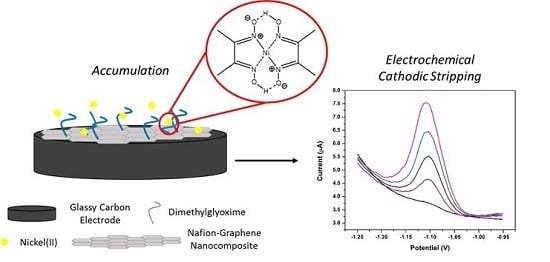
Complexation-Based Detection of Nickel(II) at a Graphene-Chelate Probe in the Presence of Cobalt and Zinc by Adsorptive Stripping Voltammetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Preparation of the Nafion-Graphene Dimethylglyoxime Suspension (NGr-DMG)
2.4. Preparation of Nafion-Graphene Dimethylglyoxime Modified Glassy Carbon Electrode (NGr-DMG-GCE)
2.5. Procedure for Square Wave Adsorptive Cathodic Stripping Voltammetric (AdCSV) Analysis
2.6. Sample Preparation
3. Results and Discussion
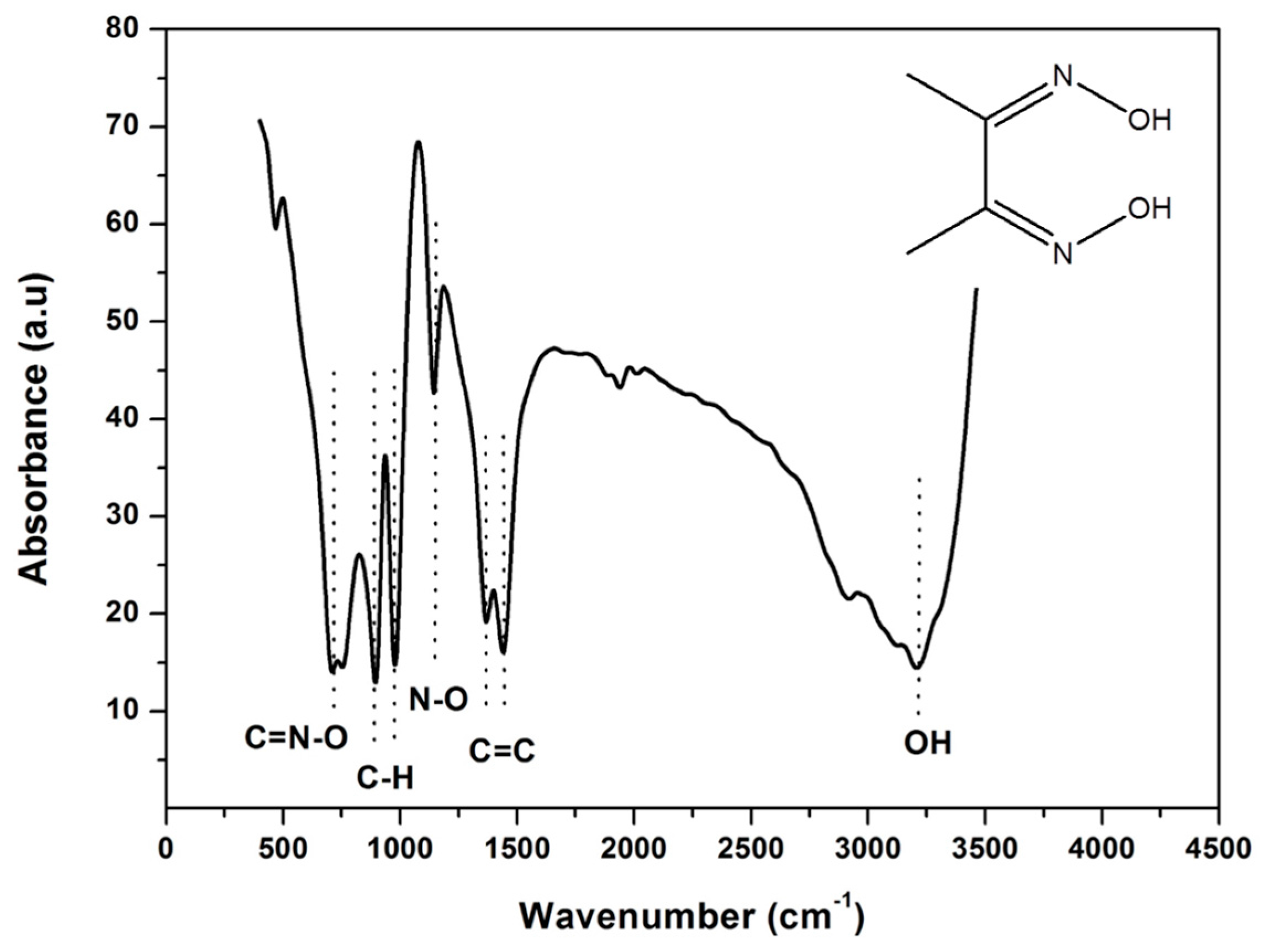
3.1. Fourier Transform Infrared Spectroscopy (FT-IR) of DMG
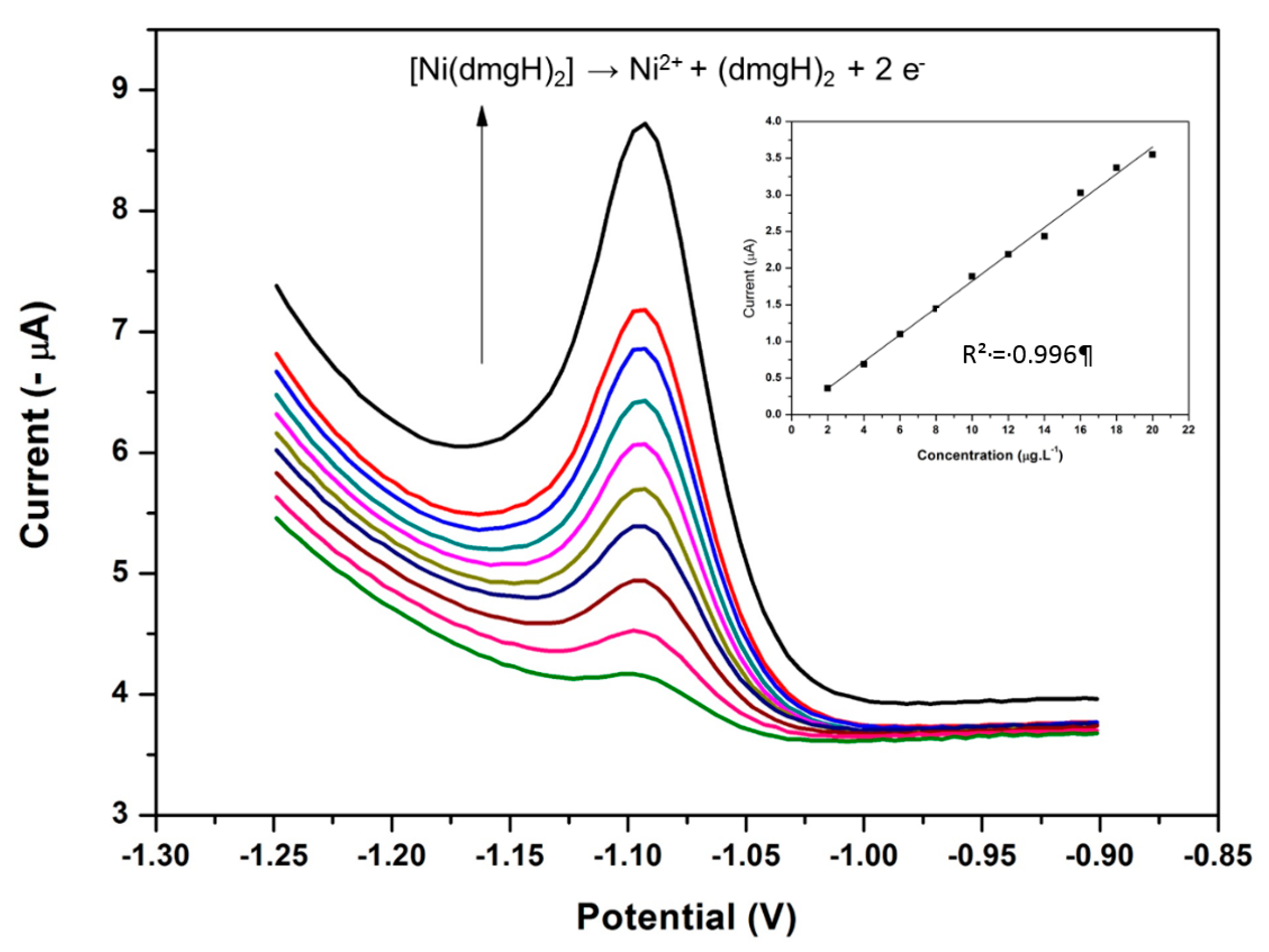
3.2. Nickel Dimethylglyoxime (Ni(DMG)2) Complex Formation and Electrochemical Stripping Reduction
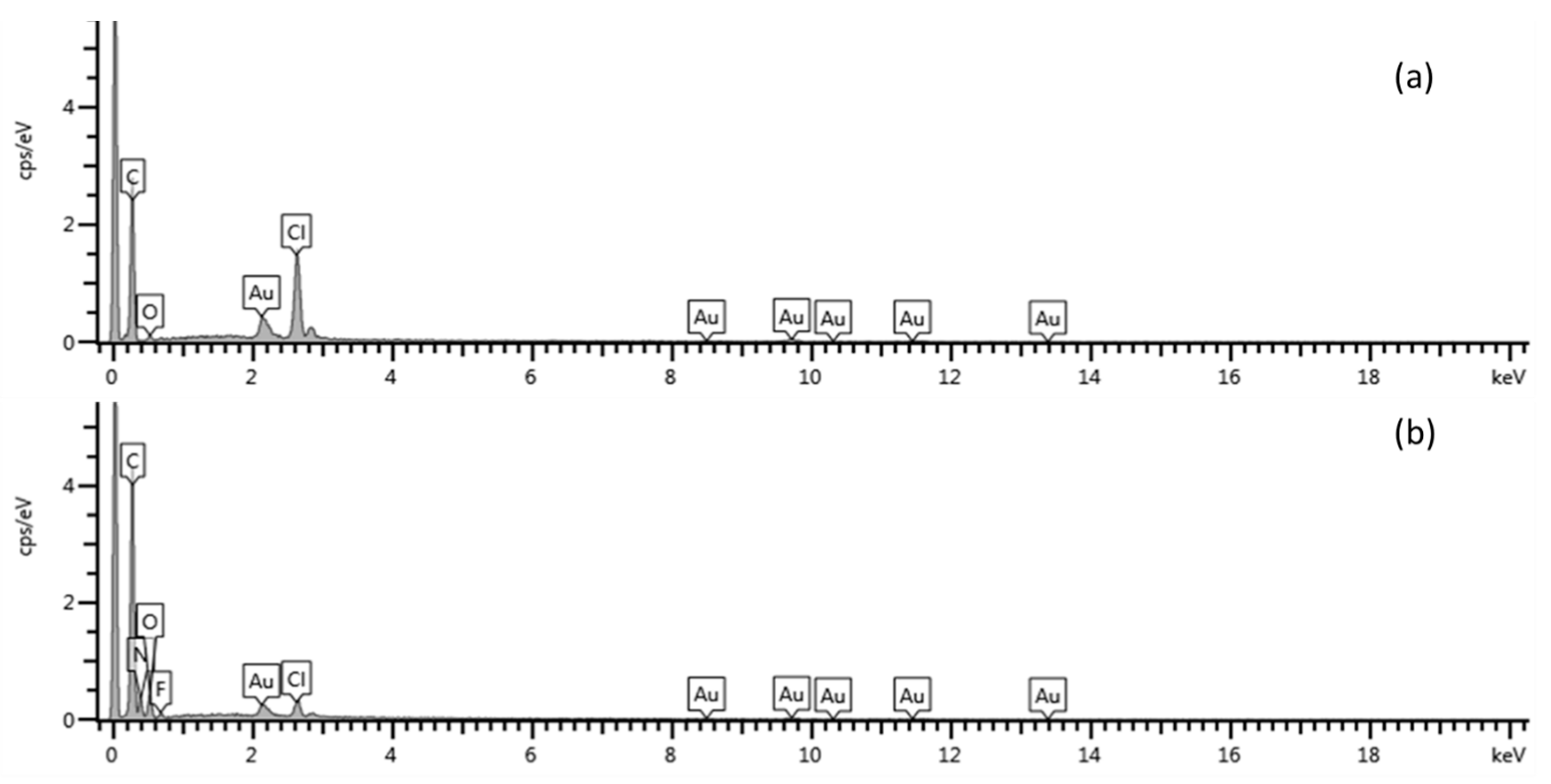
3.3. Characterization of Graphene (Gr)
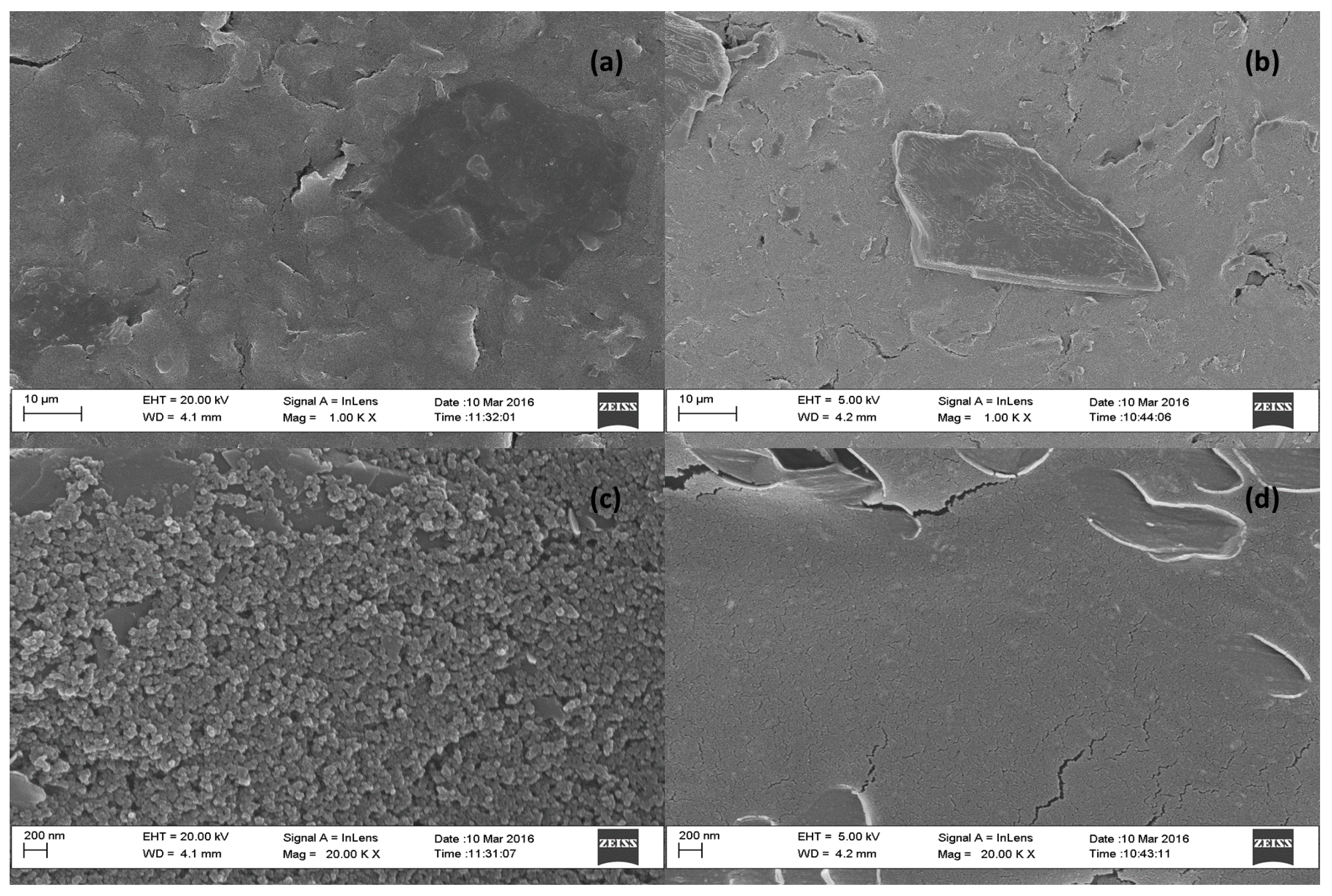
3.4. Morphological Characterization of Modified Electrode
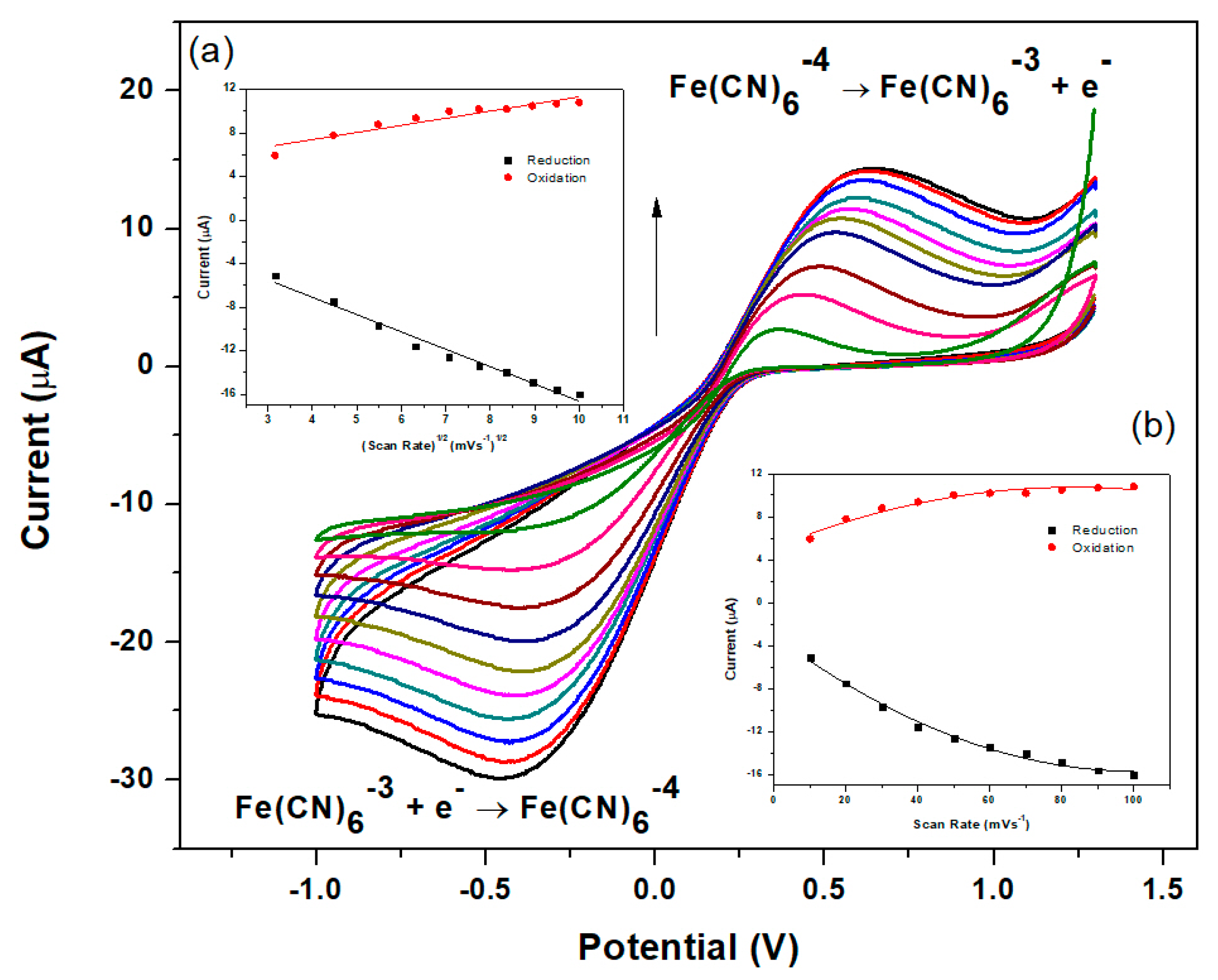
3.5. Electrochemical Characterization of the Nafion-Graphene Dimethylglyoxime Modified Glassy Carbon Electrode (NGr-DMG-GCE)
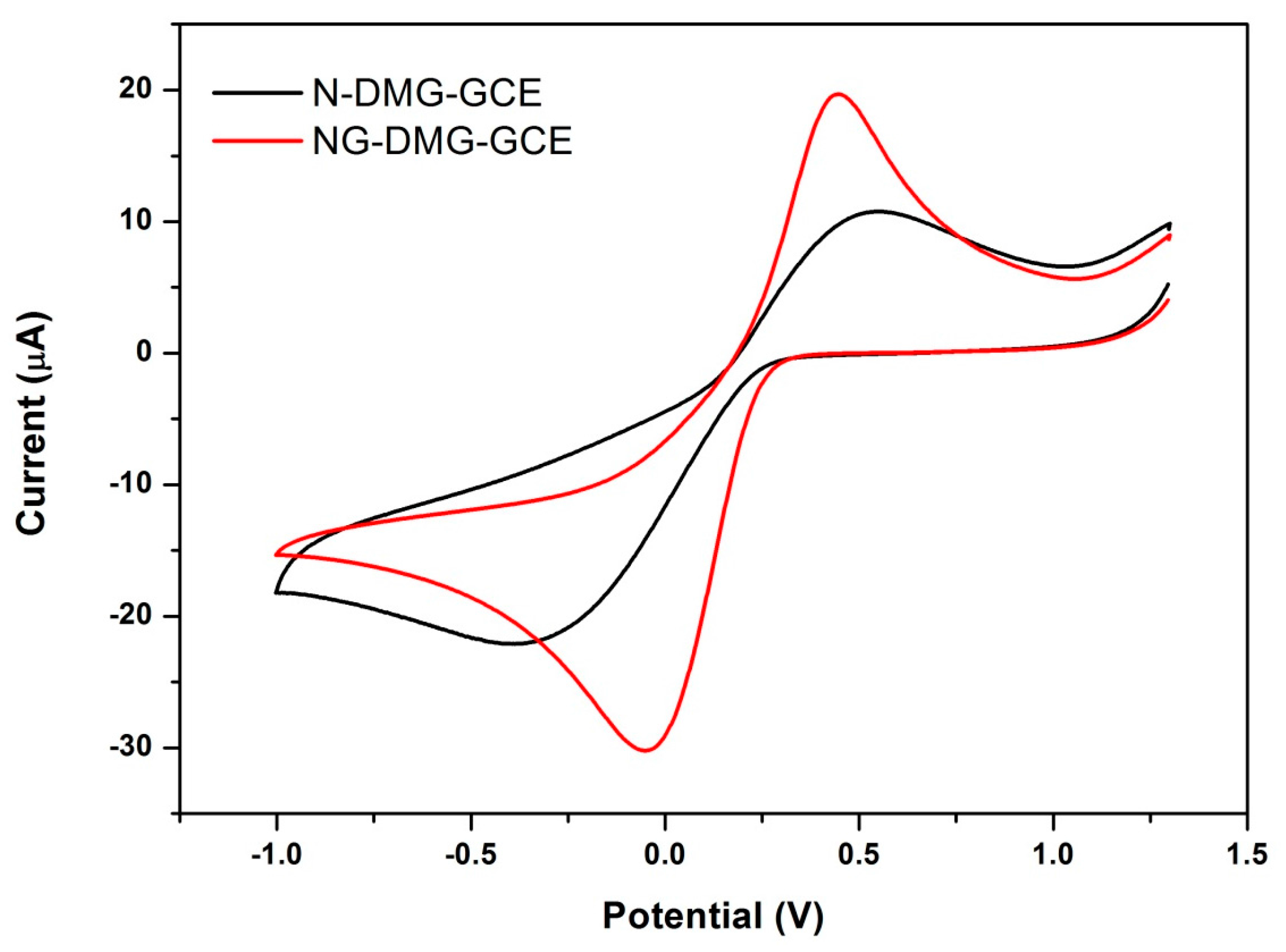
3.6. Electrochemical Behaviour of the NGr-DMG-GCE
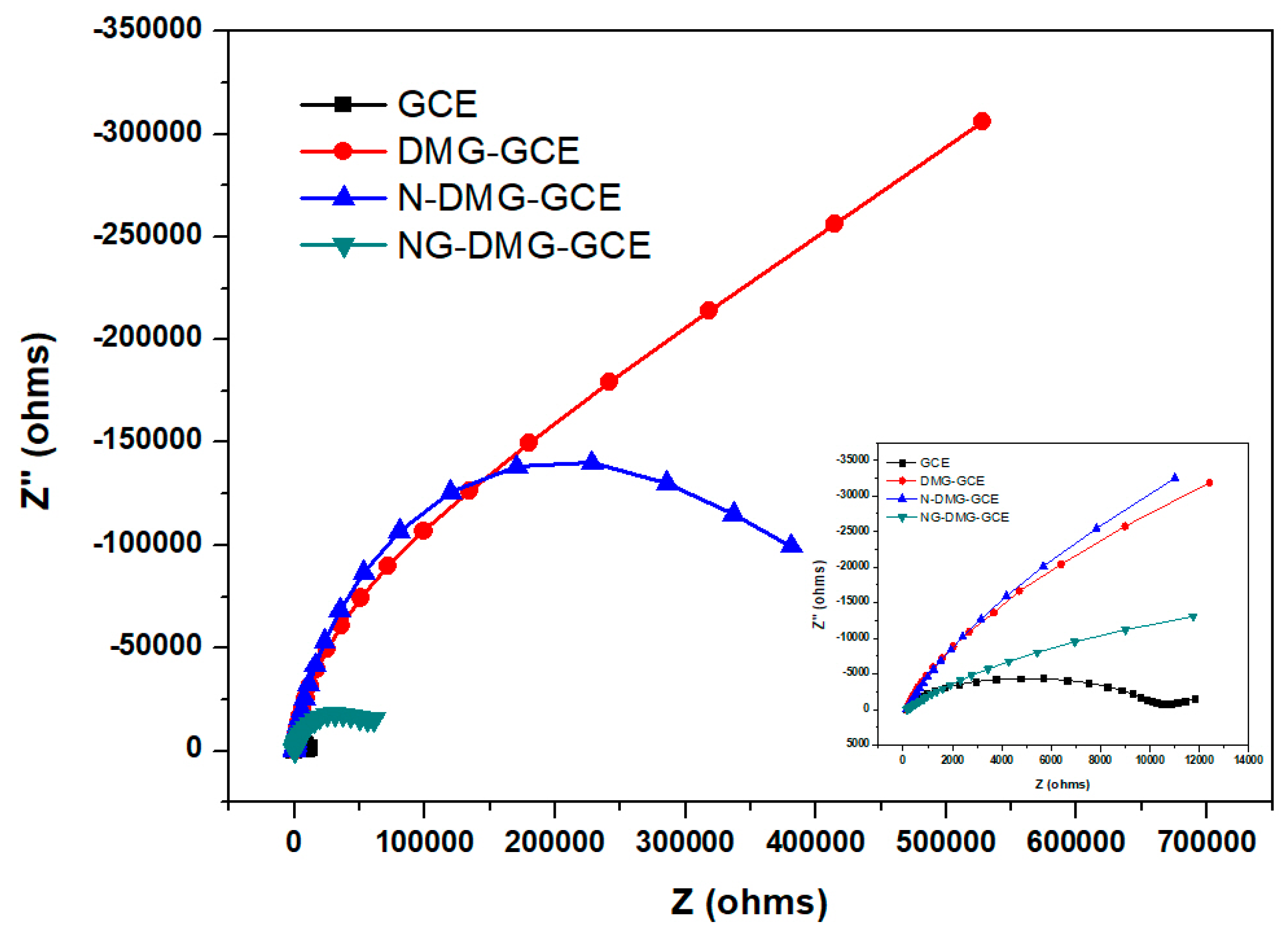
3.7. Electrochemical Impedance Spectroscopy (EIS) Analysis of the NGr-DMG-GCE
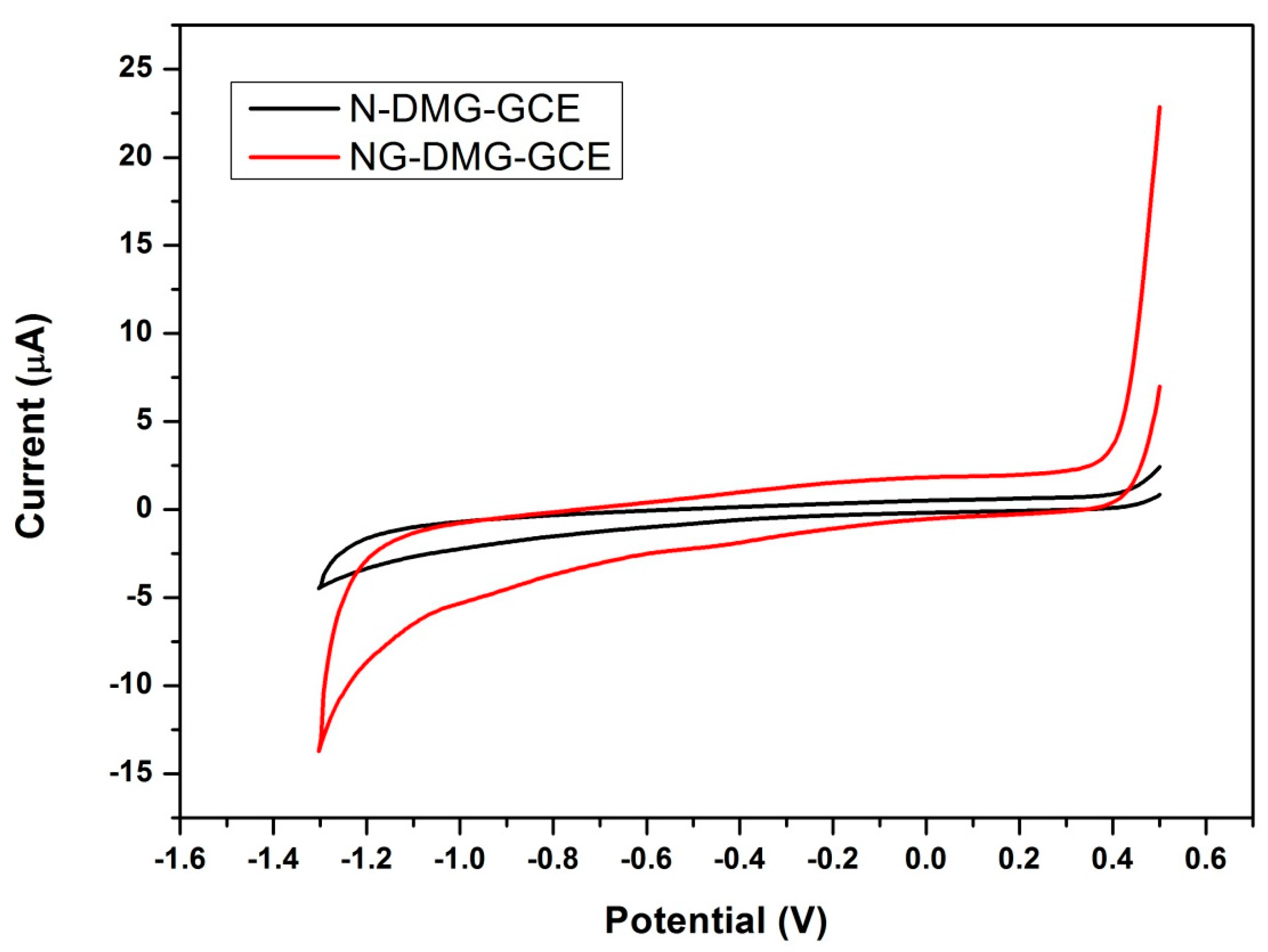
3.8. Further Electrochemical Characterization of the NGr-DMG-GCE
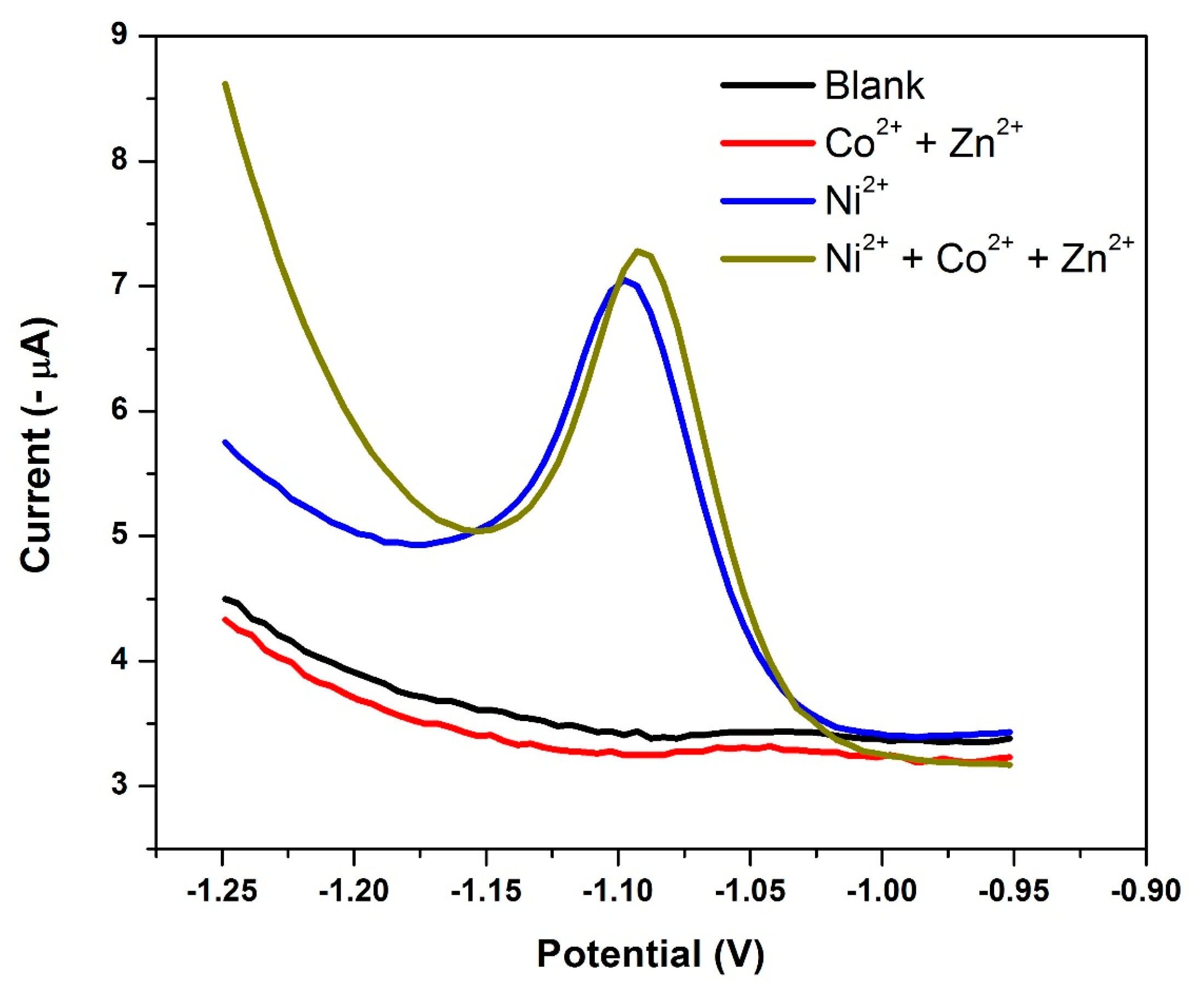
3.9. Effect of the NGr-DMG-GCE on the Stripping Response of Ni2+
3.10. Influence of Dimethylglyoxime Concentration
3.11. Optimization of Instrumental Parameters
3.12. Influence of Electrolyte pH
3.13. Influence of Oxygen Removal
3.14. Electrode Reproducibility and Interference Studies
3.15. Analytical Performance of the NGr-DMG-GCE
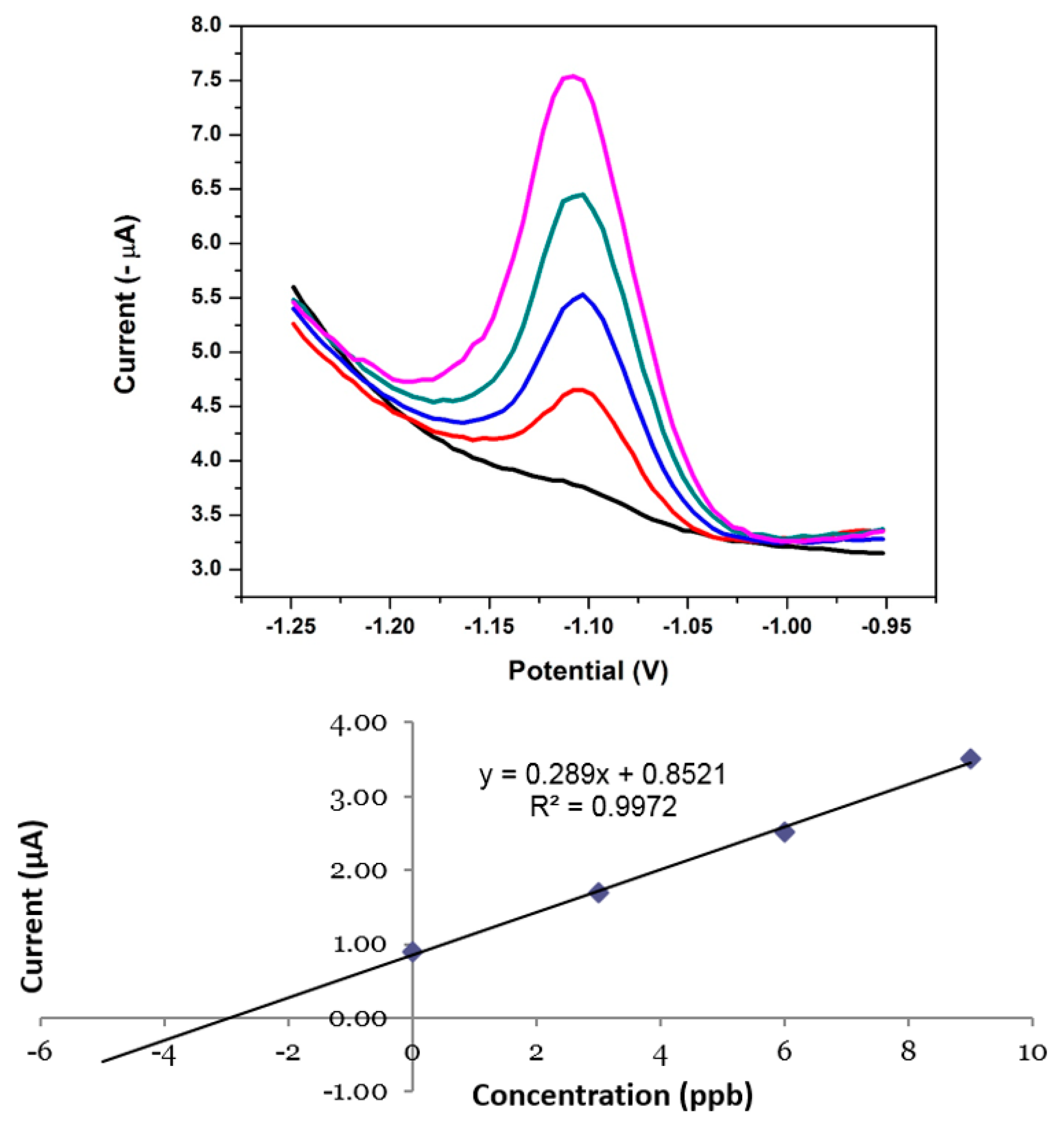
3.16. Application of the NGr-DMG-GCE to Real Water Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zou, Z.; Jang, A.; MacKnight, E.T.; Wu, P.-M.; Do, J.; Shim, J.S.; Bishop, P.L.; Ahn, C.H. An On-Site Heavy Metal Analyzer with Polymer Lab-on-a-Chips for Continuous Sampling and Monitoring. IEEE Sens. J. 2009, 9, 586–594. [Google Scholar] [CrossRef]
- March, G.; Nguyen, T.; Piro, B. Modified Electrodes Used for Electrochemical Detection of Metal Ions in Environmental Analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Keil, D.E.; Berger-Ritchie, J.; McMillin, G.A. Testing for Toxic Elements: A Focus on Arsenic, Cadmium, Lead, and Mercury. Lab. Med. 2011, 42, 735–742. [Google Scholar] [CrossRef]
- WHO. Nickel in Drinking-water. In Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO/SDE/ WSH/05.08/55; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Mamba, B.B.; Rietveld, L.C.; Verberk, J.Q.J.C. SA drinking water standards under the microscope. Water Wheel 2008, 7, 24–27. [Google Scholar]
- Brett, C.M.A.; Oliveira Brett, A.M.C.F.; Pereira, J.L.C. Adsorptive Stripping Voltammetry of Cobalt and Nickel in Flow Systems at Wall-Jet Electrodes. Electroanalysis 1991, 3, 683–689. [Google Scholar] [CrossRef]
- Wararattananurak, P.; Chooto, P.; Sherdshoopongse, P.; Chuaynukool, C.; Innuphat, C. Lead determination in canned food by square-wave adsorptive cathodic stripping voltammetry. ScienceAsia 2014, 40, 355–361. [Google Scholar] [CrossRef]
- Bing, C. Chemical accumulation and voltammetric determination of traces of nickel(II) at glassy carbon electrodes modified with dimethyl glyoxime containing polymer coatings. Talanta 1999, 49, 651–659. [Google Scholar] [CrossRef]
- Wang, J. Stripping Analysis: Principles, Instrumentation, and Applications; VCH Publishers, Inc.: Deerfield Beach, FL, USA, 1985; Available online: https://scholar.google.com/scholar?q=J+Wang+1985&btnG=&hl=en&as_sdt=0%2C26#1 (accessed on 4 August 2015).
- Ferancová, A.; Hattuniemi, M.K.; Sesay, A.M.; Räty, J.P.; Virtanen, V.T. Electrochemical Monitoring of Nickel(II) in Mine Water. Mine Water Environ. 2015. [Google Scholar] [CrossRef]
- Bobrowski, A.; Krolicka, A.; Maczuga, M.; Zarebski, J. Highly Sensitive and Selective Adsorptive Stripping Voltammetric Method Employing a Lead Film Screen-printed Electrode for Determination of Cobalt as its Nioximate Complex. Electroanalysis 2016, 28, 343–349. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, D.; Liu, Y. Rapid and sensitive determination of cobalt by adsorptive cathodic stripping voltammetry using tin–bismuth alloy electrode. Ionics (Kiel) 2016, 22, 721–729. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Metelka, R.; Vytřas, K.; Barczak, M. Lead Film Electrode Prepared with the Use of a Reversibly Deposited Mediator Metal in Adsorptive Stripping Voltammetry of Nickel. Electroanalysis 2014, 26, 2049–2056. [Google Scholar] [CrossRef]
- Bas, B.; Wegiel, K.; Jedlinska, K. The renewable bismuth bulk annular band working electrode: Fabrication and application in the adsorptive stripping voltammetric determination of nickel(II) and cobalt(II). Anal. Chim. Acta 2015, 881, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bobrowski, A.; Królicka, A.; Maczuga, M.; Zarȩbski, J. A novel screen-printed electrode modified with lead film for adsorptive stripping voltammetric determination of cobalt and nickel. Sens. Actuators B 2014, 191, 291–297. [Google Scholar] [CrossRef]
- Ferancová, A.; Hattuniemi, M.K.; Sesay, A.M.; Räty, J.P.; Virtanen, V.T. Rapid and direct electrochemical determination of Ni(II) in industrial discharge water. J. Hazard. Mater. 2016, 306, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ferancová, A.; Hattuniemi, M.K.; Sesay, A.M.; Räty, J.P.; Virtanen, V.T. Complexation of Ni(II) by Dimethylglyoxime for Rapid Removal and Monitoring of Ni(II) in Water. Mine Water Environ. 2016. [Google Scholar] [CrossRef]
- González, P.; Cortínez, V.A.; Fontán, C.A. Determination of nickel by anodic adsorptive stripping voltammetry with a cation exchanger-modified carbon paste electrode. Talanta 2002, 58, 679–690. [Google Scholar] [CrossRef]
- Rezaei, B.; Rezaei, E. Simultaneous determination of trace amounts of nickel, cobalt, and zinc in the wastewater of a galvanic workshop by using adsorptive cathodic stripping voltammetry. J. Anal. Chem. 2006, 61, 262–265. [Google Scholar] [CrossRef]
- Tartarotti, F.O.; De Oliveira, M.F.; Balbo, V.R.; Stradiotto, N.R. Determination of nickel in fuel ethanol using a carbon paste modified electrode containing dimethylglyoxime. Microchim. Acta 2006, 155, 397–401. [Google Scholar] [CrossRef]
- Thomsen, K.N.; Kryger, L.; Baldwin, R.P. Voltammetric Determination of Traces of Nickel (II) with a Medium Exchange Flow System and a Chemically Modified Carbon Paste Electrode Containing Dimethylglyoxime. Anal. Chem. 1988, 151–155. [Google Scholar] [CrossRef]
- Tien, H. The Application of Chelating Agent Incorporated Polymer Modified Electrodes in the Detection of Trace Metals. J. Chin. Chem. Soc. 1998, 45, 39–46. [Google Scholar]
- Zen, J.; Chi, N.; Hsu, F.; Chung, M. Square-wave Voltam metric Determination of Copper(ii) With a Nafion-Dimethylglyoxime Mercury-film Electrode. Analyst 1995, 120, 511–515. [Google Scholar] [CrossRef]
- Zen, J.; Lee, M. Determination of Traces of Nickel(II) at a Perfluorinated Ionomer/Dimethylglyoxime Mercury Film Electrode. Anal. Chem. 1993, 120, 3238–3243. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Hadjichari, A. Simultaneous Determination of Nickel and Cobalt in Natural Water and Sediment Samples on an in-situ Plated Mercury Film Electrode by Adsorptive Cathodic Stripping Voltammetry. Anal. Sci. 1999, 15, 95–100. [Google Scholar] [CrossRef]
- Nghit, T.V.; Tho, H.; Hung, P.; Chu, V.; Quangt, X.; Dinh, P. Adsorptive Cathodic Stripping Voltammetric Determination of Several Heavy Metals (Ni, Co, Cu, Pb) in Natural Waters, (n.d.). Available online: http://hdl.handle.net/11094/13141 (accessed on 25 July 2017).
- Korolczuk, M.; Tyszczuk, K.; Grabarczyk, M. Adsorptive stripping voltammetry of nickel and cobalt at in situ plated lead film electrode. Electrochem. Commun. 2005, 7, 1185–1189. [Google Scholar] [CrossRef]
- Dal Borgo, S.; Sopha, H.; Smarzewska, S.; Hočevar, S.B.; Švancara, I.; Metelka, R. Macroporous Bismuth Film Screen-Printed Carbon Electrode for Simultaneous Determination of Ni(II) and Co(II). Electroanalysis 2015, 27, 209–216. [Google Scholar] [CrossRef]
- Gharib Naseri, N.; Baldock, S.J.; Economou, A.; Goddard, N.J.; Fielden, P.R. Disposable electrochemical flow cells for catalytic adsorptive stripping voltammetry (CAdSV) at a bismuth film electrode (BiFE). Anal. Bioanal. Chem. 2008, 391, 1283–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutton, E.A.; Hočevar, S.B.; Ogorevc, B.; Smyth, M.R. Bismuth film electrode for simultaneous adsorptive stripping analysis of trace cobalt and nickel using constant current chronopotentiometric and voltammetric protocol. Electrochem. Commun. 2003, 5, 765–769. [Google Scholar] [CrossRef]
- Segura, R.; Pradena, M.; Pinto, D.; Godoy, F.; Nagles, E.; Arancibia, V. Adsorptive stripping voltammetry of nickel with 1-nitroso-2-napthol using a bismuth film electrode. Talanta 2011, 85, 2316–2319. [Google Scholar] [CrossRef] [PubMed]
- Segura, R.A.; Pizarro, J.A.; Oyarzun, M.P.; Castillo, A.D.; Díaz, K.J.; Placencio, A.B. Determination of Lead and Cadmium in Water Samples by Adsorptive Stripping Voltammetry Using a Bismuth film/1-Nitroso-2-Napthol/Nafion Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2016, 11, 1707–1719. [Google Scholar]
- Pokpas, K.; Zbeda, S.; Jahed, N.; Mohamed, N.; Baker, P.G.; Iwuoha, E.I.; Sensorlab, E.I.I. Electrochemically reduced graphene oxide pencil-graphite in situ plated bismuth-film electrode for the determination of trace metals by anodic stripping voltammetry. Int. J. Electrochem. Sci. 2014, 9, 736–759. [Google Scholar]
- Walch, N.J.; Davis, F.; Langford, N.; Holmes, J.L.; Collyer, S.D.; Higson, S.P.J. Enhancement of Electrode Performance by a Simple Casting Method Using Sonochemically Exfoliated Graphene. Anal. Chem. 2015, 87, 9273–9279. [Google Scholar] [CrossRef] [PubMed]
- Pokpas, K.; Jahed, N.; Tovide, O.; Baker, P.G.; Iwuoha, E.I. Nafion-graphene nanocomposite in situ plated bismuth-film electrodes on pencil graphite substrates for the determination of trace heavy metals by anodic stripping voltammetry. Int. J. Electrochem. Sci. 2014, 9, 5092–5115. [Google Scholar]
- Zbeda, S.; Pokpas, K.; Titinchi, S.; Jahed, N.; Baker, P.G.; Iwuoha, E.I. Few-layer binder free graphene modified mercury film electrode for trace metal analysis by square wave anodic stripping voltammetry. Int. J. Electrochem. Sci. 2013, 8, 11125–11141. [Google Scholar]
- Chaiyo, S.; Mehmeti, E.; Žagar, K.; Siangproh, W.; Chailapakul, O.; Kalcher, K. Electrochemical sensors for the simultaneous determination of zinc, cadmium and lead using a Nafion/ionic liquid/graphene composite modified screen-printed carbon electrode. Anal. Chim. Acta 2016, 918, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ghanei-Motlagh, M.; Taher, M.A.; Heydari, A.; Ghanei-Motlagh, R.; Gupta, V.K. A novel voltammetric sensor for sensitive detection of mercury(II) ions using glassy carbon electrode modified with graphene-based ion imprinted polymer. Mater. Sci. Eng. C 2016, 63, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lei, C.; Zhong, T.; Long, L.; Wu, Z.; Huan, S.; Zhang, Q. A graphene/ionic liquid modified selenium-doped carbon paste electrode for determination of copper and antimony. Anal. Methods 2016, 8, 1120–1126. [Google Scholar] [CrossRef]
- Punrat, E.; Maksuk, C.; Chuanuwatanakul, S.; Wonsawat, W.; Chailapakul, O. Polyaniline/graphene quantum dot-modified screen-printed carbon electrode for the rapid determination of Cr(VI) using stopped-flow analysis coupled with voltammetric technique. Talanta 2016, 150, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Chen, T.; Xu, F.; Zhao, W.; Liu, W. Screen-printed Carbon Electrode Modified with Commercial Multilayer Graphene for Lead Detection in Soybean Sauces by Differential Pulse Stripping Voltammetry. Int. J. Electrochem. Sci. 2016, 11, 1907–1917. [Google Scholar]
- William, J.; Hummers, S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Nasional, U.T. Preparation and Spectral Properties of Mixed-Ligand Complexes of VO(IV), Ni(II), Zn(II), Pd(II), Cd(II) and Pb(II) with Dimethylglyoxime and N-Acetylglycine. J. Chem. 2010, 7, 580–587. [Google Scholar]
- Jadhav, S.; Kulkarni, S.; Quadri, S. Ultrasound Assisted Synthesis and Physiochemical Investigation of Nickel-Dimethyl Glyoxime Complex. J. Chem. Chem. Sci. 2015, 5, 311–316. [Google Scholar]
- Cardoso, W.S.; Dias, V.L.N.; Costa, W.M.; De Araujo Rodrigues, I.; Marques, E.P.; Sousa, A.G.; Boaventura, J.; Bezerra, C.W.B.; Song, C.; Liu, H.; et al. Nickel-dimethylglyoxime complex modified graphite and carbon paste electrodes: Preparation and catalytic activity towards methanol/ethanol oxidation. J. Appl. Electrochem. 2009, 39, 55–64. [Google Scholar] [CrossRef]
- Godycki, L.E.; Rundle, R.E. The structure of nickel dimethylglyoxime. Acta Cryst. 1953, 1953, 487–495. [Google Scholar] [CrossRef]
- Bambenek, M.A.; Pflaum, R.T. The Reaction of Nickel with Dioximes. Inorg. Chem. 1963, 758, 24–27. [Google Scholar] [CrossRef]
- Olorundare, F.O.G.; Nkosi, D.; Arotiba, O.A. Voltammetric determination of nitrophenols at a nickel dimethylglyoxime complex-gold nanoparticle modified glassy carbon electrode. Int. J. Electrochem. Sci. 2016, 11, 7318–7332. [Google Scholar] [CrossRef]
- Anderson, S. Metal-metal bonds and physical properties of some nickel (II)-vic-dioxime complexes. Retrospective Theses and Dissertations. 1962. Paper 2035. Available online: http://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=3034&context=rtd (accessed on 25 July 2017).
- Baxter, L.; Bobrowski, A.; Bond, M.; Heath, G.; Paul, R.L.; Mrzljak, R.; Zarebski, J. Electrochemical and spectroscopic investigation of the reduction of dimethylglyoxime at mercury electrodes in the presence of cobalt and nickel. Anal. Chem. 1998, 70, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F. Voltammetric techniques of analysis: the essentials. ChemTexts 2015, 1, 17. [Google Scholar] [CrossRef]
- Taurino, I.; Carrara, S.; Giorcelli, M.; Tagliaferro, A.; De Micheli, G. Comparison of two different carbon nanotube-based surfaces with respect to potassium ferricyanide electrochemistry. Surf. Sci. 2012, 606, 156–160. [Google Scholar] [CrossRef]
- Carvalho, R.C.; Gouveia-caridade, C.; Brett, C.M.A. Glassy carbon electrodes modified by multiwalled carbon nanotubes and poly (neutral red): A comparative study of different brands and application to electrocatalytic ascorbate determination. Anal. Bioanal. Chem. 2010, 4, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.M.S.; Magalhães, J.M.C.S.; Soares, H.M.V.M. Simultaneous Determination of Nickel and Cobalt Using a Solid Bismuth Vibrating Electrode by Adsorptive Cathodic Stripping Voltammetry. Electroanalysis 2013, 25, 1247–1255. [Google Scholar] [CrossRef]








| Substrate | Analyte | Concentration (µg L−1) | Peak Current (A) | Std. Dev. (A) | % RSD |
|---|---|---|---|---|---|
| N-DMG-GCE | Co2+ and Zn2+ | 200 | N/D | N/D | N/D |
| Ni2+ | 20 | 1.12 × 10−7 | 1.12 × 10−8 | 3.72 | |
| Ni2+ in the presence of Co2+ and Zn2+ | 20 | 1.09 × 10−7 | 1.63 × 10−8 | 4.01 | |
| NGr-DMG-GCE | Co2+ and Zn2+ | 200 | N/D | N/D | N/D |
| Ni2+ | 20 | 1.94 × 10−6 | 1.06 × 10−7 | 5.46 | |
| Ni2+ in the presence of Co2+ and Zn2+ | 20 | 1.87 × 10−6 | 1.34 × 10−7 | 3.94 |
| Metal Ions | Substrate | Technique | Accumulation Time (min) | Dynamic Linear Range (µg L−1) | Detection Limit (µg L−1) | Reference | |
|---|---|---|---|---|---|---|---|
| In-situ DMG Electrodes | Ni2+ | mpBiF-SPCE | AdCSV | 180 | 1–10 | 0.027 | [28] |
| Co2+ | 1–10 | 0.094 | |||||
| Ni2+ | RBiABE | DPAdSV | 30 | 0.6–41 | 0.18 | [14] | |
| Co2+ | 0.06–4.1 | 0.018 | |||||
| Ni2+ | PbF-SPE | SWV | 60 | 0.6–2.9 | 0.2 | [15] | |
| Co2+ | 0.6–5.9 | 0.3 | |||||
| Ni2+ | SBVE | SW-AdCSV | 30 | 0–10 | 0.6 | [55] | |
| Chemically Modified DMG Electrodes | Ni2+ | NC-DMG-MFE | SWASV | 300 | 0.1–100 | 0.1 | [22] |
| Cu2+ | 1.0–80 | 1.0 | |||||
| Ni2+ | MCPE | AAdSV | 720 | 6–600 | 0.006 | [18] | |
| Ni2+ | DMG-CPE | DPV | 1500 | 80–600 | 27 | [20] | |
| Ni2+ | DMG-N/SPE | DPV | 120 | 60–500 | 30 | [16] | |
| Ni2+ | PVC-PA-DMG-GCE | SWAdCSV | 240 | 18–180 | 18 | [8] | |
| Ni2+ with Co2+ and Zn2+ | NGr-DMG-GCE | SWAdSV | 120 | 2–20 | 1.5 | This Work |
| Ni2+ Sample | Original (µg L−1) | Added (µg L−1) | Found (µg L−1) | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|
| Test Sample | N/D | 3.00 | 3.16 | 8.21 | 105 |
| Real Water Sample | N/D | 3.00 | 3.35 | 7.46 | 111 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokpas, K.; Jahed, N.; Baker, P.G.; Iwuoha, E.I. Complexation-Based Detection of Nickel(II) at a Graphene-Chelate Probe in the Presence of Cobalt and Zinc by Adsorptive Stripping Voltammetry. Sensors 2017, 17, 1711. https://doi.org/10.3390/s17081711
Pokpas K, Jahed N, Baker PG, Iwuoha EI. Complexation-Based Detection of Nickel(II) at a Graphene-Chelate Probe in the Presence of Cobalt and Zinc by Adsorptive Stripping Voltammetry. Sensors. 2017; 17(8):1711. https://doi.org/10.3390/s17081711
Chicago/Turabian StylePokpas, Keagan, Nazeem Jahed, Priscilla G. Baker, and Emmanuel I. Iwuoha. 2017. "Complexation-Based Detection of Nickel(II) at a Graphene-Chelate Probe in the Presence of Cobalt and Zinc by Adsorptive Stripping Voltammetry" Sensors 17, no. 8: 1711. https://doi.org/10.3390/s17081711