Electroanalytical Determination of Cysteine Using the Electrodes Based on Ternary Silver-Copper Sulfides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Electrode Fabrication
2.3. Apparatus and Measurements
3. Results and Discussion
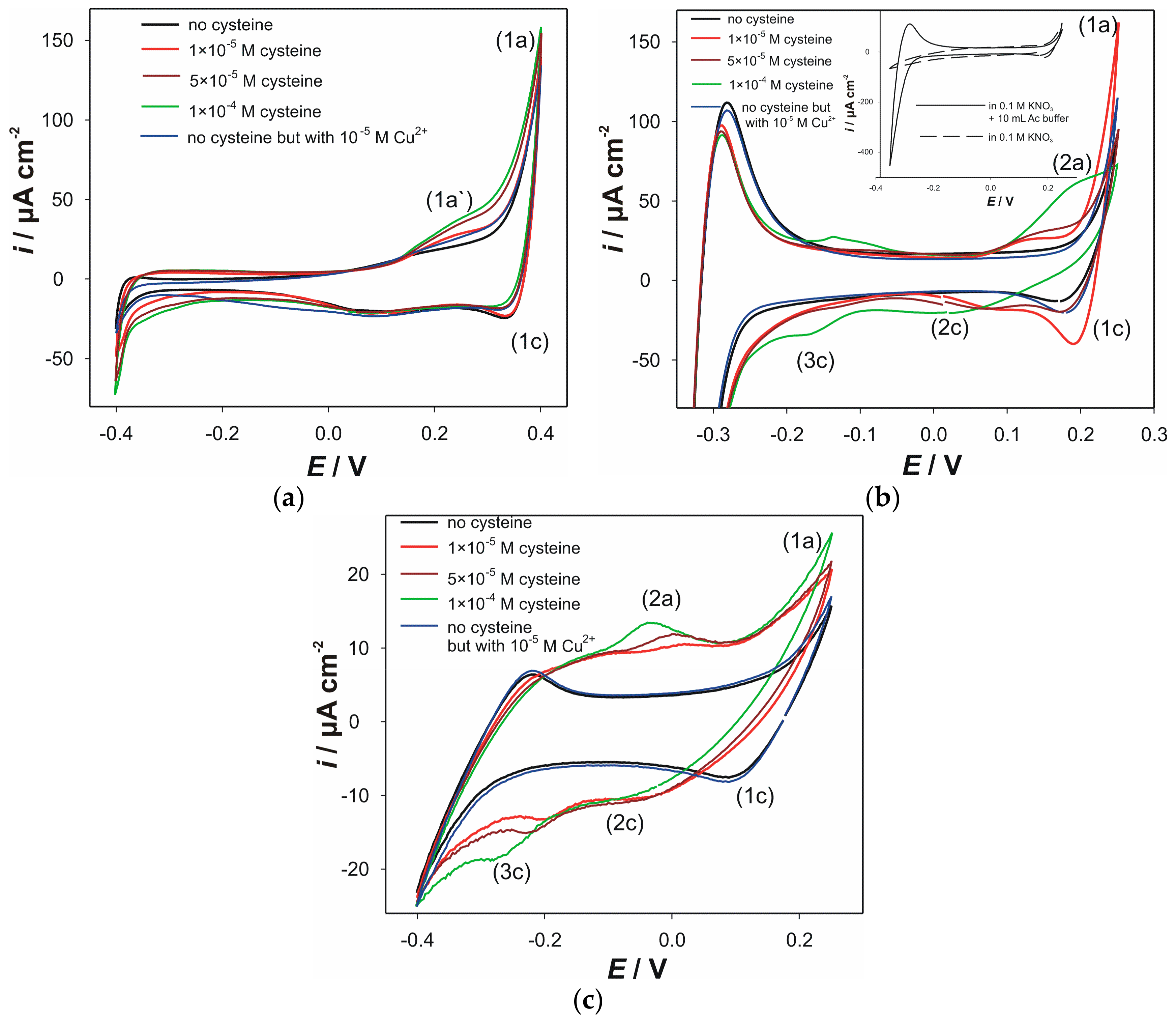
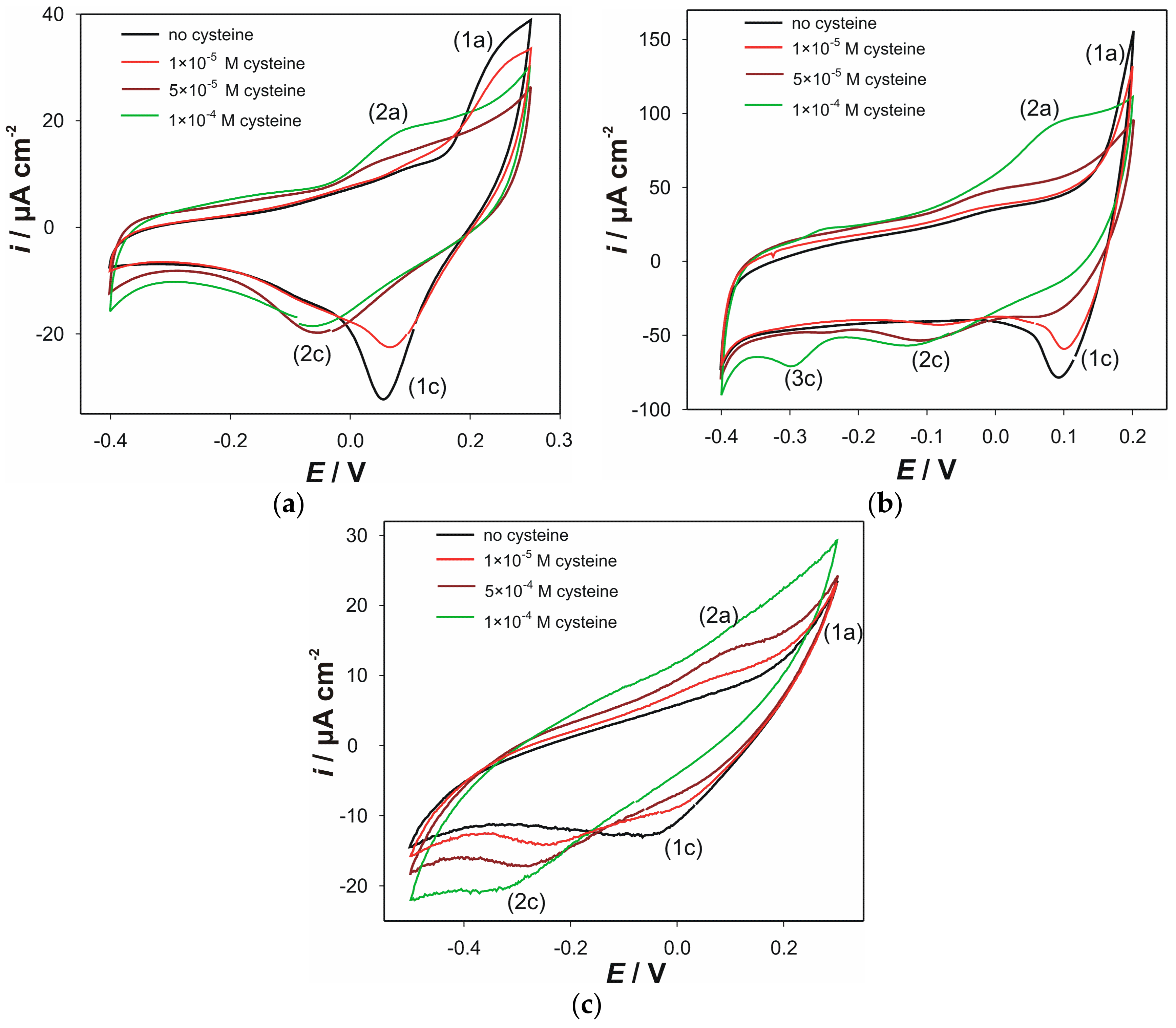
3.1. Voltammetric Characterization
3.2. Electrochemical Impedance Spectroscopy of the Electrodes
3.3. Infrared Spectra of the Electrodes
3.4. Influence of Scan Rate on CVs of Electrode “ACS”
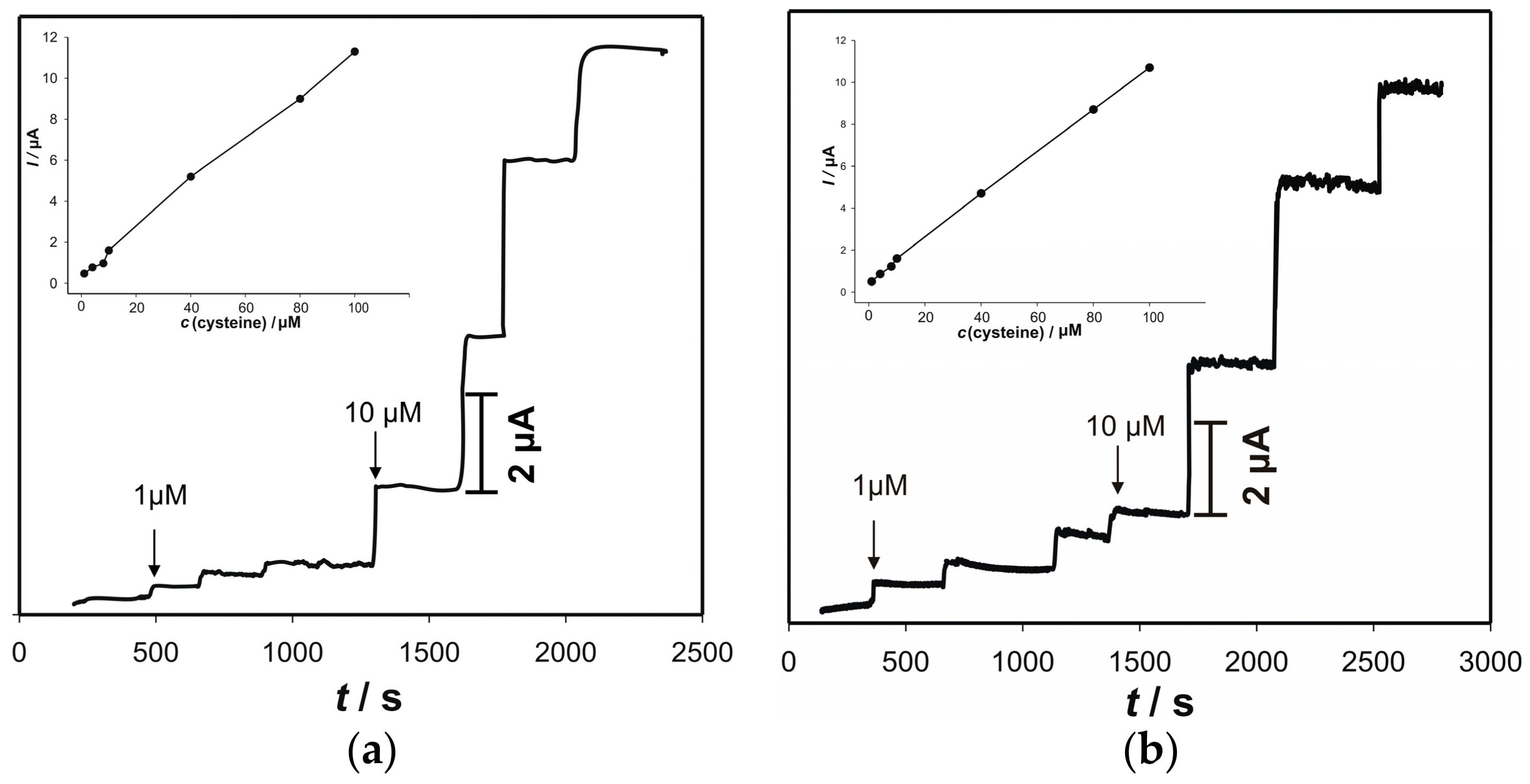
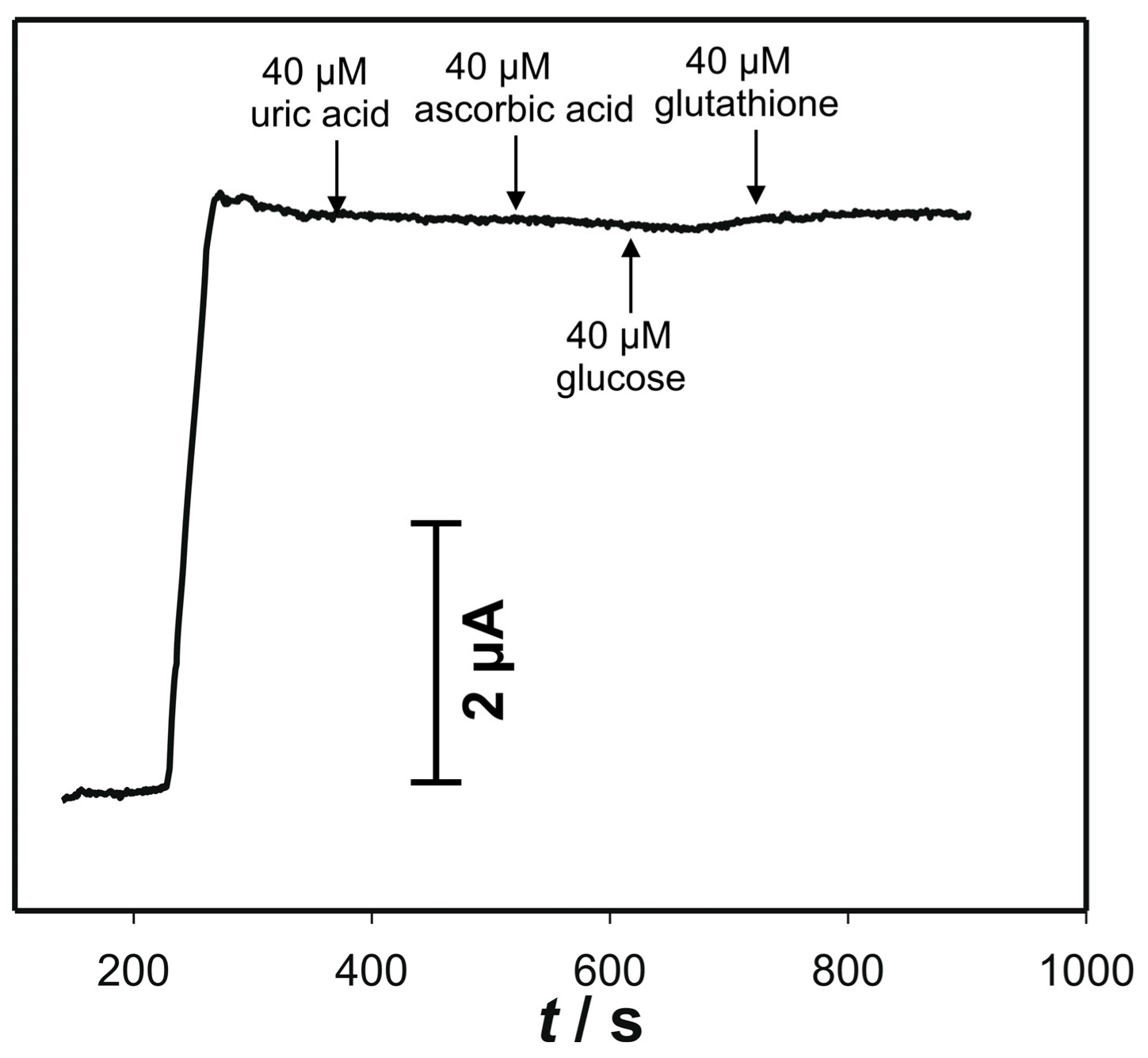
3.5. Amperometric Response to Cysteine on the Electrode “ACS”
3.6. Application of the Cysteine Determination of in a Dietary Supplement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simoes Goncalves, M.L.S.; Correia Dos Santos, M.M. Cadmium complexes of aminoacids in seawater conditions. J. Electroanal. Chem. 1984, 163, 315–326. [Google Scholar] [CrossRef]
- Fitzerald, J.W.; Hale, D.D.; Swank, W.T. Sulphur-containing amino acid metabolism in surface horizons of a hardwood forest. Soil Biol. Biochem. 1988, 20, 825–831. [Google Scholar] [CrossRef]
- Ivanov, K.; Stoimenova, A.; Obreshkova, D.; Saso, S. Biotechnology in the production of pharmaceutical industry ingredients: Amino acids. Biotechnol. Biotechnol. Equip. 2013, 27, 3620–3626. [Google Scholar] [CrossRef]
- Lau, C.; Qin, X.; Liang, J.; Lu, J. Determination of cysteine in a pharmaceutical formulation by flow injection analysis with a chemiluminescence detector. Anal. Chim. Acta 2004, 514, 45–49. [Google Scholar] [CrossRef]
- Nie, L.; Ma, H.; Sun, M.; Li, X.; Su, M.; Liang, S. Direct chemiluminescence determination of cysteine in human serum using quinine-Ce(IV) system. Talanta 2003, 59, 959–964. [Google Scholar] [CrossRef]
- Chwatko, G.; Bald, E. Determination of cysteine in human plasma by high-performance liquid chromatography and ultraviolet detection after pre-column derivatization with 2-chloro-1-methylpyridinium iodide. Talanta 2000, 52, 509–515. [Google Scholar] [CrossRef]
- Moreno, M.L.; Escobar, J.; Izquierdo-Álvarez, A.; Gil, A.; Pérez, S.; Pereda, J.; Zapico, I.; Vento, M.; Sabater, L.; Marina, A.; et al. Disulfide stress: A novel type of oxidative stress in acute pancreatitis. Free Radic. Biol. Med. 2014, 70, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Saetre, R.; Rabenstein, D.L. Determination of cysteine in plasma and urine and homocysteine in plasma by high-pressure liquid chromatography. Anal. Biochem. 1978, 90, 684–692. [Google Scholar] [CrossRef]
- Carvalho, F.D.; Remião, F.; Valet, P.; Timbrell, J.A.; Bastos, M.L.; Ferreira, M.A. Glutathione and cysteine measurement in biological samples by HPLC with a glassy carbon working detector. Biomed. Chromatogr. 2005, 8, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.E.; Lehotay, D.C.; Evrovski, J. Simplified Simultaneous Assay of Total Plasma Homocysteine and Methionine by HPLC and Pulsed Integrated Amperometry. Clin. Chem. 1998, 44, 188–190. [Google Scholar] [PubMed]
- Kusmierek, K.; Chwatko, G.; Glowacki, R.; Bald, E. Determination of endogenous thiols and thiol drugs in urine by HPLC with ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3300–3308. [Google Scholar] [CrossRef] [PubMed]
- Bald, E.; Glowacki, R. Analysis of saliva for glutathione and metabolically related thiols by liquid chromatography with ultraviolet detection. Amino Acids 2005, 28, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Leesutthiphonchai, W.; Dungchai, W.; Siangproh, W.; Ngamrojnavanich, N.; Chailapakul, O. Selective determination of homocysteine levels in human plasma using a silver nanoparticle-based colorimetric assay. Talanta 2011, 85, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Marcell, P.D.; Rodell, E.R.; Allen, R.H.; Savage, D.G.; Lindenbaum, J. Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J. Clin. Investig. 1988, 81, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Hager, G.; Brolo, A.G. Adsorption/desorption behaviour of cysteine and cystine in neutral and basic media: Electrochemical evidence for differing thiol and disulfide adsorption to a Au(111) single crystal electrode. J. Electroanal. Chem. 2003, 550–551, 291–301. [Google Scholar] [CrossRef]
- Zhou, M.; Ding, J.; Guo, L.P.; Shang, Q.K. Electrochemical Behavior of L-Cysteine and Its Detection at Ordered Mesoporous Carbon-Modified Glassy Carbon Electrode Anal. Chem. 2007, 79, 5328–5335. [Google Scholar]
- Lee, P.T.; Thomson, J.E.; Karina, A.; Salter, C.; Johnston, C.; Davies, S.G.; Compton, R.G. Selective electrochemical determination of cysteine with a cyclotricatechylene modified carbon electrode. Analyst 2015, 140, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Brinic, S.; Vladislavic, N.; Buzuk, M.; Bralic, M.; Solic, M. Bismuth film random array carbon fiber microelectrodes for determination of cysteine and N-acetyl cysteine. J. Electroanal. Chem. 2013, 705, 86–90. [Google Scholar] [CrossRef]
- Lima, P.R.; Santos, W.J.R.; Luz, R.C.S.; Damos, F.S.; Oliveira, A.B.; Goulart, M.O.F.; Kubota, L.T. An amperometric sensor based on electrochemically triggered reaction: Redox-active Ar-NO/Ar-NHOH from 4-nitrophthalonitrile-modified electrode for the low voltage cysteine detection. J. Electroanal. Chem. 2008, 612, 87–96. [Google Scholar] [CrossRef]
- Santhiago, M.; Lima, P.R.; Santos, W.J.R.; Kubota, L.T. An amperometric sensor for L-cysteine based on nanostructured platform modified with 5,5′-dithiobis-2-nitrobenzoic acid (DTNB). Sens. Actuators B Chem. 2010, 146, 213–220. [Google Scholar] [CrossRef]
- Liu, L.P.; Yin, Z.J.; Yang, Z.S. A L-cysteine sensor based on Pt nanoparticles/poly(o-aminophenol) film on glassy carbon electrode. Bioelectrochemistry 2010, 79, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hallaj, R.; Salimi, A.; Akhtari, K.; Soltanian, S.; Mamkhezri, H. Electrodeposition of guanine oxidation product onto zinc oxide nanoparticles: Application to nanomolar detection of L-cysteine. Sens. Actuators B Chem. 2009, 135, 632–641. [Google Scholar] [CrossRef]
- Rajbhandari, A.; Yadav, A.P.; Manandhar, K.; Pradhananga, R.R. Characterization and Applications of Silver Sulphide Based Membrane Electrodes. Sci. World 2009, 7, 19–23. [Google Scholar]
- Prkic, A.; Giljanovic, J.; Bralic, M. Direct Potentiometric Determination of N-acetyl-L-cysteine (NAC) in Real Samples by Using “home made” Iodide ISE. Int. J. Electrochem. Sci. 2011, 6, 5388–5395. [Google Scholar]
- Kolar, M.; Dobcnik, D.; Radic, N. Chemically Treated Silver Electrodes for the Determination of Cysteine. Microchim. Acta 2002, 138, 23–27. [Google Scholar] [CrossRef]
- Bralić, M.; Radić, N.J. Direct Potentiometric Measurements of Cysteine by Using Iodide-Selective Electrode. Croat. Chem. Acta 1994, 67, 543–551. [Google Scholar]
- Buzuk, M.; Brinic, S.; Vladislavic, N.; Bralic, M.; Buljac, M.; Škugor Rončević, I. Real-time monitoring of “self-oxidation” of cysteine in presence of Cu2+: Novel findings in the oxidation mechanism. Monatsh. Chem. 2016, 147, 359–367. [Google Scholar] [CrossRef]
- Rigo, A.; Corazza, A.; di Paolo, M.L.; Rossetto, M.; Ugolini, R.; Scarpa, M. Interaction of copper with cysteine: Stability of cuprous complexes and catalytic role of cupric ions in anaerobic thiol oxidation. J. Inorg. Biochem. 2004, 98, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Pecci, L.; Montefoschi, G.; Musci, G.; Cavallini, D. Novel findings on the copper catalysed oxidation of cysteine. Amino Acids 1997, 13, 355–367. [Google Scholar] [CrossRef]
- Bagiyan, G.A.; Koroleva, I.K.; Soroka, N.V.; Ufimtsev, A.V. Oxidation of thiol compounds by molecular oxygen in aqueous solutions. Russ. Chem. Bull. 2003, 52, 1135–1141. [Google Scholar] [CrossRef]
- Brinic, S.; Buzuk, M.; Bralic, M.; Buljac, M.; Jozić, D. Cu (II) Ion-Selective Electrode Based on Mixed Silver-Copper Sulfide: Phase Structure and Electrochemical Properties. Int. J. Electrochem. Sci. 2012, 7, 5217–5230. [Google Scholar]
- Zhu, W.; Wang, J.; Zhang, W.; Hu, N.; Wang, J.; Huang, L.; Wang, R.; Suo, Y.; Wang, J. Monolithic copper selenide submicron particulate film/copper foam anode catalyst for ultrasensitive electrochemical glucose sensing in human blood serum. J. Mater. Chem. B 2018, 6, 718–724. [Google Scholar] [CrossRef]
- Jing, B.; Xiue, J. A Facile One-Pot Synthesis of Copper Sulfide-Decorated Reduced Graphene Oxide Composites for Enhanced Detecting of H2O2 in Biological Environments. Anal. Chem. 2013, 85, 8095–8101. [Google Scholar]
- Mallappa, M.; Shivaraj, Y.; Nagaraju, K.; Vusa, C.S.R. Sensitive determination of caffeine by copper sulphide nanoparticles modified carbon paste electrode. Sens. Actuators A Phys. 2016, 248, 104–113. [Google Scholar]
- Zhang, B.; Zhang, Y.; Liang, W.; Yu, X.; Tan, H.; Wang, G.; Li, A.; Jina, J.; Huang, L. Copper sulfide-functionalized molybdenum disulfide nanohybrids as nanoenzyme mimics for electrochemical immunoassay of myoglobin in cardiovascular disease. RSC Adv. 2017, 7, 2486–2493. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Lin, Y.; He, Y.; Tang, D. Enzyme-free amperometric glucose sensor using a glassy carbon electrode modified with poly(vinyl butyral) incorporating a hybrid nanostructure composed of molybdenum disulfide and copper sulphide. Microchim. Acta 2017, 184, 807–814. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Kim, H.Y.; Kim, B.S. A novel CuS microflower superstructure based sensitive and selective nonenzymatic glucose detection. Sens. Actuators B Chem. 2016, 233, 93–99. [Google Scholar] [CrossRef]
- Giribabu, K.; Yeong Oh, S.; Suresh, R.; Praveen Kumar, S.; Manigandan, R.; Munusamy, S.; Gnanamoorthy, G.; Kim, J.Y.; Huh, Y.S.; Narayanan, V. Sensing of picric acid with a glassy carbon electrode modified with CuS nanoparticles deposited on nitrogen-doped reduced graphene oxide. Microchim. Acta 2016, 183, 2421–2430. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Sheng, Q.; Zheng, J. Electrochemical sensor for sensitive determination of nitrite based on the CuS-MWCNT nanocomposites. J. Electroanal. Chem. 2016, 769, 118–123. [Google Scholar] [CrossRef]
- Lu, W.; Sun, Y.; Dai, H.; Ni, P.; Jiang, S.; Wang, Y.; Liab, Z.; Li, Z. Fabrication of cuprous sulfide nanorods supported on copper foam for nonenzymatic amperometric determination of glucose and hydrogen peroxide. RSC Adv. 2016, 6, 90732–90738. [Google Scholar] [CrossRef]
- Li, C.Y.; Cai, Y.J.; Yang, C.H.; Wu, C.H.; Wei, Y.; Wen, T.C.; Wang, T.L.; Shieh, Y.T.; Lin, W.C.; Chen, W.J. Highly sensitive and selective electrochemical determination of dopamine and ascorbic acid at Ag/Ag2S modified electrode. Electrochim. Acta 2011, 56, 1955–1959. [Google Scholar] [CrossRef]
- Chih, Y.K.; Yang, M.C. Simultaneous detection of dopamine and ascorbic acid using silver/silver sulfide modified carbon nanotube electrodes. J. Taiwan Inst. Chem. Eng. 2014, 45, 833–839. [Google Scholar] [CrossRef]
- Sheng, Q.; Tang, H.; Wang, Y.; Zheng, Y. Direct Electrochemistry of Hemoglobin Based on Silver Sulfide Nanospheres Anchored Multiwalled Carbon Nanotubes. J. Electrochem. Soc. 2016, 163, H128–H132. [Google Scholar] [CrossRef]
- Pei, L.Z.; Yang, L.J.; Wang, J.F.; Fan, C.G.; Hu, J.L. Synthesis and Electrochemical Properties of Ag2S and Ag2S/Cu2S Crystals. e-J. Surf. Sci. Nanotech. 2010, 8, 384–387. [Google Scholar] [CrossRef]
- Pei, L.Z.; Wang, J.F.; Tao, X.X.; Wang, S.B.; Dong, Y.P.; Fan, C.G.; Zhang, Q.F. Synthesis of CuS and Cu1.1Fe1.1S2 crystals and their electrochemical properties. Mater. Charact. 2011, 62, 354–359. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Xiao, S.; Liu, J. Comparative study of interaction between pyrite and cysteine by thermogravimetric and electrochemical techniques. Hydrometallurgy 2010, 101, 88–92. [Google Scholar] [CrossRef]
- Gulens, J. Surface Effects in Relation to the Response of Solid-State Ion-Selective Electrodes. Ion-Sel. Electrode Rev. 1981, 2, 117–157. [Google Scholar]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; John Wiley & Sons Inc.: New York, NY, USA, 1987; p. 301. ISBN -10: 0471831220. [Google Scholar]
- Lukasc, Z. Evaluation of model and dispersion parameters and their effects on the formation of constant-phase elements in equivalent circuits. J. Electroanal. Chem. 1999, 464, 68–75. [Google Scholar]
- Macdonald, J.R. Power-law exponents and hidden bulk relation in the impedance spectroscopy of solids. J. Electroanal. Chem. 1994, 378, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Pawlukojć, A.; Leciejewicz, J.; Ramirez-Cuesta, A.J.; Nowicka-Scheibe, J. L-Cysteine: Neutron spectroscopy, Raman, IR and abinitio study. Spectrochim. Acta Part A 2005, 61, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Ankireddy, S.R.; Kim, J. Selective detection of dopamine in the presence of ascorbic acid via fluorescence quenching of InP/ZnS quantum dots. Int. J. Nanomed. 2015, 10, 113–119. [Google Scholar]
- Filanovsky, B. Electrochemical response of new carbon electrodes bulk modified with cobalt phthalocyanine to some thiols in the presence of heptane or human urine. Anal. Chim. Acta 1999, 394, 91–100. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Behyan, S. Sensing of L-cysteine at glassy carbon electrode using Nile blue A as a mediator. Sens. Actuators B Chem. 2007, 122, 282–288. [Google Scholar] [CrossRef]
- Salimi, A.; Hallaj, R. Catalytic oxidation of thiols at preheated glassy carbon electrode modified with abrasive immobilization of multiwall carbon nanotubes: Applications to amperometric detection of thiocytosine, L-cysteine and glutathione. Talanta 2005, 66, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Chen, J.; Chen, X.; Wang, M.; Nie, Z.; Yao, S. Electrochemical detection of L-cysteine using a boron-doped carbon nanotube-modified electrode. Electrochim. Acta 2009, 54, 3298–3302. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ding, M. Electrocatalytic oxidation and sensitive detection of cysteine at layer-by-layer assembled carbon nanotube-modified electrode. Anal. Chim. Acta 2006, 557, 52–56. [Google Scholar] [CrossRef]
- Cao, F.; Huang, Y.; Wang, F.; Kwak, D.; Dong, Q.; Song, D.; Zeng, J.; Lei, Y. A high-performance electrochemical sensor for biologically meaningful L-cysteine based on a new nanostructured L-cysteine electrocatalyst. Anal. Chim. Acta 2018, 1019, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Dong, Q.; Li, C.; Kwak, D.; Huang, Y.; Song, D.; Lei, Y. Sensitive and Selective Electrochemical Determination of L-Cysteine Based on Cerium Oxide Nanofibers Modified Screen Printed Carbon Electrode. Electroanalysis 2018, 30, 1133–1139. [Google Scholar] [CrossRef]
- Yusoff, N.; Rameshkumar, P.; Noor, A.M.; Huang, N.M. Amperometric determination of L-cysteine using a glassy carbon electrode modified with palladium nanoparticles grown on reduced graphene oxide in a Nafion matrix. Microchim. Acta 2018, 185, 246. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wen, Y.; Lu, L.; Xu, J.; Zhang, L.; Yao, Y.; He, H. A Novel L-Cysteine Electrochemical Sensor Using Sulfonated Graphene-poly(3,4-Ethylenedioxythiophene) Composite Film Decorated with Gold Nanoparticles. Electroanalysis 2014, 26, 648–655. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Liu, L.; Wang, G.; Wang, D.; Qu, L. Preparation of nickel oxide nanoparticles on N-doped reduced graphene oxide: A two-dimensional hybrid for electrocatalytic sensing of L-cysteine. J. Alloy Compd. 2017, 691, 834–840. [Google Scholar] [CrossRef]
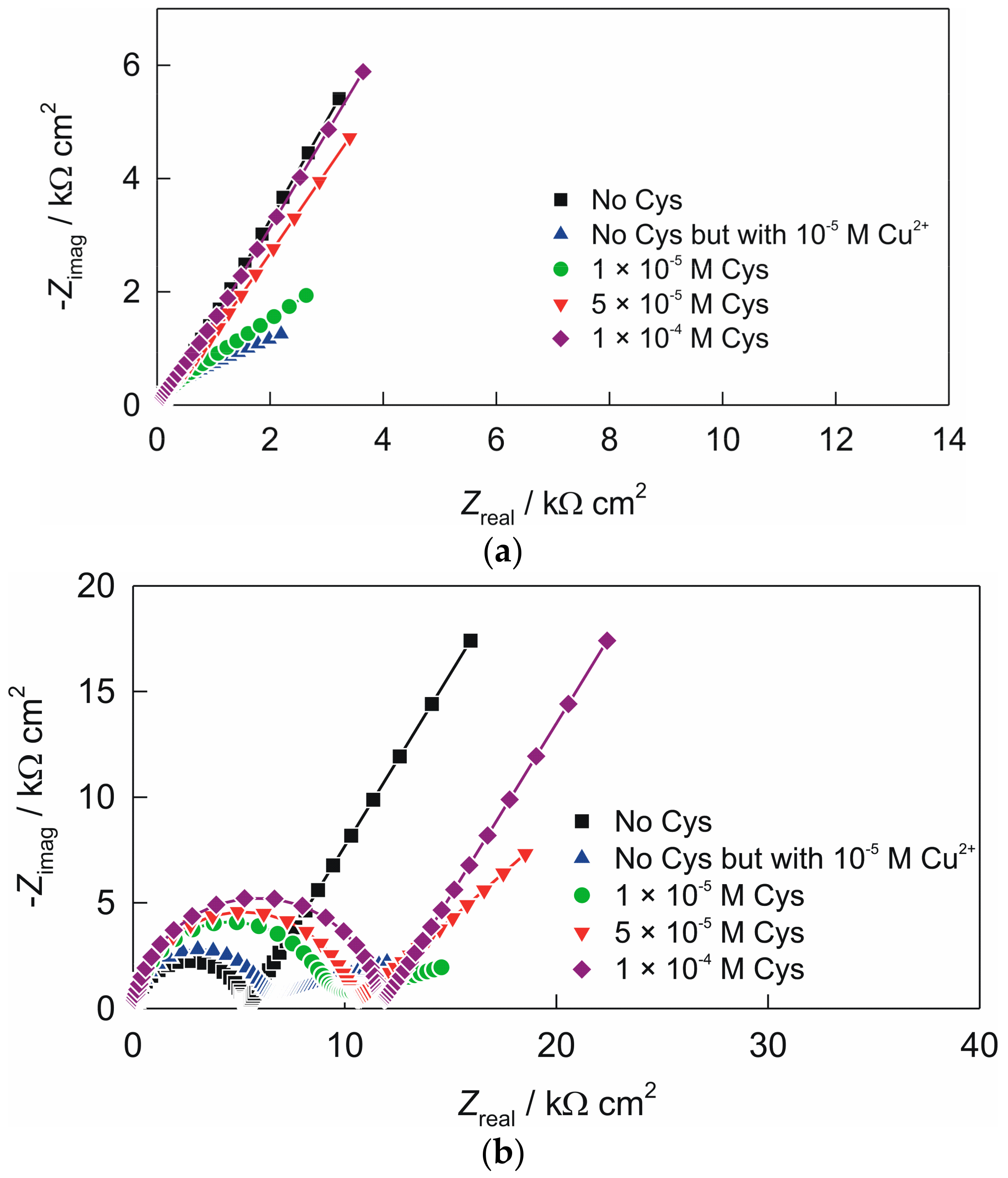
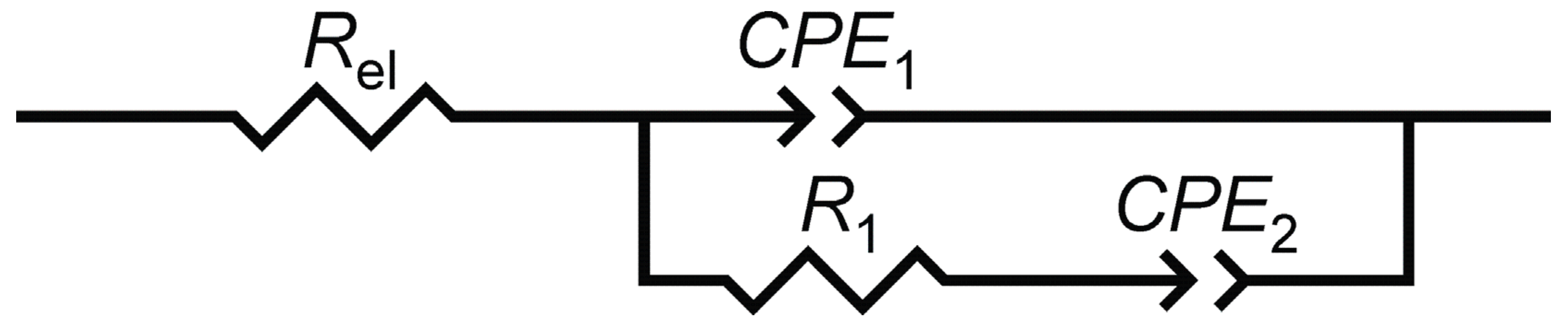
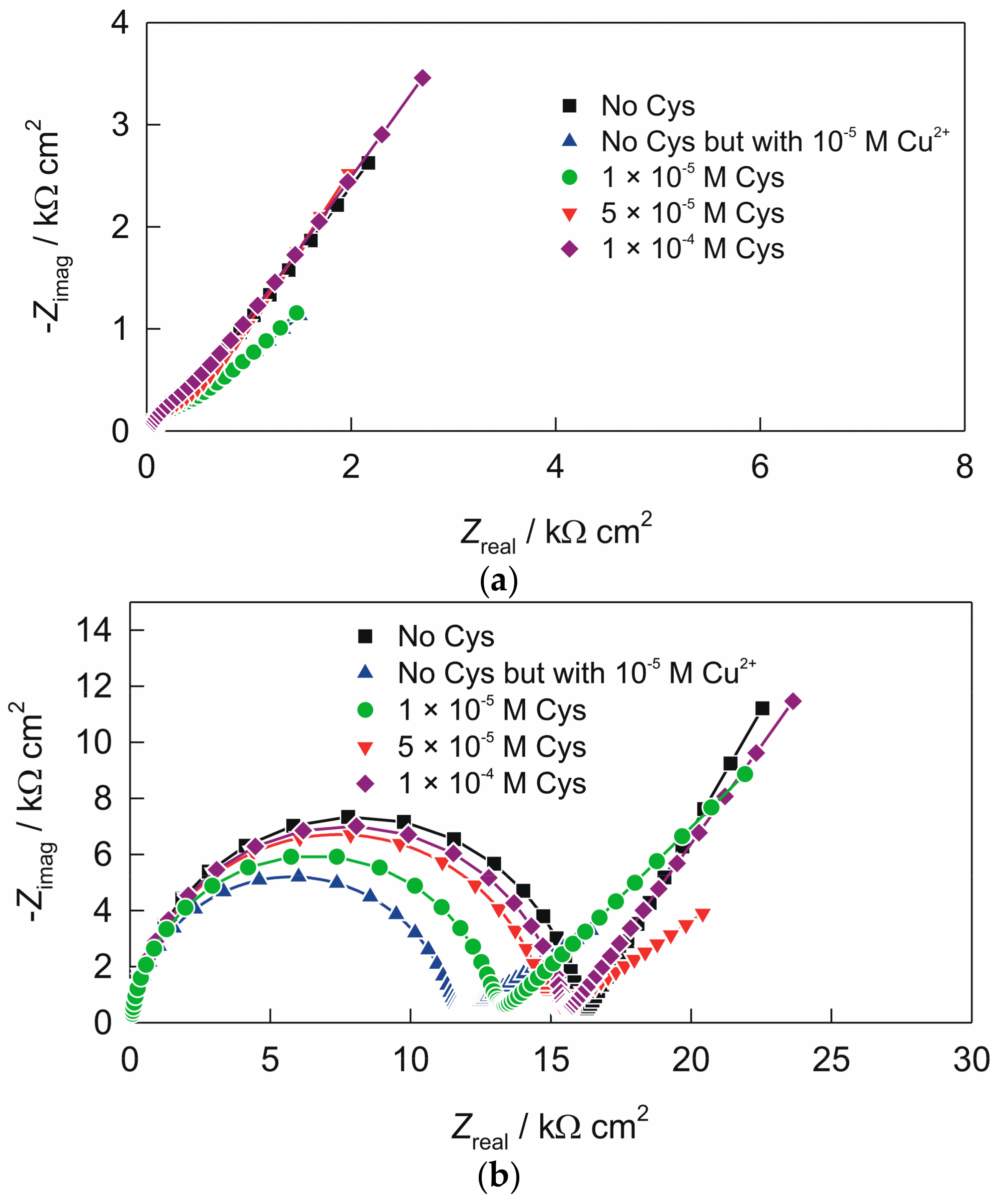


| electrode “ACS” | 105 × Q1/Ω−1 cm−2 sn | n1 | R1/Ω cm2 | 105 × Q2/Ω−1 cm−2 sn | n2 | |
| no cysteine | 12.72 | 0.853 | 713 | 25.02 | 0.629 | |
| no cysteine but with 1 × 10−5 M Cu2+ | 11.12 | 0.843 | 66 | 53.85 | 0.293 | |
| c (cys) = 1 × 10−5 M | 7.99 | 0.879 | 126 | 46.25 | 0.379 | |
| c (cys) = 5 × 10−5 M | 9.87 | 0.849 | 648 | 29.18 | 0.590 | |
| c (cys) = 1 × 10−4 M | 12.68 | 0.841 | 979 | 21.66 | 0.613 | |
| electrode “AC1.8S” | 107 × Q1/Ω−1 cm−2 sn | n1 | R1/kΩ cm2 | 105 × Q2/Ω−1 cm−2 sn | n2 | |
| no cysteine | 0.20 | 0.896 | 5.3 | 10.42 | 0.651 | |
| no cysteine but with 1 × 10−5 M Cu2+ | 0.10 | 0.953 | 5.3 | 18.06 | 0.202 | |
| c (cys) = 1 × 10−4 M | 0.11 | 0.950 | 7.9 | 17.92 | 0.182 | |
| c (cys) = 5 × 10−5 M | 0.14 | 0.928 | 10.2 | 15.40 | 0.460 | |
| c (cys) = 1 × 10−4 M | 0.13 | 0.930 | 11.7 | 10.40 | 0.650 |
| electrode “ACS” | 105 × Q1/Ω−1 cm−2 sn | n1 | R1/Ω cm2 | 105 × Q2/Ω−1 cm−2 sn | n2 | |
| no cysteine | 15.85 | 0.824 | 619 | 52.43 | 0.580 | |
| no cysteine but with 1 × 10−5 M Cu2+ | 12.94 | 0.846 | 317 | 104.72 | 0.450 | |
| c (cys) = 1 × 10−5 M | 11.36 | 0.857 | 353 | 97.87 | 0.484 | |
| c (cys) = 5 × 10−5 M | 10.84 | 0.857 | 515 | 62.95 | 0.623 | |
| c (cys) = 1 × 10−4 M | 15.85 | 0.824 | 619 | 52.43 | 0.580 | |
| electrode “AC1.8S” | 107 × Q1/Ω−1 cm−2sn | n1 | R1/kΩ cm2 | 105 × Q2/Ω−1cm−2sn | n2 | |
| no cysteine | 0.12 | 0.945 | 16.0 | 16.69 | 0.666 | |
| no cysteine but with 1 × 10−5 M Cu2+ | 0.12 | 0.942 | 11.3 | 25.22 | 0.366 | |
| c (cys) = 1 × 10−4 M | 0.12 | 0.942 | 12.9 | 22.26 | 0.378 | |
| c (cys) = 5 × 10−5 M | 0.12 | 0.940 | 14.6 | 14.30 | 0.605 | |
| c (cys) = 1 × 10−4 M | 0.11 | 0.948 | 15.4 | 14.08 | 0.496 |
| Electrode | E (V) vs. Ag/AgCl | LOD (μM−1) | Linear Range (μM−1) | Sensitivity | Ref. |
|---|---|---|---|---|---|
| Carbon paste electrode modified with 4-nitrophthalonitrile | 0.33 | 0.25 | 0.8–13.2 | 37 nA μM−1 | [3] |
| Bulk carbon electrodes modified with cobalt phthalocyanine | 0.40 | 0.2 | 1–12 | 8.89 nA μM−1 | [53] |
| Glassy carbon modified with Nile blue | −0.45 | 1.3 | 10–250 | not reported | [54] |
| Glassy carbon electrode modified with MWCNTs | 0.18 | 5.4 | 10–500 | 3 nA μM−1 | [55] |
| Oxidation product of guanine at ZnOx nanoparticles modified GCE | 0.50 | 0.05 | 0.3–20 | 28.5 nA μM−1 | [22] |
| Boron-doped carbon nanotube modified GCE | 0.47 vs. SCE | 0.26 | 0.78–200 | 0.025 nA μM−1 | [56] |
| Pt nanoparticles/poly(o-aminophenol) film on GCE | 0.41 | 0.08 | 0.4–6300 | not reported | [21] |
| Positively charged poly(diallyldimethylammonium chloride) and negatively charged MWNTs on glassy carbon | 0.80 | 0.3 | 20–1300 | not reported | [57] |
| Au/CeO2 composite nanofibers on screen printed electrodes | 0.7 | 0.01 | to 200 | 321 μA mM–1 cm–2 | [58] |
| Cerium oxide nanofibers modified screen printed carbon electrode | 0.7 | 0.02 | to 200 | 120 μA mM–1 cm–2 | [59] |
| Palladium nanoparticles grown on reduced graphene oxide in a Nafion matrix | 0.6 vs. SCE | 0.5–10 | 1.3 μA mM–1 cm–2 | [60] | |
| AuNPs/SG-PEDOT/GCE | 0.32 | 0.02 | 0.1–382 | 25.85 μA mM–1 cm–2 | [61] |
| NiO NPs/N-rGO/CPE | 0.65 | 0.1 | 0.3–1620 | 0.051 μA mM–1 | [62] |
| mixed silver-copper sulfides | 0.04 | 0.024 | 1–100 | 0.1 μA mM–1 | our work |
| Sample No. | Original Content/μM | Added/μM | Found/μM | Recovery/(%) |
|---|---|---|---|---|
| 1 | 1 | - | 0.92 | |
| 2 | 10 | 10 | 20.15 | 100.8 |
| 3 | 10 | 20 | 30.09 | 100.3 |
| 4 | 10 | 30 | 39.56 | 98.9 |
| 5 | 10 | 40 | 49.85 | 99.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladislavić, N.; Rončević, I.Š.; Buljac, M.; Brinić, S.; Krivić, D.; Buzuk, M. Electroanalytical Determination of Cysteine Using the Electrodes Based on Ternary Silver-Copper Sulfides. Sensors 2018, 18, 3753. https://doi.org/10.3390/s18113753
Vladislavić N, Rončević IŠ, Buljac M, Brinić S, Krivić D, Buzuk M. Electroanalytical Determination of Cysteine Using the Electrodes Based on Ternary Silver-Copper Sulfides. Sensors. 2018; 18(11):3753. https://doi.org/10.3390/s18113753
Chicago/Turabian StyleVladislavić, Nives, Ivana Škugor Rončević, Maša Buljac, Slobodan Brinić, Denis Krivić, and Marijo Buzuk. 2018. "Electroanalytical Determination of Cysteine Using the Electrodes Based on Ternary Silver-Copper Sulfides" Sensors 18, no. 11: 3753. https://doi.org/10.3390/s18113753





