Sulfide Species Optical Monitoring by a Miniaturized Silicon Photomultiplier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
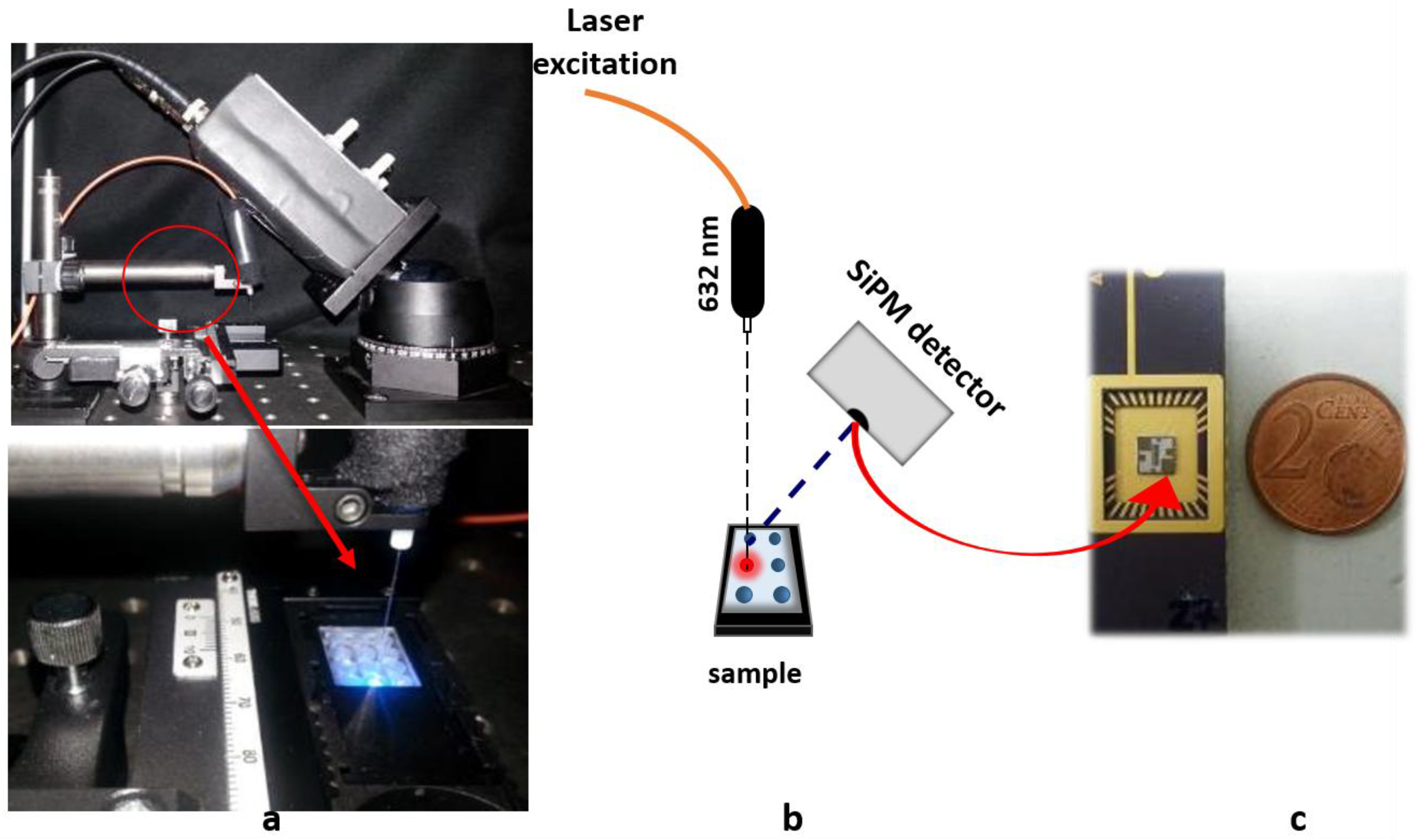
2.2. Silicon Photomultiplier System Measurement Setup
2.3. Sulfide Amount Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Petralia, S.; Castagna, M.E.; Cappello, E.; Puntoriero, F.; Trovato, E.; Gagliano, A.; Conoci, S. A miniaturized silicon based device for Nucleic Acids electrochemical Detection. Sens. Biosens. Res. 2015, 6, 90–94. [Google Scholar] [CrossRef]
- Libertino, S.; Conoci, S.; Scandurra, A.; Spinella, C. Biosensor integration on Si-based devices: Feasibility studies and examples. Sens. Actuators B Chem. 2013, 179, 240–251. [Google Scholar] [CrossRef]
- Banna, M.H.; Imran, S.; Francisque, A.; Najjaran, H.; Sadiq, R.; Rodriguez, M.; Hoorfar, M. Online drinking water quality monitoring: Review on available and emerging technologies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1370–1421. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.C.; Mason, A. (Eds.) Smart Sensors for Real-Time Water Quality Monitoring; Springer: Berlin, Germany, 2013; ISBN 978-3-642-37006-9. [Google Scholar]
- Doujaiji, B.; Al-Tawfiq, J.A. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med. 2010, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Grech, T.; Fiorenza, F.; Marletta, A.; Valenti, P.; Petralia, S. Sulfidic spring in the gypsum karst system of Monte Conca (Italy): Chemistry and microbiological evidences. Int. J. Speleol. 2015, 44, 125–139. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Davis, J.; Compton, R.G. Analytical strategies for the detection of sulfide: A review. Talanta 2000, 52, 771–784. [Google Scholar] [CrossRef]
- Chena, C.; Zhao, D.; Lu, L.; Yang, F.; Yang, X. A simple and rapid colorimetric sensor for sulfide anion detection based on redox reaction of ABTS with Au (III). Sens. Actuators B Chem. 2015, 220, 1247–1253. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.H.; Tang, K.T. A review of sensor-based methods for monitoring hydrogen sulfide. Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, C.J.; Tao, T.; Duan, M.; Fang, S.W.; Zheng, M. A miniaturized fiber-optic colorimetric sensor for nitrite determination by coupling with a microfluidic capillary waveguide. Anal. Bioanal. Chem. 2016, 408, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Mazzillo, M.; Condorelli, G.; Sanfilippo, D.; Valvo, G.; Carbone, B.; Fallica, G.; Billotta, S.; Belluso, M.; Bonanno, G.; Cosentino, L.; et al. Silicon photomultiplier technology at Stmicroelectronics. IEEE Trans. Nucl. Sci. 2009, 56, 2434–2442. [Google Scholar] [CrossRef]
- Sciacca, E.; Giudice, A.C.; Sanfilippo, D.; Zappa, F.; Lombardo, S.; Consentino, R.; Di Franco, C.; Ghioni, M.; Fallica, G.; Bonanno, G.; et al. Silicon Planar Technology for Single-Photon Optical Detectors. IEEE Trans. Electron Devices 2003, 50, 918–925. [Google Scholar] [CrossRef]
- Santangelo, M.F.; Sciuto, E.L.; Lombardo, S.A.; Busacca, A.C.; Petralia, S.; Conoci, S. Novel Si-based technologies for bio-sensing applications, S. Libertino. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 6900307. [Google Scholar] [CrossRef]
- Santangelo, M.F.; Sciuto, E.L.; Busacca, A.C.; Petralia, S.; Conoci, S.; Libertino, S. SiPM as miniaturised optical biosensor for DNA-microarray applications. Sens. Biosens. Res. 2015, 6, 95–98. [Google Scholar] [CrossRef]
- Pagano, R.; Corso, D.; Lombardo, S.; Libertino, S.; Valvo, G.; Sanfilippo, D.; Russo, A.; Fallica, P.G.; Pappalardo, A.; Finocchiaro, P. Optimized silicon photomultipliers with optical trenches. In Proceedings of the IEEE European Solid-State Device Research, Helsinki, Finland, 12–16 September 2011; p. 183. [Google Scholar] [CrossRef]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Kuban, V.; Dasgupta, P.K.; Marx, J.N. Nitroprusside and methylene blue methods for silicone membrane differentiated flow injection determination of sulfide in water and wastewater. Anal. Chem. 1992, 64, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Spanziani, M.A.; Davis, J.L.; Tinani, M.; Carroll, M.K. On-line Determination of Sulfide by the ‘Methylene Blue Method’ With Diode-laser-based Fluorescence Detection. Analyst 1997, 122, 1555–1557. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Davis, J.; Jiang, L.; Jones, T.G.J.; Davies, S.N.; Compton, R.G. The electrochemical analog of the methylene blue reaction: A novel amperometric approach to the detection of hydrogen sulfide. Electroanalysis 2000, 12, 1453–1460. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Davis, J.; Marken, F.; Jiang, L.; Jones, T.G.J.; Davies, S.N.; Compton, R.G. Electrochemical detection of sulphide: A novel dual flow cell. Sens. Actuators B Chem. 2000, 69, 189–192. [Google Scholar] [CrossRef]
- Tang, D.; Santschi, P.H. Sensitive determination of dissolved sulfide in estuarine water by solid-phase extraction and high-performance liquid chromatography of methylene blue. J. Chromatogr. A. 2000, 883, 305–309. [Google Scholar] [CrossRef]
- Liang, Z.; Tsoi, T.H.; Chan, C.F.; Dai, L.; Wu, Y.; Du, G.; Zhu, L.; Lee, C.S.; Wong, W.T.; Law, G.L.; et al. A smart “off–on” gate for the in situ detection of hydrogen sulphide with Cu(II)-assisted europium emission. Chem. Sci. 2016, 7, 2151–2156. [Google Scholar] [CrossRef]
- Italian Law D.L. 152/06. Available online: http://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2006-04-14&atto.codiceRedazionale=006G0171 (accessed on 10 January 2018).

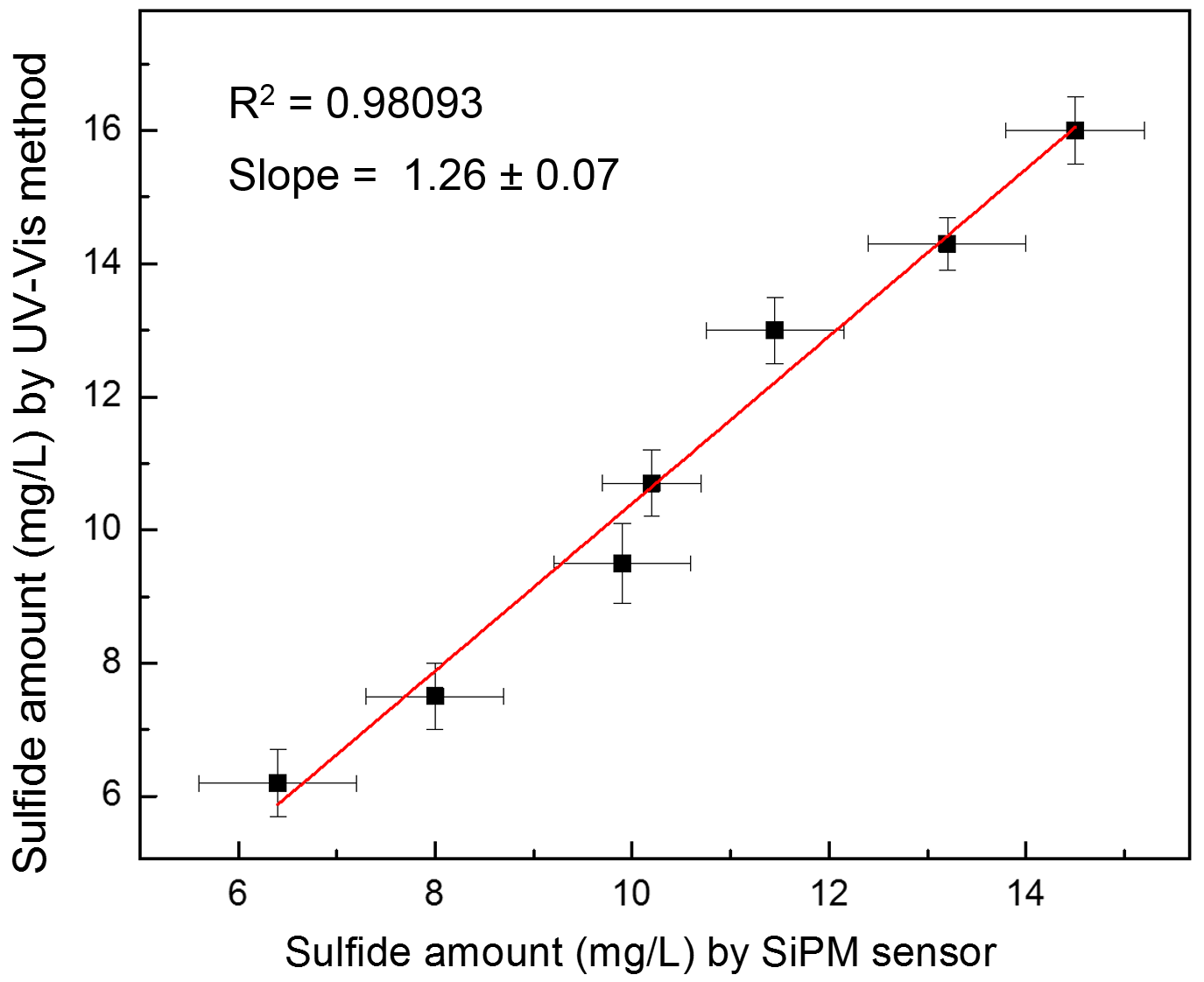
| Sample | SiPM Current Intensity (µA) | Sulfide mg/L by SiPM | Sulfide mg/L by UV-Vis |
|---|---|---|---|
| 1 | 52.5 ± 2.2 | 6.4 ± 0.8 | 6.2 ± 0.5 |
| 2 | 62.9 ± 1.9 | 8.0 ± 0.7 | 7.5 ± 0.5 |
| 3 | 77.5 ± 2.0 | 9.9 ± 0.7 | 9.5 ± 0.6 |
| 4 | 79.3 ± 2.3 | 10.2 ± 0.5 | 10.7 ± 0.5 |
| 5 * | 87.9 ± 2.1 | 11.4 ± 0.7 | 13.0 ± 0.5 |
| 6 * | 100.0 ± 2.5 | 13.2 ± 0.8 | 14.3 ± 0.4 |
| 7 * | 109.0 ± 2.4 | 14.5 ± 0.7 | 16.0 ± 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petralia, S.; Sciuto, E.L.; Santangelo, M.F.; Libertino, S.; Messina, M.A.; Conoci, S. Sulfide Species Optical Monitoring by a Miniaturized Silicon Photomultiplier. Sensors 2018, 18, 727. https://doi.org/10.3390/s18030727
Petralia S, Sciuto EL, Santangelo MF, Libertino S, Messina MA, Conoci S. Sulfide Species Optical Monitoring by a Miniaturized Silicon Photomultiplier. Sensors. 2018; 18(3):727. https://doi.org/10.3390/s18030727
Chicago/Turabian StylePetralia, Salvatore, Emanuele Luigi Sciuto, Maria Francesca Santangelo, Sebania Libertino, Maria Anna Messina, and Sabrina Conoci. 2018. "Sulfide Species Optical Monitoring by a Miniaturized Silicon Photomultiplier" Sensors 18, no. 3: 727. https://doi.org/10.3390/s18030727
APA StylePetralia, S., Sciuto, E. L., Santangelo, M. F., Libertino, S., Messina, M. A., & Conoci, S. (2018). Sulfide Species Optical Monitoring by a Miniaturized Silicon Photomultiplier. Sensors, 18(3), 727. https://doi.org/10.3390/s18030727






