Polyacrylamide Ferrogels with Magnetite or Strontium Hexaferrite: Next Step in the Development of Soft Biomimetic Matter for Biosensor Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Ferrogels
2.3. Methods
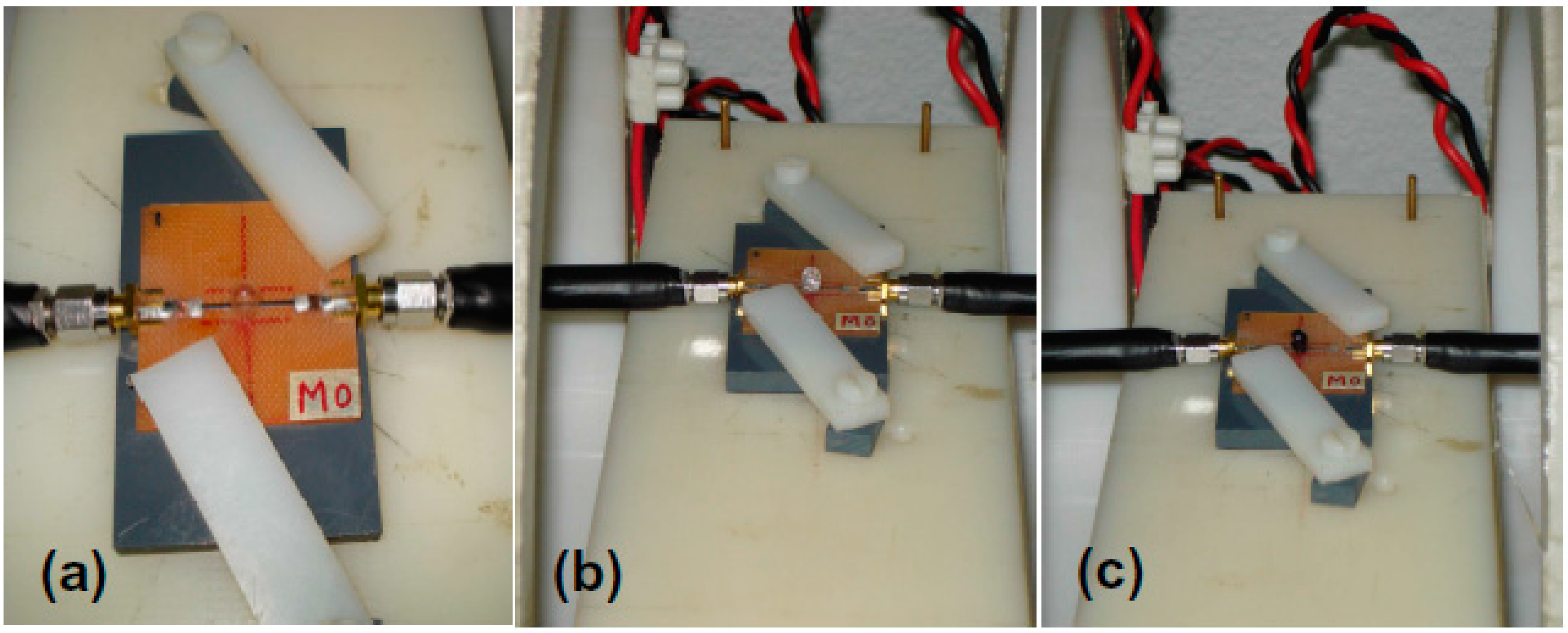
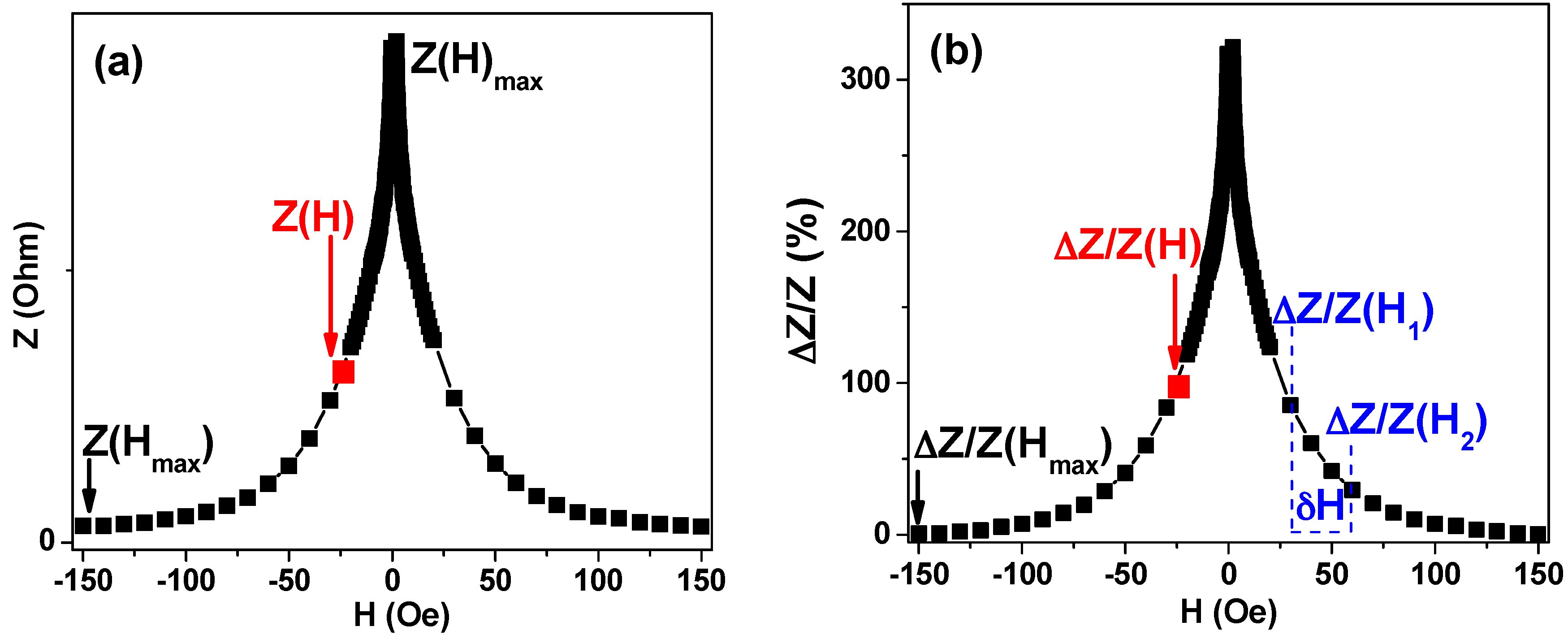
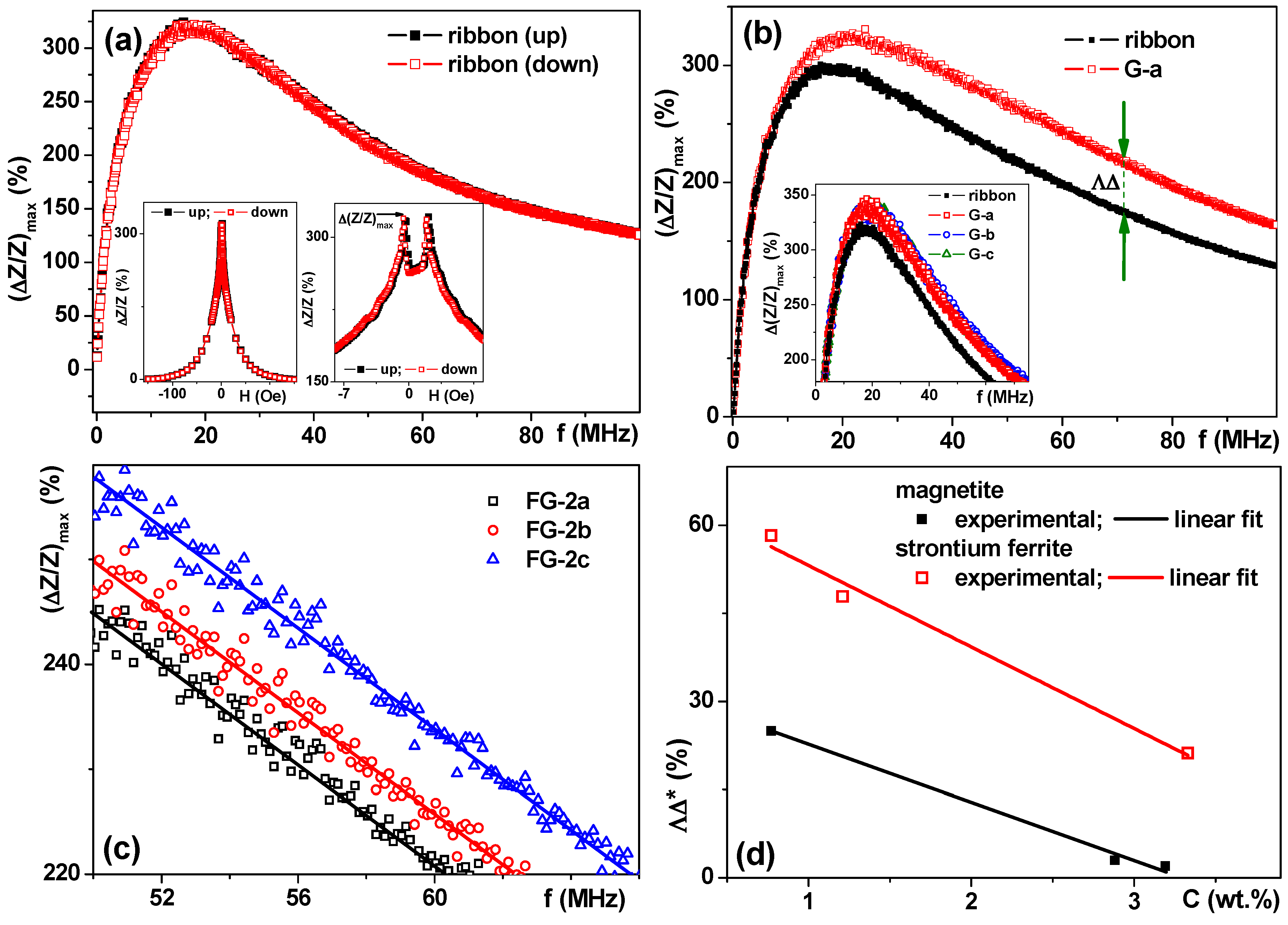
2.4. Magnetoimpedance Measurements
3. Results and Discussion
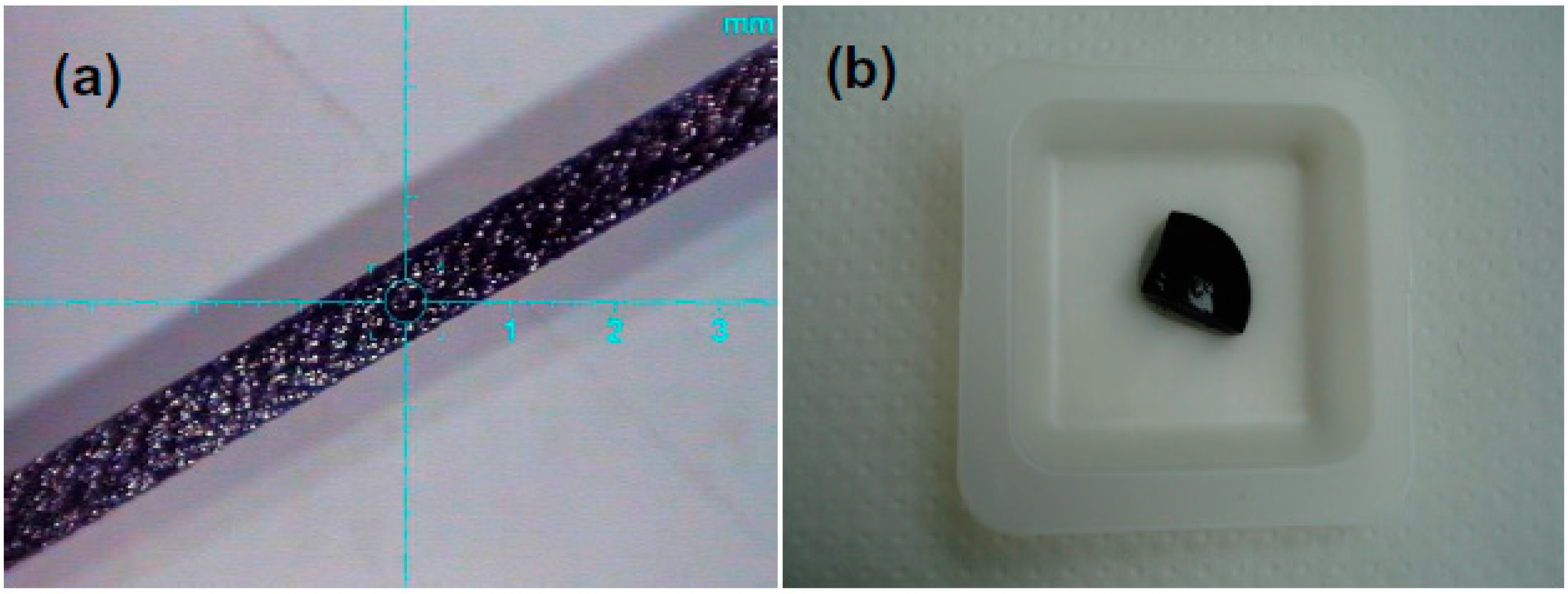
3.1. Characterization of the Ribbons
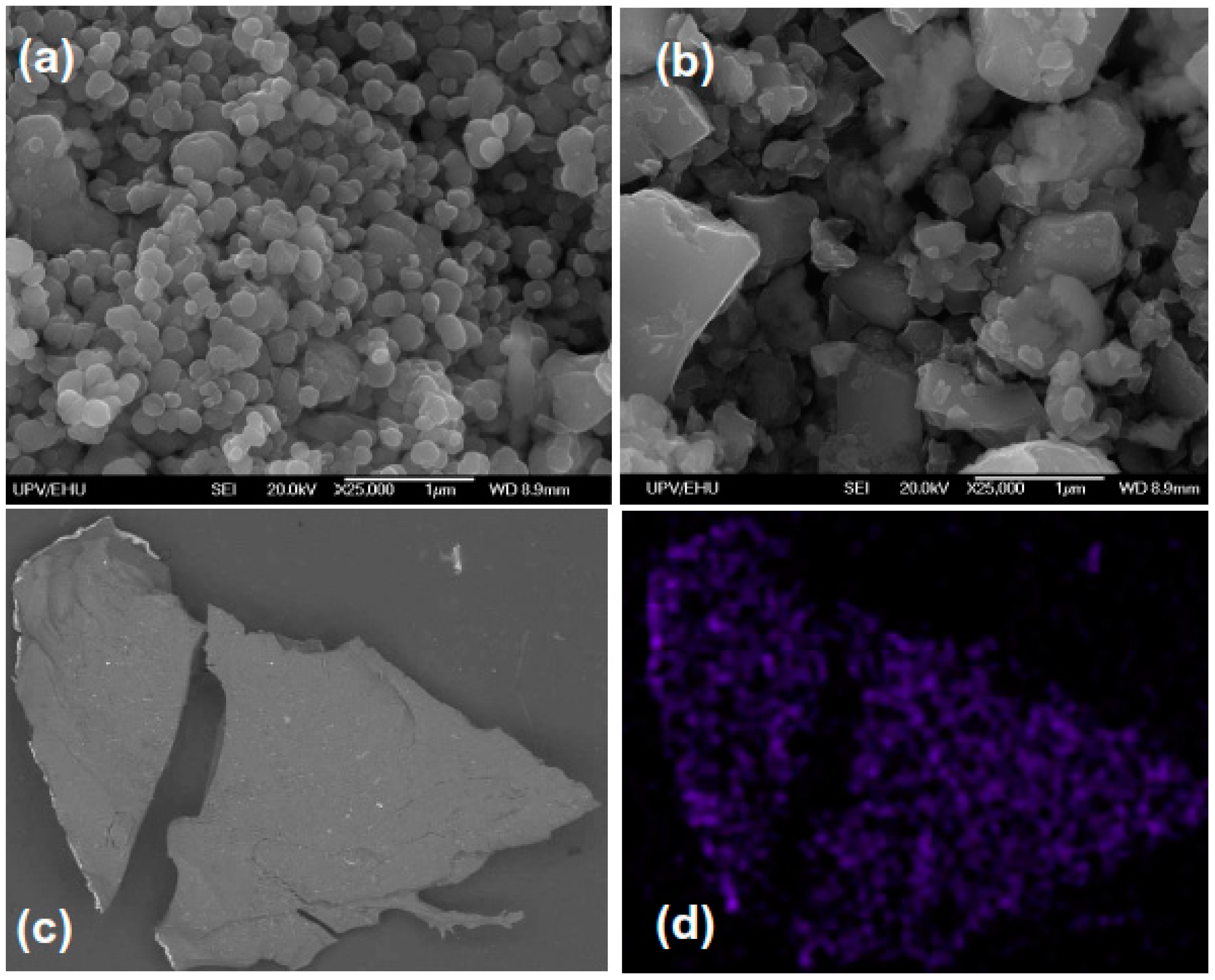
3.2. Characterization of the Magnetite and Hexaferrite Particles
3.3. Characterization of the Ferrogels
3.3.1. Ferrogels Structure
3.3.2. Young Modulus of Ferrogels
3.3.3. Deformation of Ferrogels in a Uniform Magnetic Field
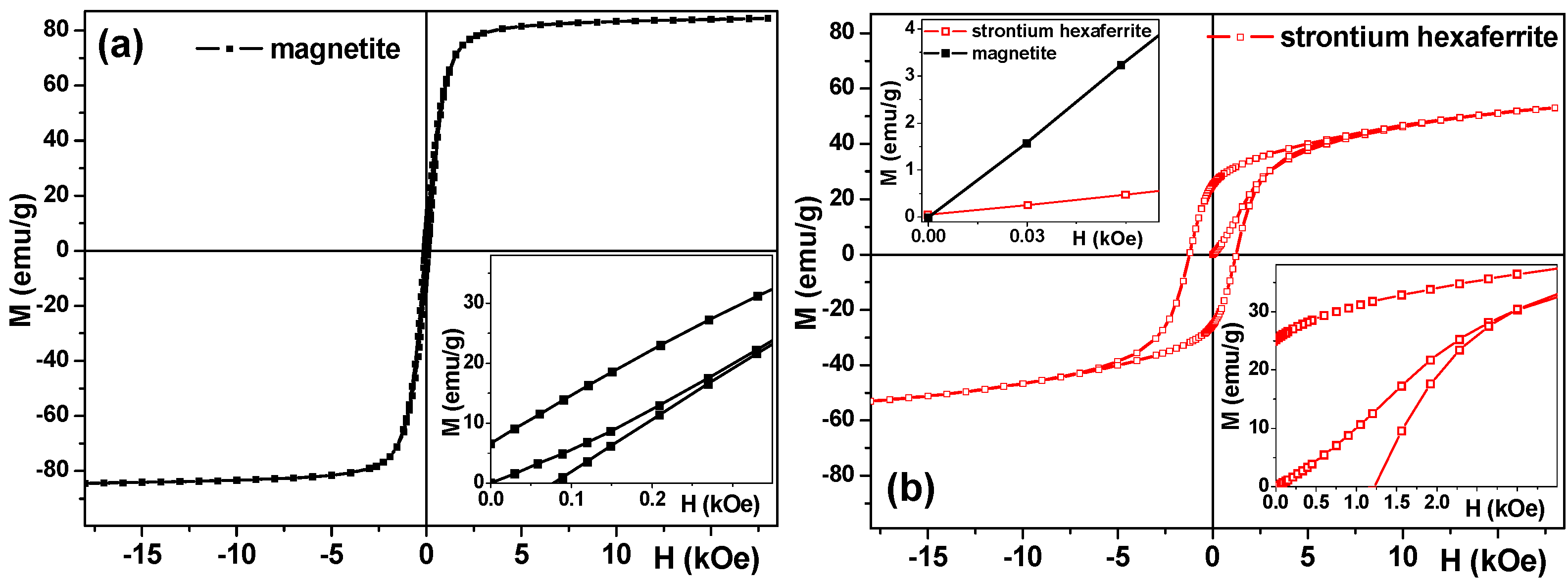
3.4. Magnetic Properties of Ferrogels
3.5. Magnetoimpedance Measurements in Configuration of Biosensor Prototype
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bucak, S.; Yavuzturk, B.; Sezer, A.D. Magnetic nanoparticles: Synthesis, surface modification and application. In Recent Advances in Novel Drug Carrier Systems; Sezer, A.D., Ed.; InTech: Rijeka, Croatia, 2012; pp. 165–200. [Google Scholar]
- Moroz, P.; Jones, S.K.; Gray, B.N. Status of hyperthermia in the treatment of advanced liver cancer. J. Surg. Oncol. 2001, 77, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.M.; Gabriel, S.A.; Sukhorukov, G.B.; Gould, D.J. Improved and targeted delivery of bioactive molecules to cells with magnetic layer-by-layer assembled microcapsules. Nanoscale 2015, 7, 9686–9693. [Google Scholar] [CrossRef] [PubMed]
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.J. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Fernandez, E.; Safronov, A.P.; Svalov, A.V.; Beketov, I.V.; Burgoa Beitia, A.; Garcıa-Arribas, A.; Blyakhman, F.A. Giant magnetoimpedance biosensor for ferrogel detection: Model system to evaluate properties of natural tissue. Appl. Phys. Lett. 2015, 106, 193702. [Google Scholar] [CrossRef]
- Blanc-Béguin, F.; Nabily, S.; Gieraltowski, J.; Turzo, A.; Querellou, S.; Salaun, P.Y. Cytotoxicity and GMI bio-sensor detection of maghemite nanoparticles internalized into cells. J. Magn. Magn. Mater. 2009, 321, 192–197. [Google Scholar] [CrossRef]
- Wang, T.; Guo, L.; Lei, C.; Zhou, Y. Ultrasensitive determination of carcinoembryonic antigens using a magnetoimpedance immunosensor. RSC Adv. 2015, 5, 51330–51336. [Google Scholar] [CrossRef]
- Coisson, M.; Barrera, G.; Celegato, F.; Martino, L.; Vinai, F.; Martino, P.; Ferraro, G.; Tiberto, P. Specific absorption rate determination of magnetic nanoparticles through hyperthermia measurements in non-adiabatic conditions. J. Magn. Magn. Mater. 2016, 415, 2–7. [Google Scholar] [CrossRef]
- Safronov, A.P.; Beketov, I.V.; Tyukova, I.S.; Medvedev, A.I.; Samatov, O.M.; Murzakaev, A.M. Magnetic nanoparticles for biophysical applications synthesized by high-power physical dispersion. J. Magn. Magn. Mater. 2015, 383, 281–287. [Google Scholar] [CrossRef]
- Kumar, A.; Mohapatra, S.; Fal-Miyar, V.; Cerdeira, A.; Garcia, J.A.; Srikanth, H.; Gass, J.; Kurlyandskaya, G.V. Magnetoimpedance biosensor for Fe3O4 nanoparticle intracellular uptake evaluation. Appl. Phys. Lett. 2007, 91, 143902. [Google Scholar] [CrossRef]
- Tomizawa, S.; Kawabata, S.; Komori, S.; Hoshino, H.; Fukuoka, Y.; Shinomiya, K. Evaluation of segmental spinal cord evoked magnetic field after sciatic nerve stimulation. Clin. Neurophys. 2008, 119, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Mohri, K.; Honkura, Y.; Panina, L.V. Recent advances of pico-Tesla resolution magneto-impedance sensor based on amorphous wire CMOS IC MI Sensor. IEEE Trans. Magn. 2012, 48, 3833–3839. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Garcia-Arribas, A.; Barandiaran, J.M.; Kisker, E. Giant magnetoimpedance stripe and coil sensors. Sens. Actuators A 2001, 91, 116–119. [Google Scholar] [CrossRef]
- Miller, M.M.; Prinz, G.A.; Cheng, S.-F.; Bounnak, S. Detection of a micron-sized magnetic sphere using a ring-shaped anisotropic magnetoresistance-based sensor: A model for a magnetoresistance-based biosensor. Appl. Phys. Lett. 2002, 81, 2211–2213. [Google Scholar] [CrossRef]
- Ferreira, H.A.; Graham, D.L.; Freitas, P.P.; Cabral, J.M.S. Biodetection using magnetically labeled biomolecules and arrays of spin valve sensors. J. Appl. Phys. 2002, 93, 7281–7286. [Google Scholar] [CrossRef]
- Besse, P.-A.; Boero, G.; Demierre, M.; Pott, V.; Popovic, R. Detection of single magnetic microbead using a miniaturized silicon Hall sensor. Appl. Phys. Lett. 2002, 80, 4199–4201. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; de Cos, D.; Volchkov, S.O. Magnetosensitive Transducers for Nondestructive Testing Operating on the Basis of the Giant Magnetoimpedance Effect: A Review. Russ. J. Non-Destr. Test. 2009, 45, 377–398. [Google Scholar] [CrossRef]
- Chiriac, H.; Herea, D.D.; Corodeanu, S. Microwire array for giant magnetoimpedance detection of magnetic particles for biosensor prototype. J. Magn. Magn. Matter. 2007, 311, 425–428. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Levit, V.I. Advanced materials for drug delivery and biosensors based on magnetic label detection. Mater. Sci. Eng. C 2007, 27, 495–503. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V. Giant magnetoimpedance for biosensing: Advantages and shortcomings. J. Magn. Magn. Matter. 2009, 321, 659–662. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.; Pu, H. Magnetic impedance biosensor: A review. Biosens. Bioelectron. 2017, 90, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Kavaldzhiev, M.N.; Kosel, J. Flexible magnetoimpedance sensor. J. Magn. Magn. Mater. 2015, 378, 499–505. [Google Scholar] [CrossRef]
- Shcherbinin, S.V.; Volchkov, S.O.; Lepalovskii, V.N.; Chlenova, A.A.; Kurlyandskaya, G.V. System based on a ZVA-67 vector network analyzer for measuring high-frequency parameters of magnetic film structures. Russ. J. Nondestruct. 2017, 53, 204–212. [Google Scholar] [CrossRef]
- Svalov, A.V.; Fernandez, E.; Arribas, A.; Alonso, J.; Fdez-Gubieda, M.L.; Kurlyandskaya, G.V. FeNi-based magnetoimpedance multilayers: Tailoring of the softness by magnetic spacers. Appl. Phys. Lett. 2012, 100, 162410. [Google Scholar] [CrossRef]
- Beato-López, J.J.; Pérez-Landazábal, J.I.; Gómez-Polo, C. Magnetic nanoparticle detection method employing non-linear magnetoimpedance effects. J. Appl. Phys. 2017, 121, 163901. [Google Scholar] [CrossRef]
- Beach, R.; Berkowitz, A. Sensitive field-and frequency-dependent impedance spectra of amorphous FeCoSiB wire and ribbon. J. Appl. Phys. 1994, 76, 6209–6213. [Google Scholar] [CrossRef]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in cancer medicine. Phys. Today 2012, 65, 38–42. [Google Scholar] [CrossRef]
- Volchkov, S.O.; Dukhan, A.E.; Dukhan, E.I.; Kurlyandskaya, G.V. Computer-Aided Inspection Center for Magnetoimpedance Spectroscopy. Russ. J. Nondestruct. 2016, 52, 647–652. [Google Scholar] [CrossRef]
- Helminger, M.; Wu, B.; Kollmann, T.; Benke, D.; Schwahn, D.; Pipich, V.; Faivre, D.; Zahn, D.; Cölfen, H. Synthesis and Characterization of Gelatin-Based Magnetic Hydrogels. Adv. Funct. Mater. 2014, 24, 3187–3196. [Google Scholar] [CrossRef] [PubMed]
- Blyakhman, F.A.; Safronov, A.P.; Zubarev, A.Y.; Shklyar, T.F.; Makeyev, O.G.; Makarova, E.B.; Melekhin, V.V.; Larrañaga, A.; Kurlyandskaya, G.V. Polyacrylamide ferrogels with embedded maghemite nanoparticles for biomedical engineering. Results Phys. 2017, 7, 3624–3633. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hu, S.-H.; Liu, T.Y.; Liu, D.-M.; Chen, S.Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Osipov, V.V.; Platonov, V.V.; Uimin, M.A.; Podkin, A.V. Laser synthesis of magnetic iron oxide nanopowders. Tech. Phys. 2012, 57, 543–549. [Google Scholar] [CrossRef]
- Novoselova, I.P.; Safronov, A.P.; Samatov, O.M.; Beketov, I.V.; Medvedev, A.I.; Kurlyandskaya, G.V. Water based suspensions of iron oxide obtained by laser target evaporation for biomedical applications. J. Magn. Magn. Mater. 2016, 415, 35–38. [Google Scholar] [CrossRef]
- O’Handley, R.C. Modern Magnetic Materials; John Wiley & Sons: New York, NY, USA, 1972; p. 740. [Google Scholar]
- Qu, J.; Liu, G.; Wang, Y.; Hong, R. Preparation of Fe3O4-chitosan nanoparticles used for hypothermia. Adv. Powder Technol. 2010, 21, 421–427. [Google Scholar] [CrossRef]
- McHenry, M.E.; Willard, M.A.; Laughlin, D.E. Amorphous and nanocrystalline materials for applications as soft magnets. Prog. Mater. Sci. 1999, 44, 291–433. [Google Scholar] [CrossRef]
- Spano, M.; Hathaway, K.; Savage, H. Magnetostriction and magnetic anisotropy of field annealed Metglas* 2605 alloys via dc M-H loop measurements under stress. J. Appl. Phys. 1982, 53, 2667–2669. [Google Scholar] [CrossRef]
- Lotfollahi, Z.; García-Arribas, A.; Amirabadizadeh, A.; Orue, I.; Kurlyandskaya, G.V. Comparative study of magnetic and magnetoimpedance properties of CoFeSiB-based amorphous ribbons of the same geometry with Mo or W additions. J. Alloy. Compd. 2017, 693, 767–776. [Google Scholar] [CrossRef]
- Brunchi, C.-E.; Morariu, S.; Bercea, M. Intrinsic viscosity and conformational parameters of xanthan inaqueous solutions: Salt addition effect. Colloids Surf. B Biointerfaces 2014, 122, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Picout, D.R.; Ross-Murphy, S.B. On the Mark–Houwink parameters for galactomannans. Carbohydr. Polym. 2007, 70, 145–148. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgensrahlen (Determination of the size and internal structure of colloidal particles using X-rays). Nachr. Ges. Wiss. Göttingen. 1918, 2, 98–102. [Google Scholar]
- Galicia, J.A.; Sandre, O.; Cousin, F.; Guemghar, D.; Ménager, C.; Cabuil, V. Designing magnetic composite materials using aqueous magnetic fluids. J. Phys. Condens. Matter 2003, 15, S1379–S1402. [Google Scholar]
- Safronov, A.P.; Terziyan, T.V.; Istomina, A.S.; Beketov, I.V. Swelling and Contraction of Ferrogels Based on Polyacrylamide in a Magnetic Field. Polym. Sci. Ser. A 2012, 54, 26–33. [Google Scholar] [CrossRef]
- Knobel, M. Giant magnetoimpedance in soft magnetic amorphous and nanocrystalline materials. J. Phys. IV 1998, 8, 212–220. [Google Scholar] [CrossRef]
- Spizzo, F.; Sgarbossa, P.; Sieni, E.; Semenzato, A.; Dughiero, F.; Forzan, M.; Bertani, R.; Del Bianco, L. Synthesis of ferrofluids made of iron oxide nanoflowers: Interplay between carrier fluid and magnetic properties. Nanomaterials 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Galicia, J.A.; Cousin, F.; Dubois, E.; Sandre, A.; Cabuila, V.; Perzynski, R. Static and dynamic structural probing of swollen polyacrylamide ferrogels. Soft Matter 2009, 5, 2614–2624. [Google Scholar] [CrossRef]
- Quesada-Perez, M.; Maroto-Centeno, J.A.; Forcada, J.; Hidalgo-Alvarez, R. Gel swelling theories: The classical formalism and recent approaches. Soft Matter 2011, 7, 10536–10547. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003; p. 59. [Google Scholar]
- Safronov, A.P.; Tyukova, I.S.; Kurlyandskaya, G.V. Coil-to-helix transition of gellan in dilute solutions is a two-step process. Food Hydrocoll. 2018, 74, 108–114. [Google Scholar] [CrossRef]
- Dickinson, E. Food Polymers, Gels and Colloids; Royal Society of Chemistry: Cambridge, UK, 1991. [Google Scholar]
- Amici, E.; Clark, A.H.; Normand, V.; Johnson, N.B. Interpenetrating Network Formation in Gellan–Agarose Gel Composites. Biomacromolecules 2000, 1, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Xu, L.; Wang, H.; Fu, Y.; Zhang, Z.; Liu, L.; Wang, C.; Zhao, J.; Rong, J. Dual Physically Cross-Linked Double Network Hydrogels with High Mechanical Strength, Fatigue Resistance, Notch-Insensitivity and Self-Healing Properties. ACS Appl. Mater. Interfaces 2016, 8, 34034–34044. [Google Scholar] [CrossRef] [PubMed]
- Filipcsei, G.; Zrinyi, M. Magnetodeformation effects and the swelling of ferrogels in a uniform magnetic field. J. Phys. Condens. Matter 2010, 22, 276001. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R. Biophysics; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1999. [Google Scholar]
- Makhotkin, V.E.; Shurukhin, B.P.; Lopatin, V.A.; Marchukov, P.Y.; Levin, Y.K. Magnetic field sensors based on amorphous ribbons. Sens. Actuators A Phys. 1991, 27, 759–762. [Google Scholar] [CrossRef]
- Semirov, A.V.; Bukreev, D.A.; Moiseev, A.A.; Derevyanko, M.S.; Kudryavtsev, V.O. Relationship Between the Temperature Changes of the Magnetostriction Constant and the Impedance of Amorphous Elastically Deformed Soft Magnetic Cobalt-Based Ribbons. Russ. Phys. J. 2013, 55, 977–982. [Google Scholar] [CrossRef]
- Machado, F.L.A.; de Araujo, A.E.P.; Puca, A.A.; Rodrigues, A.R.; Rezende, S.M. Surface magnetoimpedance measurements in soft-ferromagnetic materials. Phys. Status Solidi A Appl. Res. 1999, 173, 135–144. [Google Scholar] [CrossRef]
- Megens, M.; Prins, M. Magnetic biochips: A new option for sensitive diagnostics. J. Magn. Magn. Mat. 2005, 293, 702–708. [Google Scholar] [CrossRef]
- Chiriac, H.; Tibu, M.; Moga, A.-E.; Herea, D.D. Magnetic GMI sensor for detection of biomolecules. J. Magn. Magn. Mater. 2005, 293, 671–676. [Google Scholar] [CrossRef]
- Li, G.; Wang, S. Analytical and micromagnetic modeling for detection of a single magnetic microbead or nanobead by spin valve sensors. IEEE Trans. Magn. 2003, 39, 3313–3315. [Google Scholar]



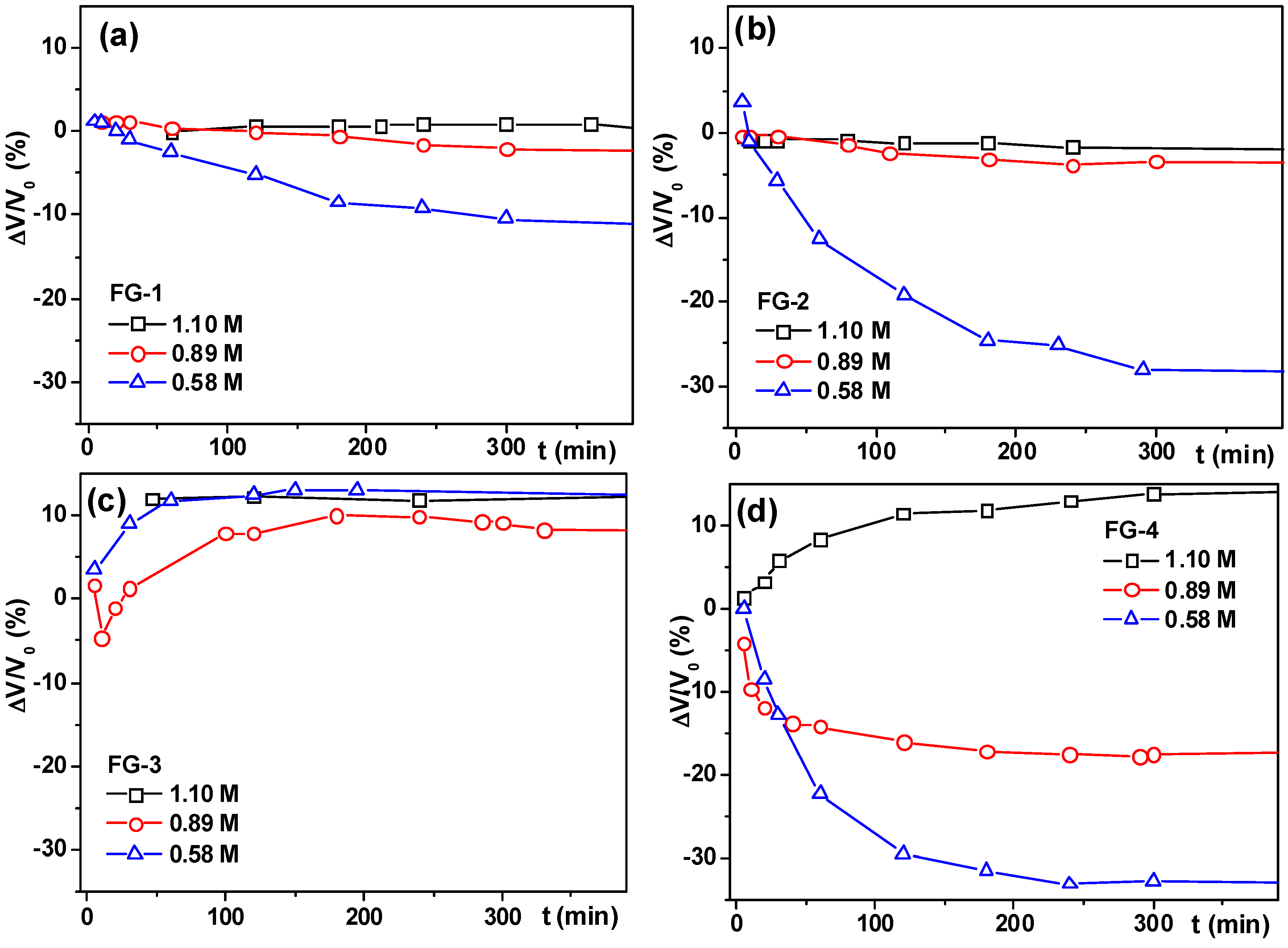
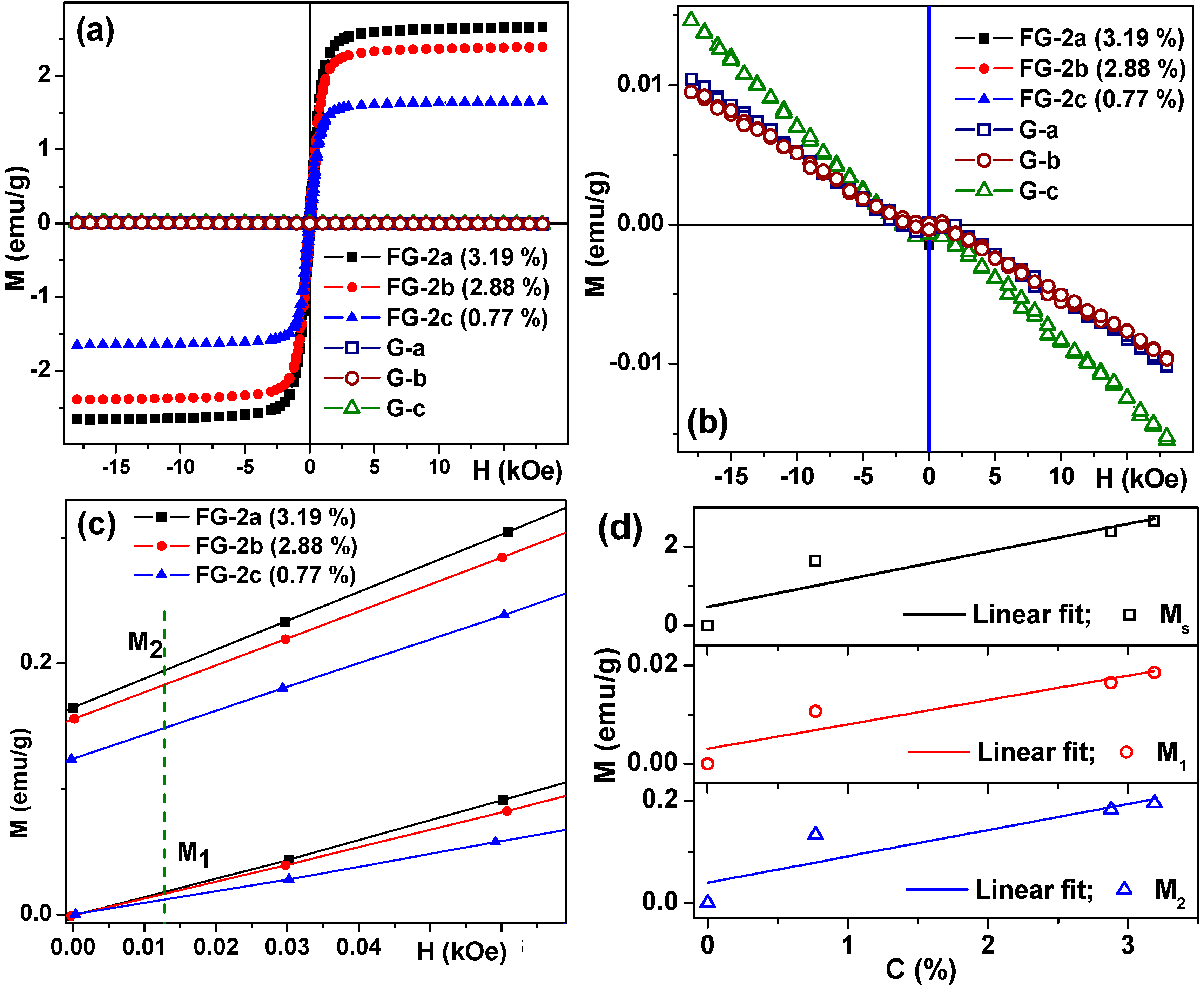
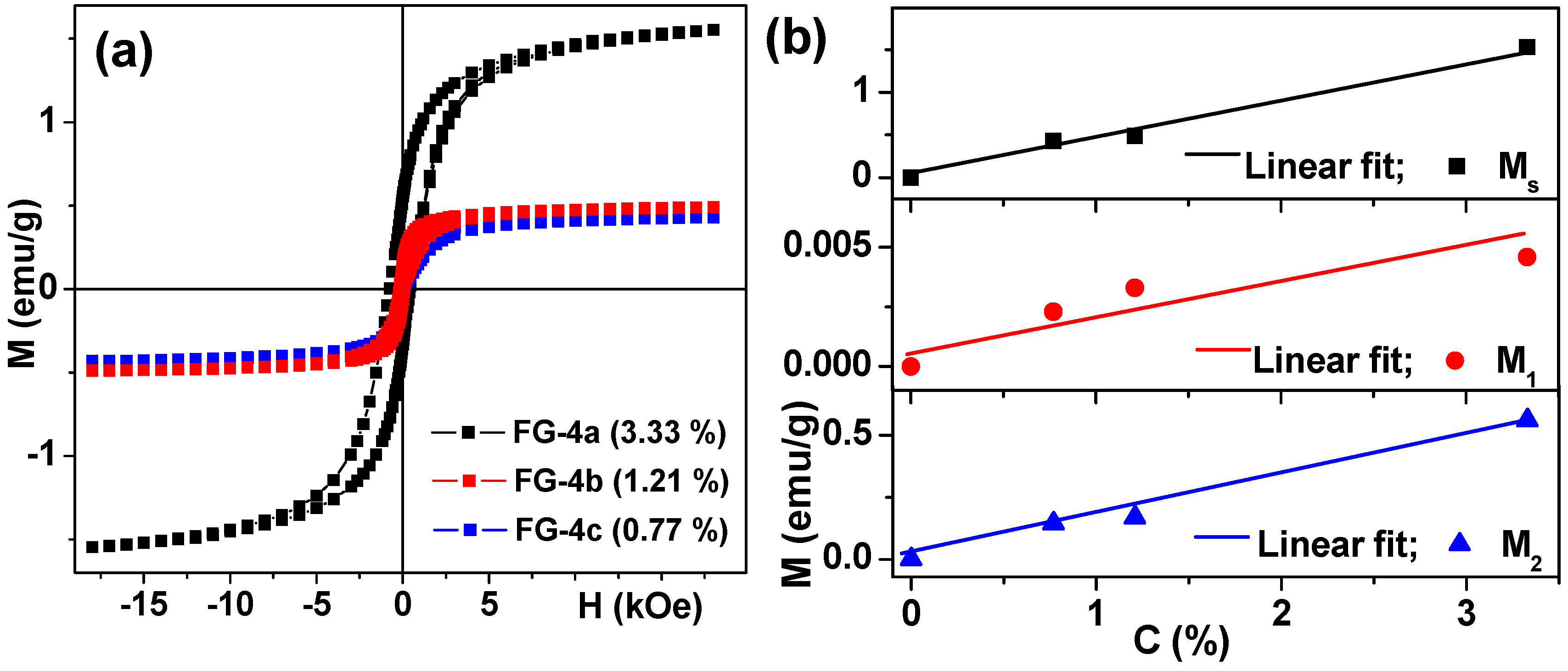
| G/FG Series | Magnetic Particles | Concentration of AAm (M) | Thickener | Swelling Ratio, α | Weight fraction of Magnetic Particles, ϑ (%) |
|---|---|---|---|---|---|
| FG-1a | Fe3O4 | 1.10 | Guar | 10 | 5.20 |
| FG-1b | Fe3O4 | 0.85 | Guar | 15 | 4.12 |
| FG-1c | Fe3O4 | 0. 58 | Guar | 27 | 2.59 |
| FG-2a | Fe3O4 | 1.10 | Xanthan | 17 | 3.19 |
| FG-2b | Fe3O4 | 0.85 | Xanthan | 21 | 2.88 |
| FG-2c | Fe3O4 | 0. 58 | Xanthan | 93 | 0.77 |
| FG-3a | SrFe12O19 | 1.10 | Guar | 15 | 3.70 |
| FG-3b | SrFe12O19 | 0.85 | Guar | 51 | 1.24 |
| FG-3c | SrFe12O19 | 0. 58 | Guar | 54 | 1.31 |
| FG-4a | SrFe12O19 | 1.10 | Xanthan | 16 | 3.33 |
| FG-4b | SrFe12O19 | 0.85 | Xanthan | 52 | 1.21 |
| FG-4c | SrFe12O19 | 0. 58 | Xanthan | 92 | 0.77 |
| G-a | - | 1.10 | Xanthan | 31 | 0 |
| G-b | - | 0.85 | Xanthan | 90 | 0 |
| G-c | - | 0. 58 | Xanthan | 303 | 0 |
| Particles Type | Phases | 2θ (˚) | FWHMav (˚) | Intensity | Phases Content (%) | DXRD (nm) |
|---|---|---|---|---|---|---|
| “Magnetite” | Fe3O4 | 35.631 | 0.164 | 2799.33 | 94 | 130 |
| Fe2O3 | 33.354 | 0.209 | 27.61 | 1 | 75 | |
| FeO(OH) | 21.369 | 0.216 | 75.64 | 5 | 70 | |
| “Strontium hexaferrite” | SrFe12O19 | 34.349 | 0.136 | 712.19 | 87 | 230 |
| Fe2O3 | 33.319 | 0.262 | 166.16 | 13 | 50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safronov, A.P.; Mikhnevich, E.A.; Lotfollahi, Z.; Blyakhman, F.A.; Sklyar, T.F.; Larrañaga Varga, A.; Medvedev, A.I.; Fernández Armas, S.; Kurlyandskaya, G.V. Polyacrylamide Ferrogels with Magnetite or Strontium Hexaferrite: Next Step in the Development of Soft Biomimetic Matter for Biosensor Applications. Sensors 2018, 18, 257. https://doi.org/10.3390/s18010257
Safronov AP, Mikhnevich EA, Lotfollahi Z, Blyakhman FA, Sklyar TF, Larrañaga Varga A, Medvedev AI, Fernández Armas S, Kurlyandskaya GV. Polyacrylamide Ferrogels with Magnetite or Strontium Hexaferrite: Next Step in the Development of Soft Biomimetic Matter for Biosensor Applications. Sensors. 2018; 18(1):257. https://doi.org/10.3390/s18010257
Chicago/Turabian StyleSafronov, Alexander P., Ekaterina A. Mikhnevich, Zahra Lotfollahi, Felix A. Blyakhman, Tatyana F. Sklyar, Aitor Larrañaga Varga, Anatoly I. Medvedev, Sergio Fernández Armas, and Galina V. Kurlyandskaya. 2018. "Polyacrylamide Ferrogels with Magnetite or Strontium Hexaferrite: Next Step in the Development of Soft Biomimetic Matter for Biosensor Applications" Sensors 18, no. 1: 257. https://doi.org/10.3390/s18010257





