Design and Characterization of Dicyanovinyl Reactive Dyes for the Colorimetric Detection of Thiols and Biogenic Amines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Starting Materials
2.2. Physical Measurements
2.3. Spectrophotometric Measurements
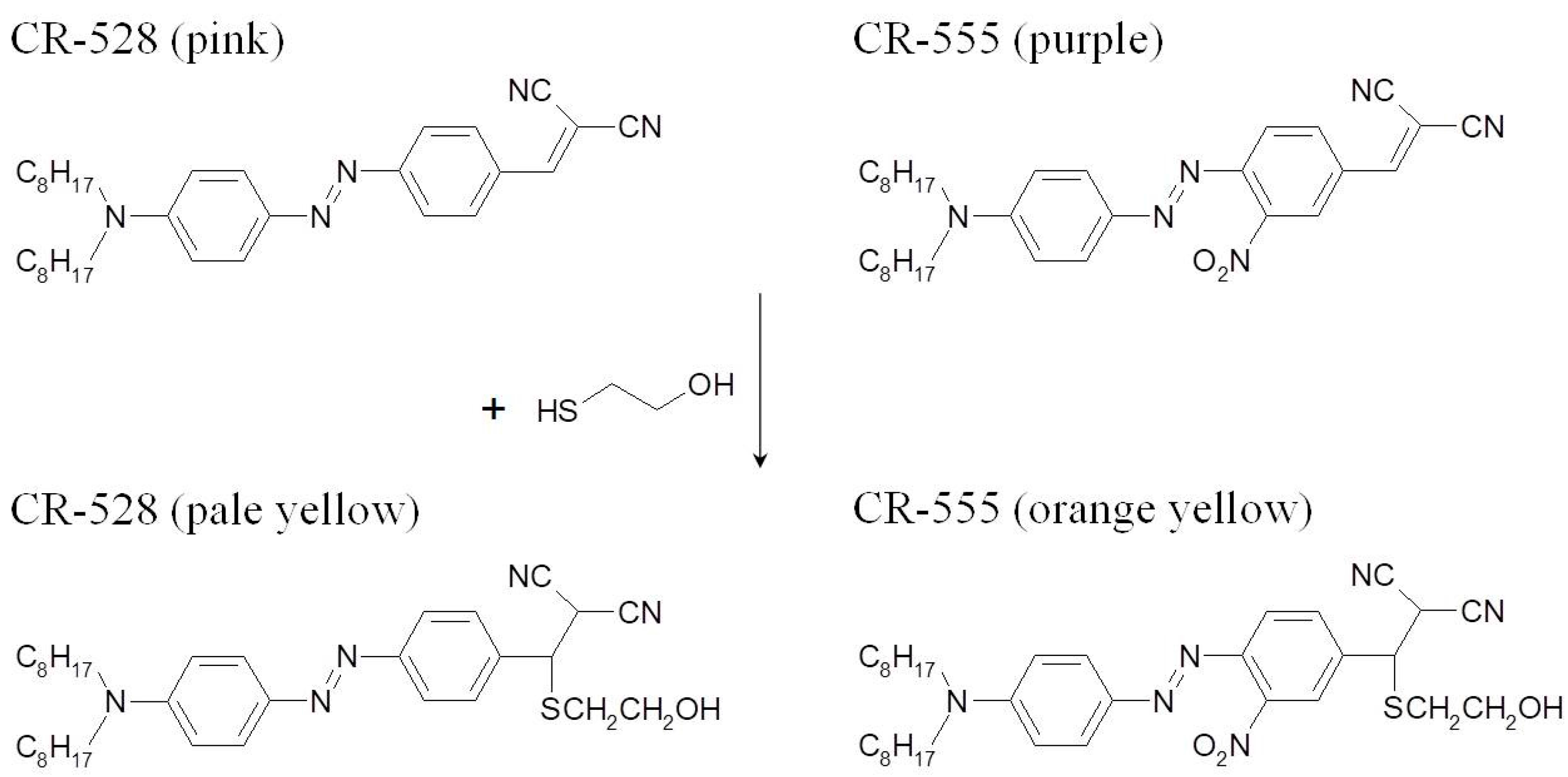
2.4. Synthesis
2.4.1. 4-N,N-dioctylamino-4′-dicyanovinylazobenzene (CR-528)
2.4.2. 4-N,N-dioctylamino-2′-nitro-4′-dicyanovinylazobenzene (CR-555)
3. Results and Discussion
3.1. Spectral Properties of CR-528 and CR-555 in Ethanol
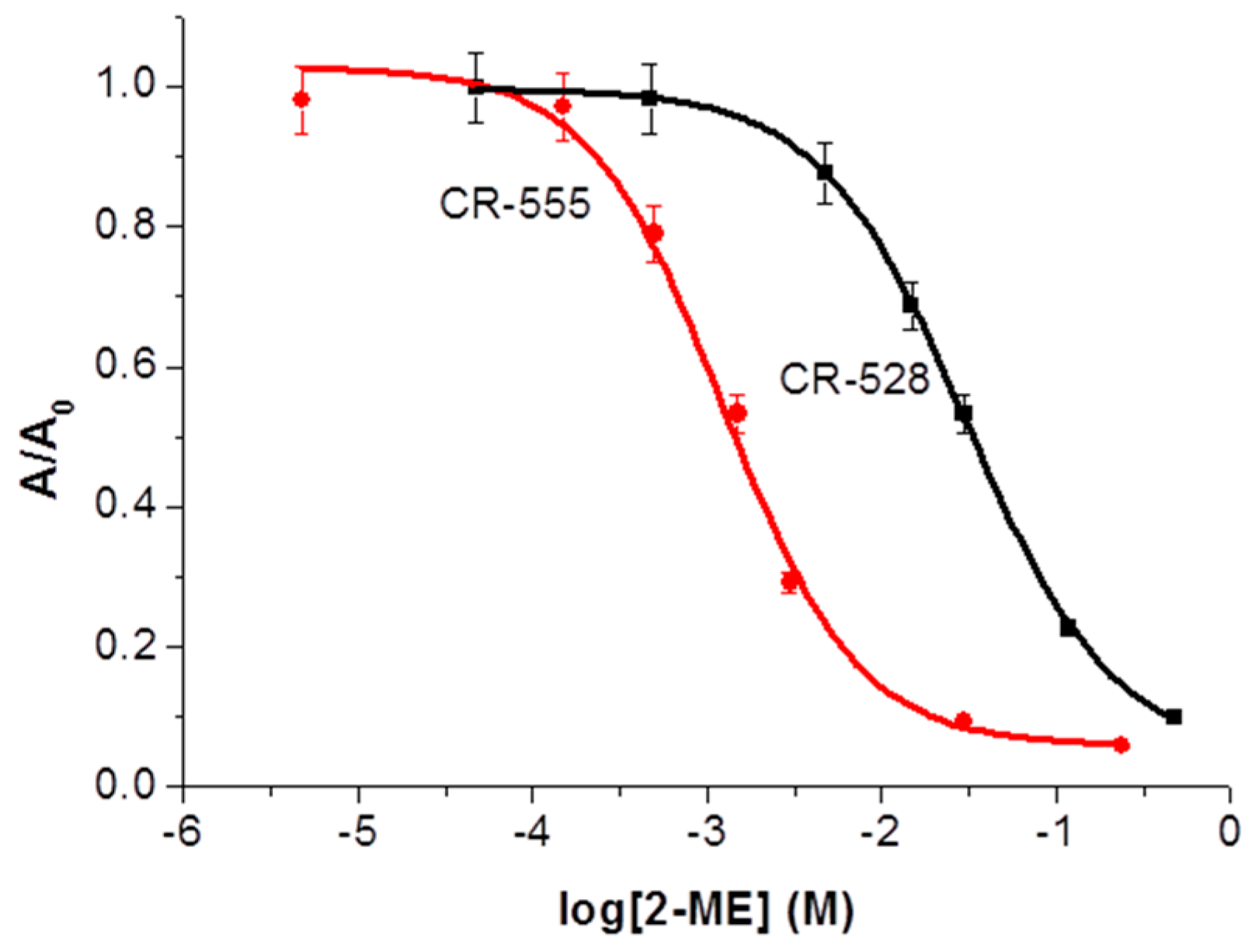
3.2. Optical Response of CR-528 and CR-555 towards 2-ME
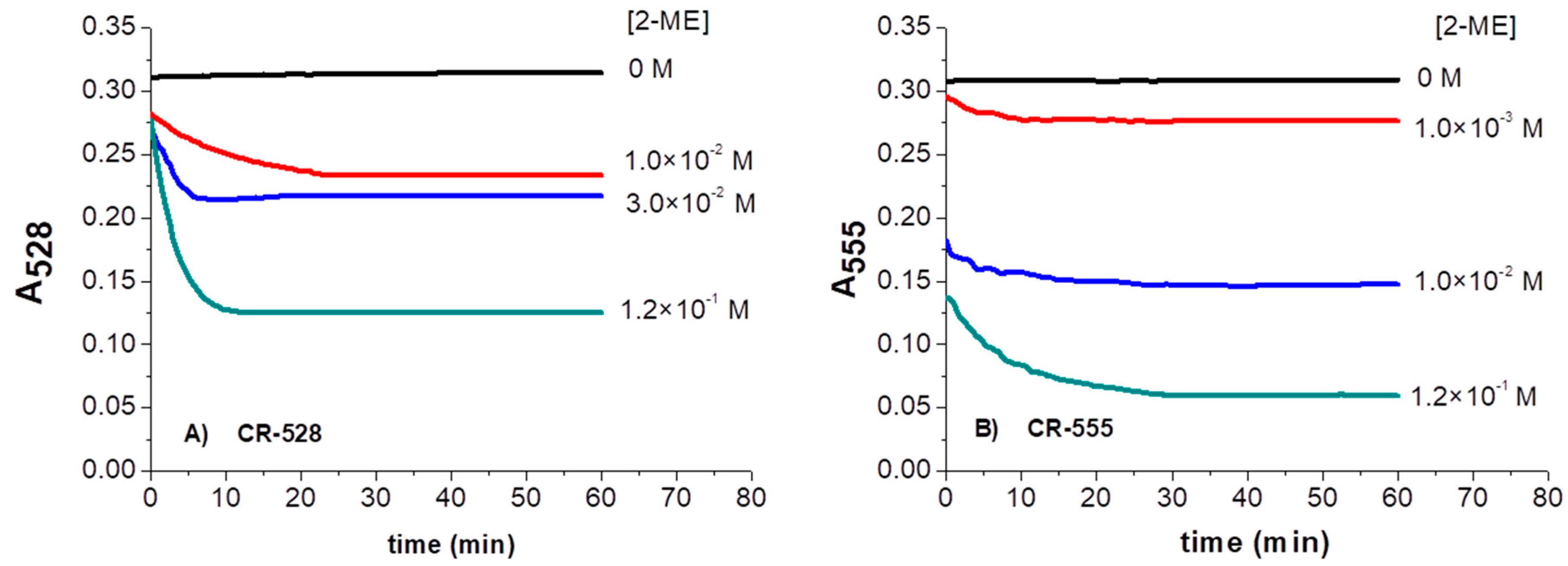
3.3. Time-Dependent Absorbance Changes of CR-528/CR-555 upon Exposure to 2-ME in Ethanol
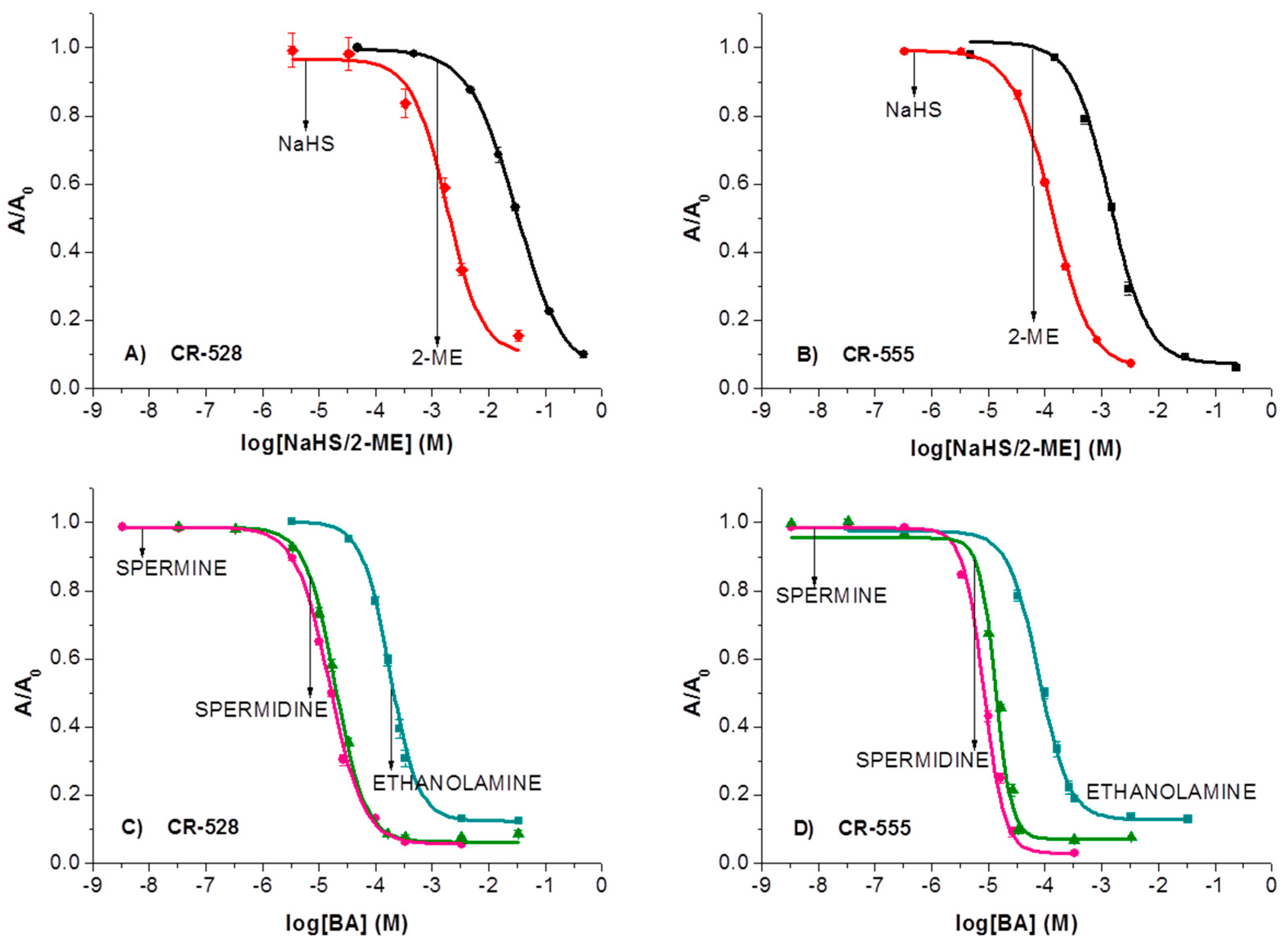
3.4. Optical Response of CR-528 and CR-555 towards Sulfur- and Amine-Analytes
3.5. Comparison with Developed Probes for the Optical Determination of Relevant Sulfur-Containing Analytes and Amines
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prakash, M.; Shetty, M.S.; Tilak, P.; Anwar, N. Total thiols: Biomedical importance and their alteration in various disorders. Online J. Health Allied Sci. 2009, 8, 1–9. [Google Scholar]
- Rouhiera, N.; Cerveauc, D.; Couturiera, J.; Reichheldf, J.P.; Reyc, P. Involvement of thiol- based mechanisms in plant development. Biochim. Biophys. Acta 2015, 1850, 1479–1496. [Google Scholar] [CrossRef] [PubMed]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A Central Role for Thiols in Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [PubMed]
- Dulsat-Serra, N.; Quintanilla-Casas, B.; Vichi, S. Volatile thiols in coffee: A review on their formation, degradation, assessment and influence on coffee sensory quality. Food Res. Int. 2016, 89, 982–988. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Exarchou, V.; Ordoudi, S.A.; Fiamegos, Y.; Stalikas, C. Ion-pair assisted extraction followed by 1H NMR determination of biogenic amines in food and biological matrices. Food Chem. 2016, 202, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, H.M.; Sameer, H.; Parameswar, K.I. Aggregation-Induced FRET via Polymer–Surfactant Complexation: A New Strategy for the Detection of Spermine. Anal. Chem. 2016, 88, 7358–7364. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; González-Béjar, M.; Moliner-Martínez, Y.; Campíns-Falcó, P.; Pérez-Prieto, J. Sensitive and Selective Plasmonic Assay for Spermine as Biomarker in Human Urine. Anal. Chem. 2014, 86, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Xu, Y.; Zhang, X.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Physiological and iTRAQ-Based Proteomic Analyses Reveal the Function of Spermidine on Improving Drought Tolerance in White Clover. J. Proteome Res. 2016, 15, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Ehmer, A.; Chaize, D.; Rimbach, G. Quantitative Determination of Spermidine in 50 German Cheese Samples on a Core−Shell Column by High-Performance Liquid Chromatography with a Photodiode Array Detector Using a Fully Validated Method. J. Agric. Food Chem. 2016, 64, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Park, J.H.; Choi, A.; Hwang, H.J.; Mah, J.H. Validation of an HPLC Analytical Method for Determination of Biogenic Amines in Agricultural Products and Monitoring of Biogenic Amines in Korean Fermented Agricultural Products. Toxicol. Res. 2015, 31, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.P.; Huang, X.; Zou, Y.C.; Zhou, L.J.; Wang, X.; Zhang, F.; Wang, D.; Li, G.D. Effective organic amine detection by nanoparticle-assembled tin dioxide microspheres: The importance of interparticle porosity on sensing properties. Sens. Actuators B: Chem. 2016, 224, 381–390. [Google Scholar] [CrossRef]
- Yanxiao, L.; Xiao-bo, Z.; Xiao-wei, H.; Ji-yong, S.; Jie-wen, Z.; Holmes, M.; Hao, L. A new room temperature gas sensor based on pigment-sensitized TiO2 thin film for amines determination. Biosens. Bioelectron. 2015, 67, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Xu, Y.M.; Zhang, X.F.; Cheng, X.L.; Gao, S.; Zhao, H.; Cai, Z.; Huo, L.H. Construction of three-dimensional flower-like α-MoO3 with hierarchical structure for highly selective triethylamine sensor. Sens. Actuators B: Chem. 2015, 208, 406–414. [Google Scholar] [CrossRef]
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z.; Xu, S.; Zhang, W.; Yu, H.; Li, M.; Huang, Z.; Zang, J.; et al. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Agarwal, V.; Kumar, P.; Gupta, G.; Khatri, M.; Kumar, A. Diagnosis of Oral Malodor: A Review of the Literature. Indian J. Dent. Sci. 2013, 5, 88–93. [Google Scholar]
- Loutfi, A.; Coradeschi, S.; Kumar Mani, G.; Shankar, P.; Balaguru Rayappan, J.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Liu, T.; Huo, F.; Li, J.; Chao, J.; Zhang, Y.; Yin, C. A fast response and high sensitivity thiol fluorescent probe in living cells. Sens. Actuators B: Chem. 2016, 232, 619–624. [Google Scholar] [CrossRef]
- Xu, J.; Yu, H.; Hu, Y.; Chen, M.; Shao, S. A gold nanoparticle-based fluorescence sensor for high sensitive and selective detection of thiols in living cells. Biosens. Bioelectron. 2016, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huo, F.; Yin, C.; Li, J.; Chao, J.; Zhang, Y. A triphenylamine as a fluorophore and maleimide as a bonding group selective turn-on fluorescent imaging probe for thiols. Dyes Pigments 2016, 128, 209–214. [Google Scholar] [CrossRef]
- Huo, F.J.; Sun, Y.Q.; Su, J.; Chao, J.B.; Zhi, H.J.; Yin, C.X. Colorimetric Detection of Thiols Using a Chromene Molecule. Org. Lett. 2009, 11, 4918–4921. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Dai, H.; Fu, Y.; Ying, Y.; Li, Y. Colorimetric Sensor Array for Thiols Discrimination Based on Urease−Metal Ion Pairs. Anal. Chem. 2016, 88, 8542–8547. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, J.H.; Kim, M.I.; Park, H.G. Label-free colorimetric detection of biological thiols based on target-triggered inhibition of photoinduced formation of AuNPs. Nanotechnology 2016, 27, 055501. [Google Scholar] [CrossRef] [PubMed]
- Henthorn, H.A.; Pluth, M.D. Mechanistic Insights into the H2S-Mediated Reduction of Aryl Azides Commonly Used in H2S Detection. J. Am. Chem. Soc. 2015, 137, 15330–15336. [Google Scholar] [CrossRef] [PubMed]
- Montoya, L.A.; Pearce, T.F.; Hansen, R.J.; Zakharov, L.N.; Pluth, M.D. Development of Selective Colorimetric Probes for Hydrogen Sulfide Based on Nucleophilic Aromatic Substitution. J. Org. Chem. 2013, 78, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Li, D.P.; Zhang, J.F.; Cui, J.; Ma, X.F.; Liu, J.T.; Miao, J.Y.; Zhao, B.X. A ratiometric fluorescent probe for fast detection of hydrogen sulfideand recognition of biological thiols. Sens. Actuators B: Chem. 2016, 234, 231–238. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, K.; Kim, H.J. Coumarin–malonitrile conjugate as a fluorescence turn-on probe for biothiols and its cellular expression. Chem. Commun. 2011, 47, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- Kumpf, J.; Schwaebel, S.T.; Bunz, U.H.F. Amine Detection with Distyrylbenzenedialdehyde-Based Knoevenagel Adducts. J. Org. Chem. 2015, 80, 5159–5166. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.A.; Bhamore, J.R.; Singhal, R.K.; Kailasa, S.K. Microwave assisted synthesis of tyrosine protected gold nanoparticles for dual (colorimetric and fluorimetric) detection of spermine and spermidine in biological samples. Biosens. Bioelectron. 2016, 88, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Singh, J.; Kaur, H.; Singh, N.; Kaur, N. Estimation of biogenic amines and biothiols by metal complex of fluorescent organic nanoparticles acting as single receptor multi-analyte sensor in aqueous medium. Sens. Actuators B: Chem. 2015, 220, 295–301. [Google Scholar] [CrossRef]
- Leng, P.Q.; Zhao, F.L.; Yin, B.C.; Ye, B.C. A novel, colorimetric method for biogenic amine detection based on arylalkylamine N-acetyltransferase. Chem. Commun. 2015, 51, 8712–8714. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadian, F.; Gholami, M.D.; Abdi, A.A. Determination of Vaporous Aliphatic Amines by Colorimetric Responses of a Molecular Receptor Based on Azobenzothiazole-Polyene Dye. IEEE Sens. J. 2015, 15, 6485–6490. [Google Scholar] [CrossRef]
- Nedeljko, P.; Turel, M.; Lobnik, A. Fluorescence-based determination of agmatine in dietary supplements. Anal. Lett. 2015, 48, 1619–1628. [Google Scholar] [CrossRef]
- Nedeljko, P.; Turel, M.; Košak, A.; Lobnik, A. Synthesis of hybrid thiol-functionalized SiO2 particles used for agmatine determination. J. Sol-Gel Sci. Technol. 2016, 79, 487–496. [Google Scholar] [CrossRef]
- Nedeljko, P.; Turel, M.; Lobnik, A. Hybrid sol-gel based sensor layers for optical determination of biogenic amines. Sens. Actuators B: Chem. 2017, 246, 1066–1073. [Google Scholar] [CrossRef]
- Basurto, S.; Torroba, T.; Comes, M.; Martinez-Manez, R.; Sancenon, F.; Villaescusa, L.; Amoros, P. New Chromogenic Probes into Nanoscopic Pockets in Enhanced Sensing Protocols for Amines in Aqueous Environments. Org. Lett. 2005, 7, 5469–5472. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wu, W.; Guo, H.; Zhao, J.; Ji, S.; Zhang, X.; Yuan, X.; Zhang, C. Colorimetric and Ratiometric Fluorescent Chemosensor Based on Diketopyrrolopyrrole for Selective Detection of Thiols: An Experimental and Theoretical Study. J. Org. Chem. 2011, 76, 9294–9304. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Y.; Wu, Z.; Zhang, H. Highly Sensitive and Naked Eye Dual-readout Method for L-Cysteine Detection Based on the NSET of Fluorophore Functionalized Gold Nanoparticles. Bull. Korean Chem. Soc. 2014, 35, 1159–1164. [Google Scholar] [CrossRef]
- Khajehsharifi, H.; Sheini, A. A selective naked-eye detection and determination of cysteine using an indicator-displacement assay in urine sample. Sens. Actuators B: Chem. 2014, 199, 457–462. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, X.; Zhu, B.; Jia, H.; Yang, W.; Li, Y.; Xue, J. A colorimetric and ratiometric fluorescent probe for quantitative detection of GSH at physiologically relevant levels. Sens. Actuators B: Chem. 2011, 159, 142–147. [Google Scholar] [CrossRef]
- Mohr, G.J. A chromoreactand for the selective detection of HSO3− based on the reversible bisulfite addition reaction in polymer membranes. Chem. Commun. 2002, 2646–2647. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, M.; Liu, Z.; Yu, M.; Li, F.; Yi, T.; Huang, C. Highly selective colorimetric sensor for cysteine and homocysteine based on azo derivatives. Tetrahedron Lett. 2006, 47, 7093–7096. [Google Scholar] [CrossRef]
- Chopra, S.; Singh, J.; Kaur, H.; Singh, A.; Singh, N.; Kaur, N. Colorimetric Detection of Spermine by the CuII Complex of Imine-Based Organic Nanoaggregates in Aqueous Medium. Eur. J. Inorg. Chem. 2015, 2015, 4437–4442. [Google Scholar] [CrossRef]
- Mohr, G.J. A tricyanovinyl azobenzene dye used for the optical detection of amines via a chemical reaction in polymer layers. Dyes Pigments 2004, 62, 77–81. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Kan, H.; Wang, W.; Zhu, B.; Dua, B.; Zhang, X. A highly selective colorimetric chemodosimeter for fast and quantitative detection of hydrogen sulfide. Analyst 2012, 137, 5576–5580. [Google Scholar] [CrossRef] [PubMed]
- Schaude, C.; Meindl, C.; Fröhlich, E.; Attard, J.; Mohr, G.J. Developing a sensor layer for the optical detection of amines during food spoilage. Talanta 2017, 170, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Schilling, M.L.; Katz, H.E.; Cox, D.I. Synthesis and reactions of cyanovinyl-substituted benzenediazonium salts for nonlinear optics. J. Org. Chem. 1988, 53, 5538–5540. [Google Scholar] [CrossRef]
- Chu, K.Y.; Griffiths, J. Cyanovinyl substituted azo dyes. An unusual example of negative halochromism in a monosubstituted aminoazobenzene. Tetrahedron Lett. 1976, 17, 405–406. [Google Scholar] [CrossRef]
- Mohr, G.J.; Citterio, D.; Spichiger-Keller, U.E. Development of chromogenic reactands for optical sensing of alcohols. Sens. Actuators B: Chem. 1998, 49, 226–234. [Google Scholar] [CrossRef]
- Bello, K.A.; Griffiths, J. Violet to cyan azo dyes derived from 4-amino-3-nitrobenzaldehydeas diazo component. Dyes Pigments 1989, 11, 65–76. [Google Scholar] [CrossRef]
- Lams, Y.Y.; Nkeonye, P.O.; Bello, K.A.; Yakubu, M.K.; Lawal, A.O. Synthesis of malononitrile-condensed disperse dyes and application on polyester and nylon fabrics. J. Text. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Khuhawar, M.Y.; Querski, G.A. Polyamines as cancer markers: Applicable separation methods. J. Chromatogr. B 2001, 764, 385–407. [Google Scholar] [CrossRef]


| Analyte | Analyte Structure | CR-528 | CR-555 | ||
|---|---|---|---|---|---|
| X0 a (M) | λmax b (nm) | X0 a (M) | λmax b (nm) | ||
| 2-ME |  | 3.00 × 10−2 | 434 | 1.35 × 10−3 | 474 |
| Sodium hydrosulfide | 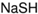 | 1.66 × 10−3 | 390 | 1.29 × 10−4 | 464 |
| Ethanolamine | 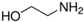 | 1.73 × 10−4 | 460 | 1.37 × 10−5 | 490 |
| Spermine | 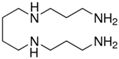 | 1.46 × 10−5 | 437 | 8.37 × 10−5 | 475 |
| Spermidine |  | 2.03 × 10−5 | 443 | 8.77 × 10−6 | 469 |
| Indicator | Analyte | Working Range (molL−1) | Response Time (min) | Remark | [Ref.] |
|---|---|---|---|---|---|
| CR-528 | Spermine | 3 × 10−6–1.2 × 10−4 | 30 | A, ethanol solution | our work |
| Spermidine | 3 × 10−6–1.2 × 10−4 | ||||
| Ethanolamine | 5 × 10−5–1 × 10−3 | ||||
| NaSH | 2 × 10−4–3 × 10−2 | ||||
| 2-ME | 3 × 10−3–3 × 10−1 | ||||
| CR-555 | Spermine | 2 × 10−6–2 × 10−5 | 30 | A, ethanol solution | our work |
| Spermidine | 5 × 10−6–2.5 × 10−5 | ||||
| Ethanolamine | 2 × 10−5–3.1 × 10−4 | ||||
| NaSH | 1.2 × 10−5–2.5 × 10−4 | ||||
| 2-ME | 1.5 × 10−4–1 × 10−2 | ||||
| 4-methyl-7-azidocoumarin | NaSH | 2 × 10−5, single concentration in a mechanism study | 60 | F, 50 mM PIPES, 100 mM KCl, pH 7.4 | [24] |
| 4-chloro-7-nitrobenzofura-zan (NBD-Cl) | H2S | ~1 × 10−6–3.3 × 10−5 | 30 | A, 50 mM PIPES, 100 mM KCl, pH 7.4 | [25] |
| Fluorescent probe (HF-PBA) constructed from 3-HF and 2-(fluorine-2-yl-disulfanyl)benzoic acid (PBA) | H2S | ~2 × 10−6–8 × 10−6 | 30 | F, PBS (pH 7.4) containing 40% ethanol (v/v) | [26] |
| Coumarin–malonitrile conjugate | 2-ME | 2 × 10−3, single concentration in kinetics study | 330 | F, DMSO–HEPES buffer (1:2, v/v; 0.10 M pH 7.4) | [27] |
| Tyrosine-protected gold nanoparticles (Tyr-Au NPs) | Spermine, Spermidine | 1 × 10−10–5 × 10−5 | n.d. | A,F; PBS buffer at pH 6.0 | [29] |
| Aggregates from poly[9,9-bis(6′-methyl imidazoliumbromide)hexyl)fluorene-co-4,7-(2,1,3-benzothiadiazole)](PFBT-MI) and surfactant | Spermine | 0–1.2 × 10−4 | n.d. | F, aqueous solution | [6] |
| Cu(II) complex of Schiff-base receptor organic nanoaggregates | Spermine | ~2 × 10−4–1.4 × 10−3 | 3 | A, DMF/water (1/99, v/v) solvent system | [43] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastnak, T.; Lobnik, A.; Mohr, G.J.; Turel, M. Design and Characterization of Dicyanovinyl Reactive Dyes for the Colorimetric Detection of Thiols and Biogenic Amines. Sensors 2018, 18, 814. https://doi.org/10.3390/s18030814
Mastnak T, Lobnik A, Mohr GJ, Turel M. Design and Characterization of Dicyanovinyl Reactive Dyes for the Colorimetric Detection of Thiols and Biogenic Amines. Sensors. 2018; 18(3):814. https://doi.org/10.3390/s18030814
Chicago/Turabian StyleMastnak, Tinkara, Aleksandra Lobnik, Gerhard J. Mohr, and Matejka Turel. 2018. "Design and Characterization of Dicyanovinyl Reactive Dyes for the Colorimetric Detection of Thiols and Biogenic Amines" Sensors 18, no. 3: 814. https://doi.org/10.3390/s18030814




