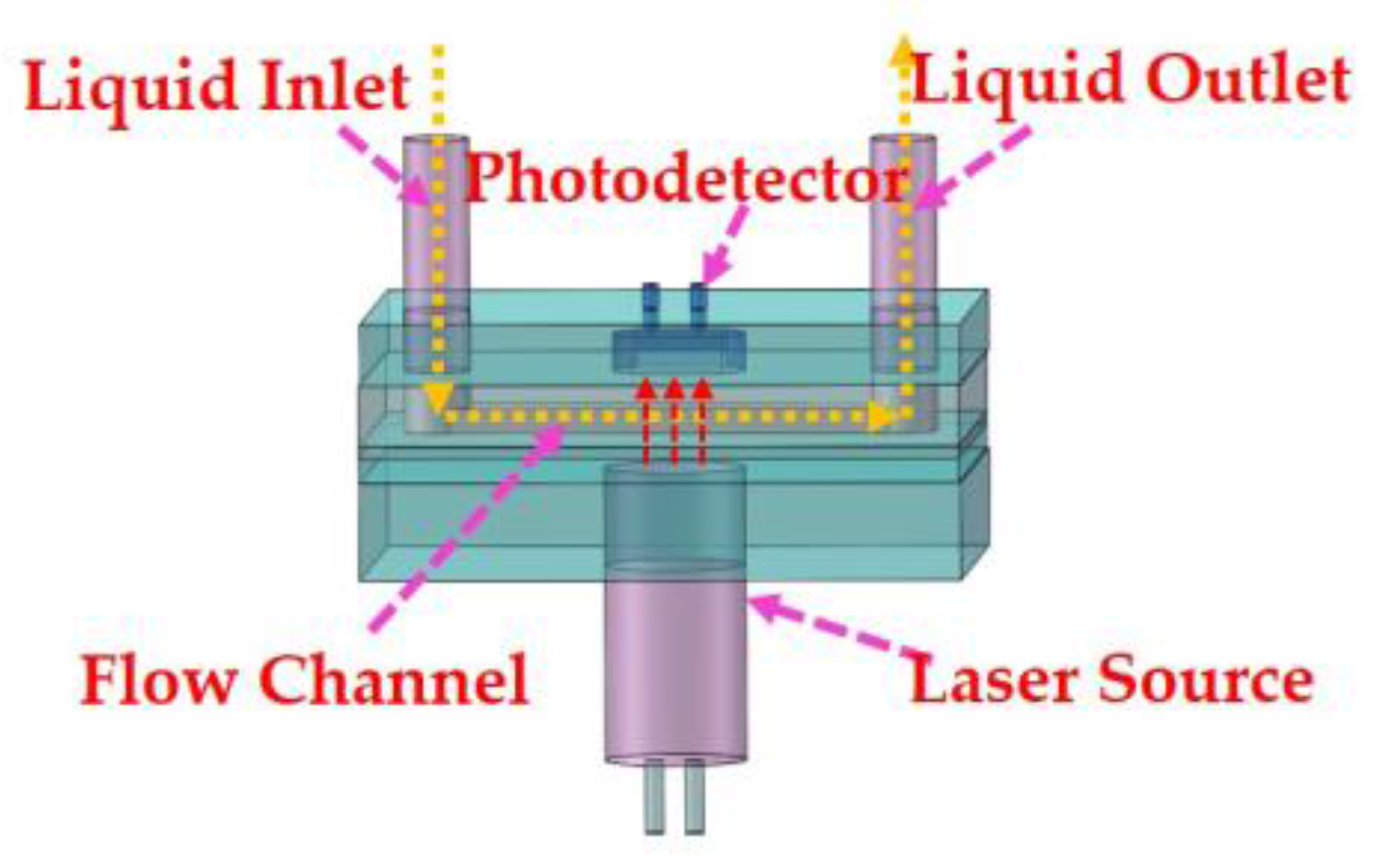
2.2.1. Light Source and Photodetector
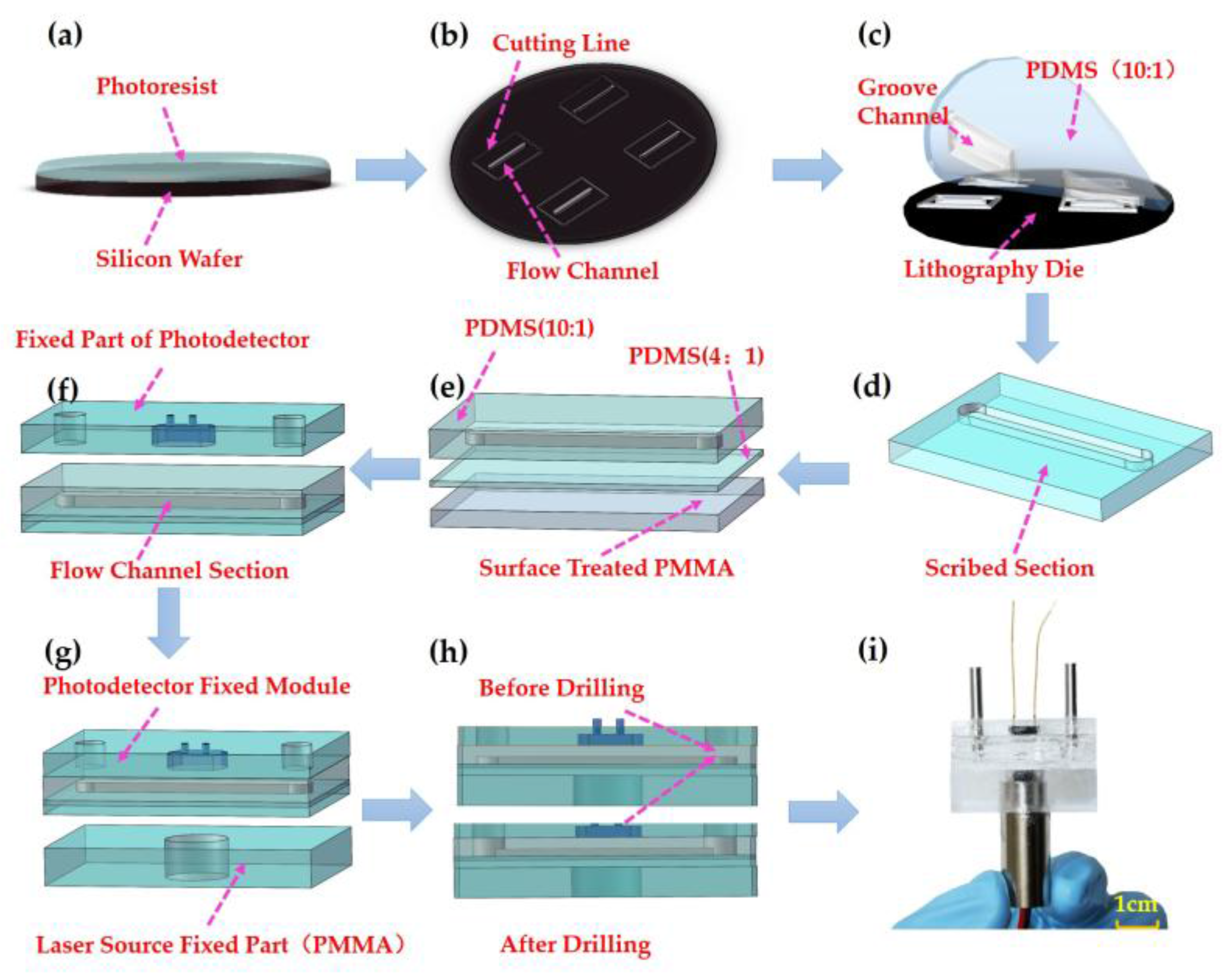
2.2.2. Fabrication of Microflow Cell
2.2.3. Integrated Design of the Colorimeter
2.3. Equipment and Materials
2.4. Preparation of Reagent Solution
2.5. Chromogenic Reaction and Experimental Section
2.6. Experimental Procedure
3. Results and Discussion
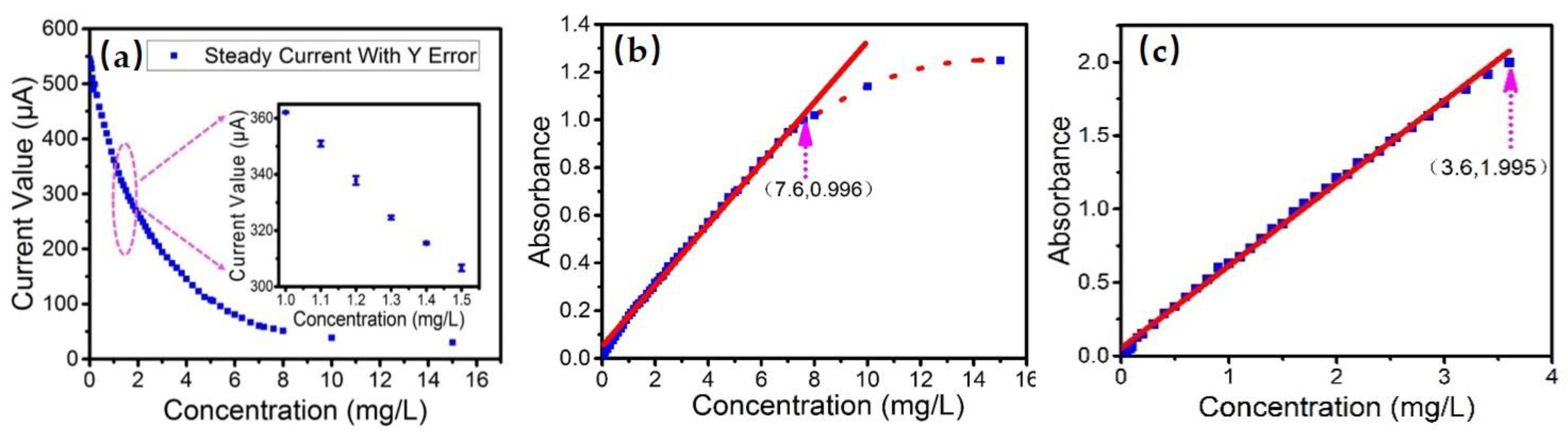
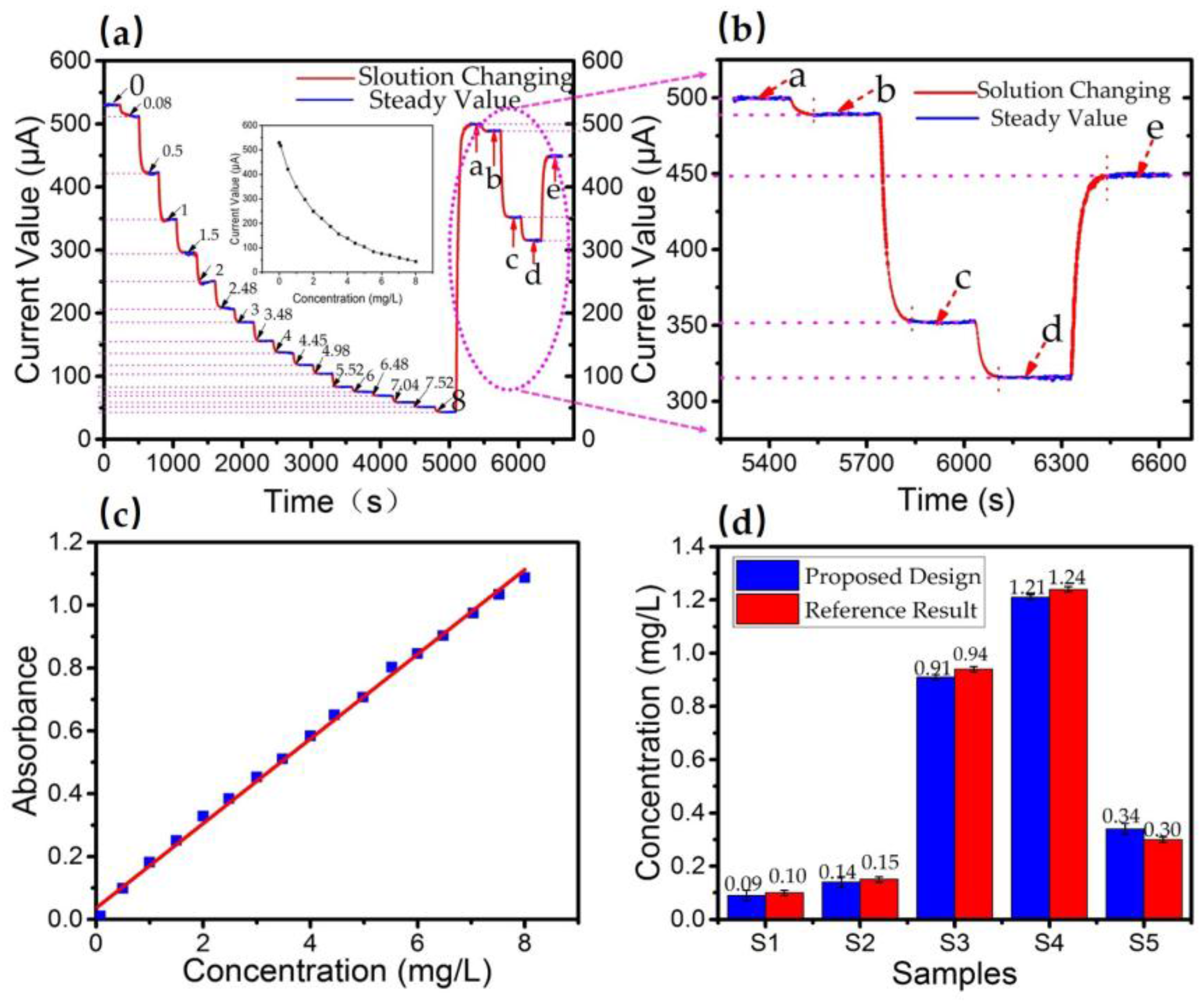
3.1. Determination of Linear Response Range
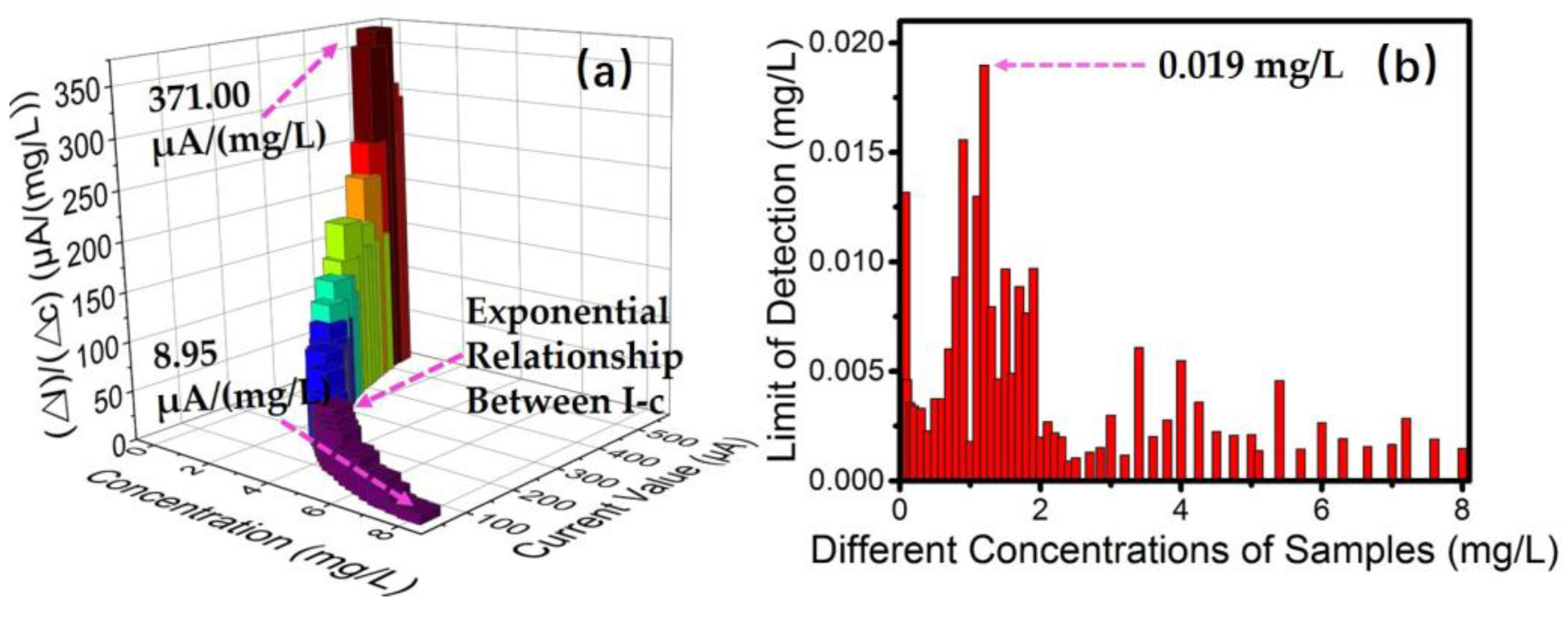
3.2. Sensitivity and Detection Limit
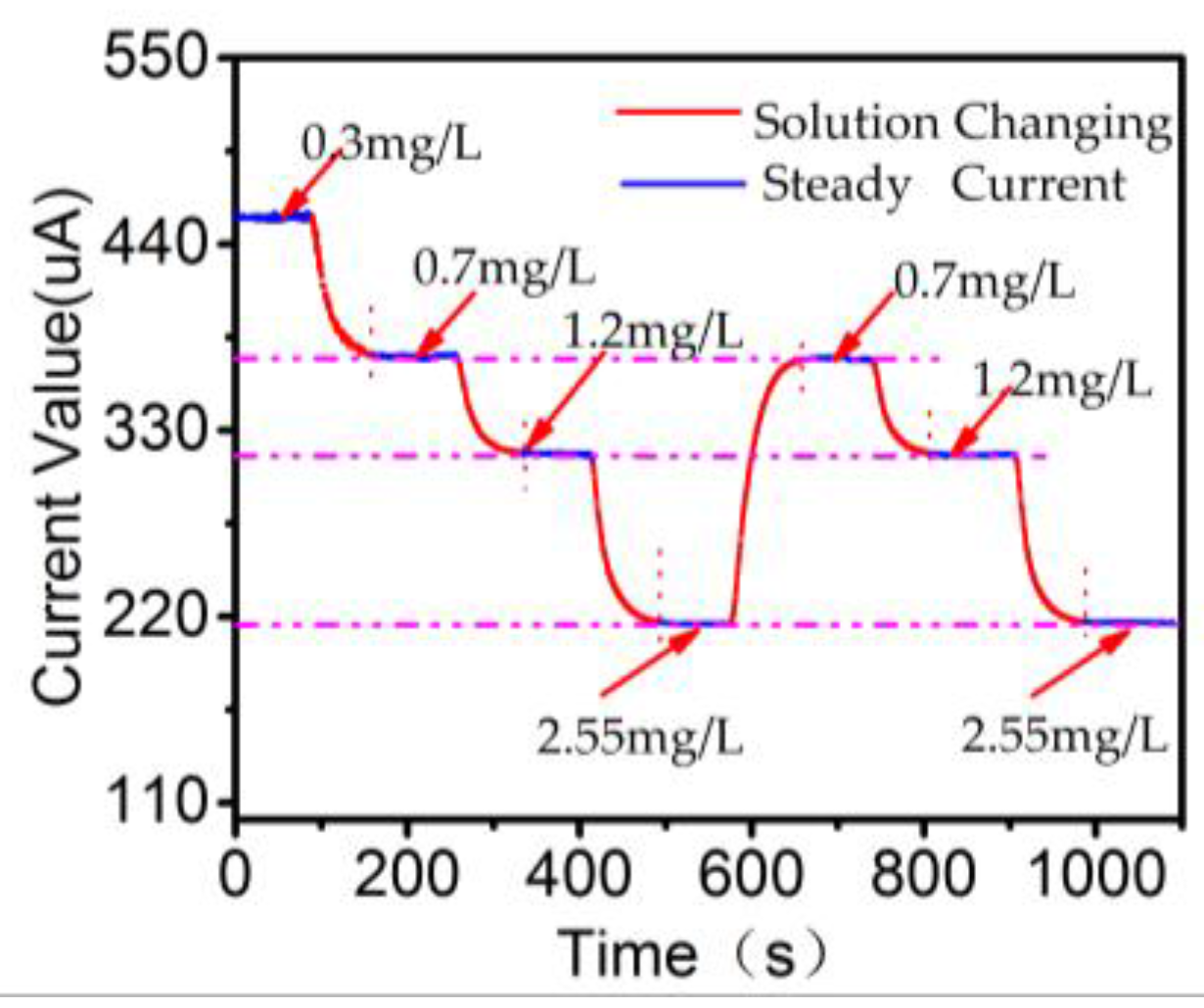
3.3. Data Consistency Analysis
3.4. Study of Interferences
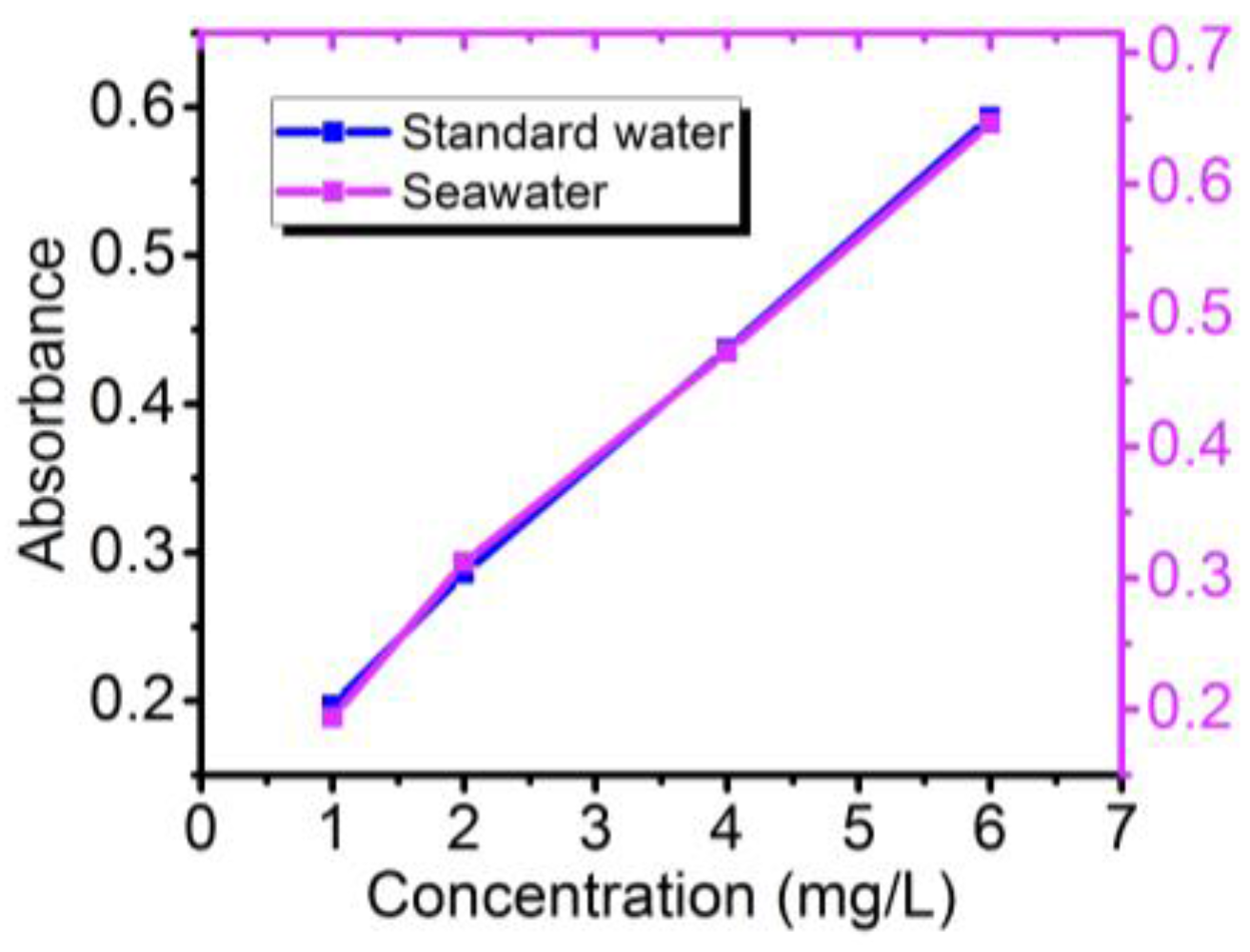
3.5. Sample Processing
3.6. Effect of the Residual Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stedmon, C.A.; Seredyńska-Sobecka, B.; Boe-Hansen, R.; Le Tallec, N.; Waul, C.K.; Arvin, E. A potential approach for monitoring drinking water quality from groundwater systems using organic matter fluorescence as an early warning for contamination events. Water Res. 2011, 45, 6030–6038. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Singh, B.; Gaur, S.; Garg, V.K.; Kushwaha, H.S. Analysis of groundwater quality using fuzzy synthetic evaluation. J. Hazard. Mater. 2007, 147, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Babin, S.M.; Burkom, H.S.; Mnatsakanyan, Z.R.; Ramac-Thomas, L.C.; Thompson, M.W.; Wojcik, R.A.; Lewis, S.H.; Yund, C. Drinking Water Security and Public Health Disease Outbreak Surveillance. Johns Hopkins APL Tech. Dig. 2008, 27, 403–411. [Google Scholar]
- Kroll, D.; King, K. Laboratory and flow loop validation and testing of the operational effectiveness of an on-line security platform for the water distribution system. In Proceedings of the Water Distribution Systems Analysis Symposium 2006, Cincinnati, OH, USA, 27–30 August 2007; Volume 173. [Google Scholar] [CrossRef]
- Hall, J.; Zaffiro, A.D.; Marx, R.B.; Kefauver, P.C.; Radha Krishnan, E.; Haught, R.C.; Herrmann, J.G. On-line water quality parameters as indicators of distribution system contamination. Am. Water Work. Assoc. 2007, 99, 66–77. [Google Scholar]
- De Marco, R.; Pejcic, B.; Chen, Z. Flow injection potentiometric determination of phosphate in waste waters and fertilisers using a cobalt wire ion-selective electrode. Analyst 1998, 123, 1635–1640. [Google Scholar] [CrossRef]
- Law Al, A.T.; Adeloju, S.B. Progress and recent advances in phosphate sensors: A review. Talanta 2013, 114, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Kolacinska, K. Recent Advances in Flow Injection Analysis. Analyst 2016, 141, 2085–2139. [Google Scholar] [CrossRef] [PubMed]
- Fiedoruk-Pogrebniak, M.; Koncki, R. Multicommutated flow analysis system based on fluorescence microdetectors for simultaneous determination of phosphate and calcium ions in human serum. Talanta 2015, 144, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Jeyaseelan, C.; Joshi, A.P. Trace determination of dexamethasone sodium phosphate in pharmaceutical formulations by differential pulse polarography. Anal. Bioanal. Chem. 2002, 373, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pagliano, E. Direct Determination of Dissolved Phosphate and Silicate in Seawater by Ion Exclusion Chromatography Sector Field Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2014, 86, 3222–3226. [Google Scholar] [CrossRef] [PubMed]
- Shyla, B.; Nagendrappa, G. A simple spectrophotometric method for the determination of phosphate in soil, detergents, water, bone and food samples through the formation of phosphomolybdate complex followed by its reduction with thiourea. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Warwick, C.; Guerreiro, A.; Soares, A. Sensing and analysis of soluble phosphates in environmental samples: A review. Biosens. Bioelectron. 2013, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Botev, I. A new conception of Bouguer-Lambert-Beer’s law. Fresenius Z. Anal. Chem. 1979, 297, 419. [Google Scholar] [CrossRef]
- Al Nabulsi, A.; Abdallah, O.; Angermann, L.; Bolz, A. New Modification of Lambert-beer’s Law using Simulation of Light Propagation in Tissue for Accurate Non-invasive Hemoglobin Measurements. In Proceedings of the International Conference on Applied Mathematics and Pharmaceutical Sciences, Singapore, 29–30 April 2012; pp. 601–608. [Google Scholar]
- Chen, X.; Chen, J.; Wang, F.; Xiang, X.; Luo, M.; Ji, X.; He, Z. Determination of glucose and uric acid with bienzyme colorimetry on microfluidic paper-based analysis devices. Biosens. Bioelectron. 2012, 35, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Masuda, Y.; Yamada, Y.; Itoh, M.; Takahashi, M.; Yatagai, T. Explanation of Human Skin Color by Multiple Linear Regression Analysis Based on the Modified Lambert-Beer Law. Opt. Rev. 2000, 7, 348–352. [Google Scholar] [CrossRef]
- Zephaniah Phillips, V.; Kim, E.; Kim, J.G. Gender-based brain lateralization using multichannel near infrared spectroscopy. In Proceedings of the International Winter Workshop on Brain-Computer Interface (BCI), Jeongsun-kun, Korea, 17–19 February 2014; pp. 3–6. [Google Scholar] [CrossRef]
- Novo, P.; França Prazeres, D.M.; Chu, V.; Conde, J.P. Microspot-based ELISA in microfluidics: Chemiluminescence and colorimetry detection using integrated thin-film hydrogenated amorphous silicon photodiodes. Lab Chip 2011, 11, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Uludag, K.; Kohl, M.; Steinbrink, J.; Obrig, H.; Villringer, A. Cross talk in the Lambert-Beer calculation for near-infrared wavelengths estimated by Monte Carlo simulations. J. Biomed. Opt. 2002, 7, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bunaciu, A.A.; Aboul-enein, H.Y.; Fleschin, S. Recent Applications of Fourier Transform Infrared Spectrophotometry in Herbal Medicine Analysis Recent Applications of Fourier Transform Infrared Spectrophotometry in Herbal Medicine Analysis. Appl. Spectrosc. Rev. 2017, 46, 251–260. [Google Scholar] [CrossRef]
- Kuriakose, S.; Joe, I.H. Feasibility of using near infrared spectroscopy to detect and quantify an adulterant in high quality sandalwood oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Denzer, W.; Hancock, G.; Islam, M.; Langley, C.E.; Peverall, R.; Ritchie, G.A.D.; Taylor, D. Trace species detection in the near infrared using Fourier transform broadband cavity enhanced absorption spectroscopy: Initial studies on potential breath analytes. Analyst 2011, 136, 801–806. [Google Scholar] [CrossRef] [PubMed]
- GBT 11893-89, Water Quality—Determination of Total Phosphorus—Ammonium Molybdate Spectrophotometric Method; GBT: South San Francisco, CA, USA, 1989.
- O’Toole, M.; Diamond, D. Absorbance based light emitting diode optical sensors and sensing devices. Sensors 2008, 8, 2453–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentle, B.S.; Ellis, P.S.; Faber, P.A.; Grace, M.R.; McKelvie, I.D. A compact portable flow analysis system for the rapid determination of total phosphorus in estuarine and marine waters. Anal. Chim. Acta 2010, 674, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Raimundo, I.M.; Rohwedder, J.J.R.; Lima, R.S.; Araújo, M.C.U. A microfluidic device with integrated fluorimetric detection for flow injection analysis. Anal. Bioanal. Chem. 2010, 396, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Wang, W. From cleanroom to desktop: Emerging micro-nanofabrication technology for biomedical applications. Ann. Biomed. Eng. 2011, 39, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, S.; Pan, T. Lab-on-a-print: From a single polymer film to three-dimensional integrated microfluidics. Lab Chip 2009, 9, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- AlSuhaimi, A.; Greenway, G.M.; McCreedy, T.; Youngvises, N. PDMS Microfluidic Device with an Integrated Optical Sensor for Determination of Zinc (II) in Pharmaceuticals: Toward the Production of Miniaturized Green Chemistry Analytical Systems. Spectrosc. Lett. 2009, 42, 370–375. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhang, J.Z. Kinetic spectrophotometric determination of submicromolar orthophosphate by molybdate reduction. Microchem. J. 2008, 89, 58–71. [Google Scholar] [CrossRef]
- Silveira, E.L.C.; De Caland, L.B.; Tubino, M. Molecular absorption spectrophotometric method for the determination of phosphorus in biodiesel. Fuel 2011, 90, 3485–3488. [Google Scholar] [CrossRef]
- Lima, M.B.; Barreto, I.S.; Andrade, S.I.E.; Almeida, L.F.; Araújo, M.C.U. A micro-flow-batch analyzer with solenoid micro-pumps for the photometric determination of iodate in table salt. Talanta 2012, 100, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Koronkiewicz, S.; Kalinowski, S. A novel direct-injection photometric detector integrated with solenoid pulse-pump flow system. Talanta 2011, 86, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Pan, T. A vacuum-driven peristaltic micropump with valved actuation chambers. J. Micromech. Microeng. 2011, 21, 065034. [Google Scholar] [CrossRef]
- Lima, M.B.; Barreto, I.S.; Andrade, S.I.E.; Neta, M.S.S.; Almeida, L.F.; Araújo, M.C.U. Photometric determination of phosphorus in mineralized biodiesel using a micro-flow-batch analyzer with solenoid micro-pumps. Talanta 2012, 98, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.K.; Reis, B.F.; Morales-Rubio, A.; de la Guardia, M. Speciation of chromium in natural waters by micropumping multicommutated light emitting diode photometry. Talanta 2007, 72, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.R.; Araujo, M.C.; Honorato, R.S.; Zagatto, E.A.; Lima, J.F.; Lapa, R.A. An improved leaping detector for flow analysis applied to iron speciation in drugs. J. Autom. Methods Manag. Chem. 2000, 22, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.P.; Perdigão, S.R.W.; Fiore, M.F.; Reis, B.F. Development of a high sensitive automatic setup for screening of microcystins in surface waters by employing a LED-based photometric detector. Sens. Actuators B Chem. 2012, 161, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Borges, S.S.; Peixoto, J.D.S.; Feres, M.A.; Reis, B.F. Downscaling a multicommuted flow injection analysis system for the photometric determination of iodate in table salt. Anal. Chim. Acta 2010, 668, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ródenas-Torralba, E.; Rocha, F.R.P.; Reis, B.F.; Morales-Rubio, Á.; De La Guardia, M. Evaluation of a multicommuted flow system for photometric environmental measurements. J. Autom. Methods Manag. Chem. 2006, 2006, 20384. [Google Scholar] [CrossRef] [PubMed]
- Camarillo-Escobedo, R.M.; Valdés-Perezgasga, F.; Rodríguez-Rivera, R. Micro-analyzer with optical detection and wireless communications. Sens. Actuators A Phys. 2013, 199, 181–186. [Google Scholar] [CrossRef]
- Lin, B.S.; Yang, Y.C.; Ho, C.Y.; Yang, H.Y.; Wang, H.Y. A PDMS-based cylindrical hybrid lens for enhanced fluorescence detection in microfluidic systems. Sensors (Switz.) 2014, 14, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jaffrezic-Renault, N.; Bessueille, F.; Leonard, D.; Xia, S.; Wang, X.; Chen, L.; Zhao, J. Development of a conductometric phosphate biosensor based on tri-layer maltose phosphorylase composite films. Anal. Chim. Acta 2008, 615, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Harmon, R.S.; DeLucia, F.C.; McManus, C.E.; McMillan, N.J.; Jenkins, T.F.; Walsh, M.E.; Miziolek, A. Laser-induced breakdown spectroscopy—An emerging chemical sensor technology for real-time field-portable, geochemical, mineralogical, and environmental applications. Appl. Geochem. 2006, 21, 730–747. [Google Scholar] [CrossRef]
- Prudnikov, E.D.; Barnes, R.M. Estimation of detection limits in inductively coupled plasma mass spectrometry. Fresenius J. Anal. Chem. 1998, 362, 465–468. [Google Scholar] [CrossRef]
- Boumans, P.W.J.M.; Maessen, F.J.M.J. Evaluation of detection limits in emission spectroscopy. Fresenius Z. Anal. Chem. 1966, 220, 241–260. [Google Scholar] [CrossRef]
- GB_T 5750.3 Standard Examination Methods for Drinking Water—Water Analysis Quality Control; GBT: South San Francisco, CA, USA, 2006.
- Connor, B.F.; Foreman, W.T.; Mayer, R.P.; Pritt, J.W.; Zayhowski, E.J.; Ritz, G.F.; Schertz, T.L. New Reporting Procedures Based on Long-Term Method Detection Levels and Some Considerations for Interpretations of Water-Quality Data Provided by the U.S. Geological Survey National Water Quality Laboratory; US Geological Survey Open-File; USGS: Reston, VA, USA, 1999. [Google Scholar]
- Spectroscopy, A.F.; Procedures, A.N.; Press, P. Nomenclature, Symbols, Units and their Usage in Spectrochemical Analysis—III. Analytical Flame Spectroscopy and Associated Non-Flame Procedures. Pure Appl. Chem. 1976, 45, 105–123. [Google Scholar] [CrossRef]
- Plates, A.C. Standard Methods for the Examination of Water and Wastewater; Plate 28; Taste and Odor Algae; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- Dias, T.R.; Brasil, M.A.S.; Feres, M.A.; Reis, B.F. A flow cell with a new design to improve the utilization of the radiation emitted by LED and employed as a radiation source for photometric detection. Sens. Actuators B Chem. 2014, 198, 448–454. [Google Scholar] [CrossRef]
- Miranda, J.C.; Kamogawa, M.Y.; Reis, B.F. Development of a portable setup and a multicommuted flow analysis procedure for the photometric determination of Fe(III) and Fe(II) in fresh water. Sens. Actuators B Chem. 2015, 207, 811–818. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hashigaya, S.; Kajiya, T.; Hirata, S. Determination of trace amounts of phosphate by flow-injection photometry. Anal. Bioanal. Chem. 2004, 378, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, G.; Gledhill, M.; Fletcher, P.J.; Worsfold, P.J. High temporal resolution field monitoring of phosphate in the River Frome using flow injection with diode array detection. Anal. Chim. Acta 2001, 440, 55–62. [Google Scholar] [CrossRef]


| Interference | Absorbance at 0 mg/L | Absorbance at 100 mg/L |
|---|---|---|
| Barium | 0.499 | 0.512 |
| Lead | 0.417 | 0.408 |
| Silver | 0.495 | 0.519 |
| Iron | 0.509 | 0.530 |
| Arsenate | 0.496 | 0.997 |
| Magnesium | 0.468 | 0.446 |
| Chlorine | 0.529 | 0.551 |
| Kalium | 0.489 | 0.505 |
| Sulfur | 0.499 | 0.505 |
| Interference | 0 mg/L | 1.3 mg/L | 2.6 mg/L | 3.9 mg/L | 5.2 mg/L |
| Arsenate | 0.498 | 0.507 | 0.519 | 0.536 | 0.561 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.-C.; Chen, Y.; Pang, Y.; Slavik, J.; Zhao, Y.-F.; Wu, X.-M.; Yang, Y.; Yang, S.-F.; Ren, T.-L. A Miniaturized Colorimeter with a Novel Design and High Precision for Photometric Detection. Sensors 2018, 18, 818. https://doi.org/10.3390/s18030818
Yan J-C, Chen Y, Pang Y, Slavik J, Zhao Y-F, Wu X-M, Yang Y, Yang S-F, Ren T-L. A Miniaturized Colorimeter with a Novel Design and High Precision for Photometric Detection. Sensors. 2018; 18(3):818. https://doi.org/10.3390/s18030818
Chicago/Turabian StyleYan, Jun-Chao, Yan Chen, Yu Pang, Jan Slavik, Yun-Fei Zhao, Xiao-Ming Wu, Yi Yang, Si-Fan Yang, and Tian-Ling Ren. 2018. "A Miniaturized Colorimeter with a Novel Design and High Precision for Photometric Detection" Sensors 18, no. 3: 818. https://doi.org/10.3390/s18030818






