The Regular Interaction Pattern among Odorants of the Same Type and Its Application in Odor Intensity Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stimuli and Assessors
2.2. The Modified Vector Model
2.3. Experimental Apparatus
2.4. Experimental Procedures
3. Results and Discussion
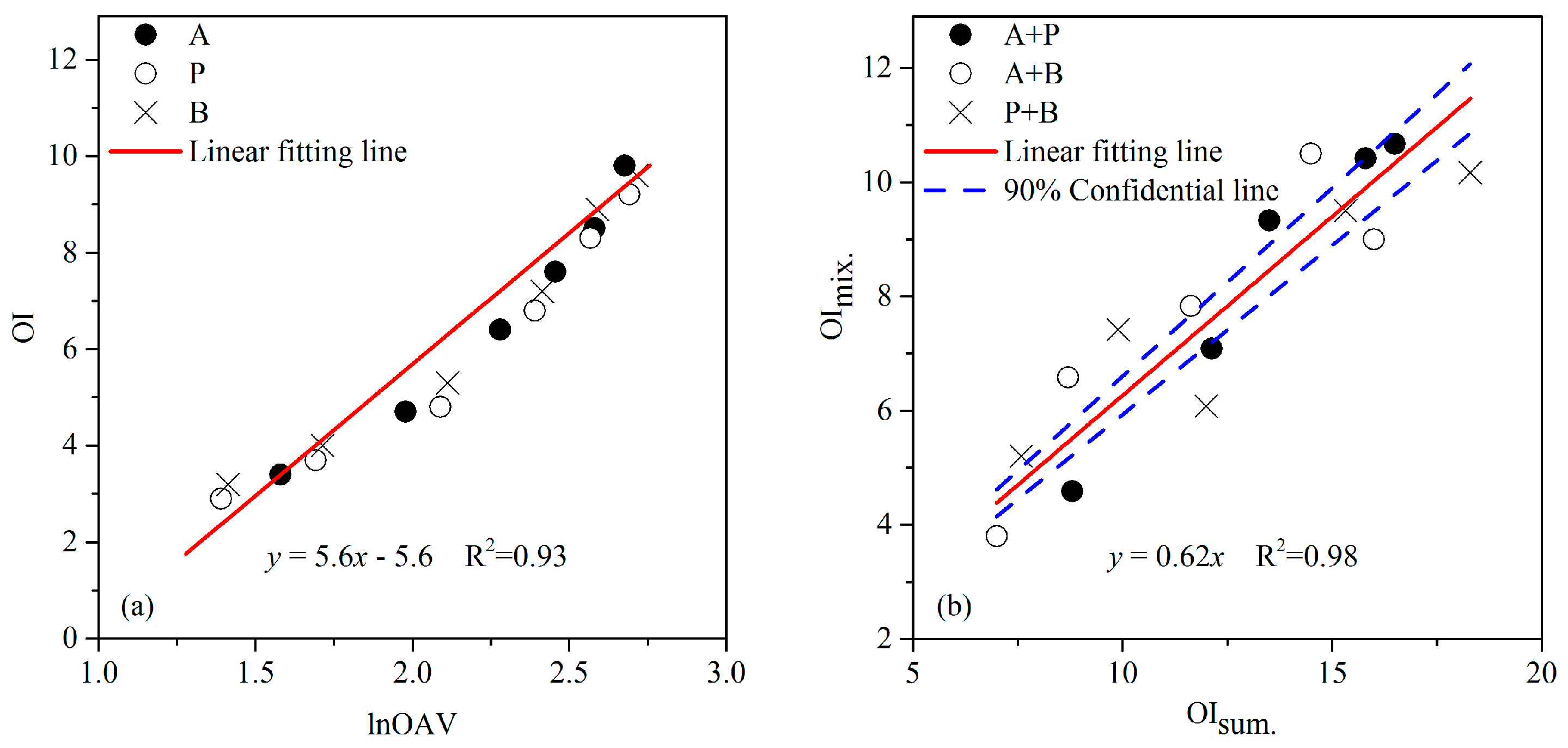
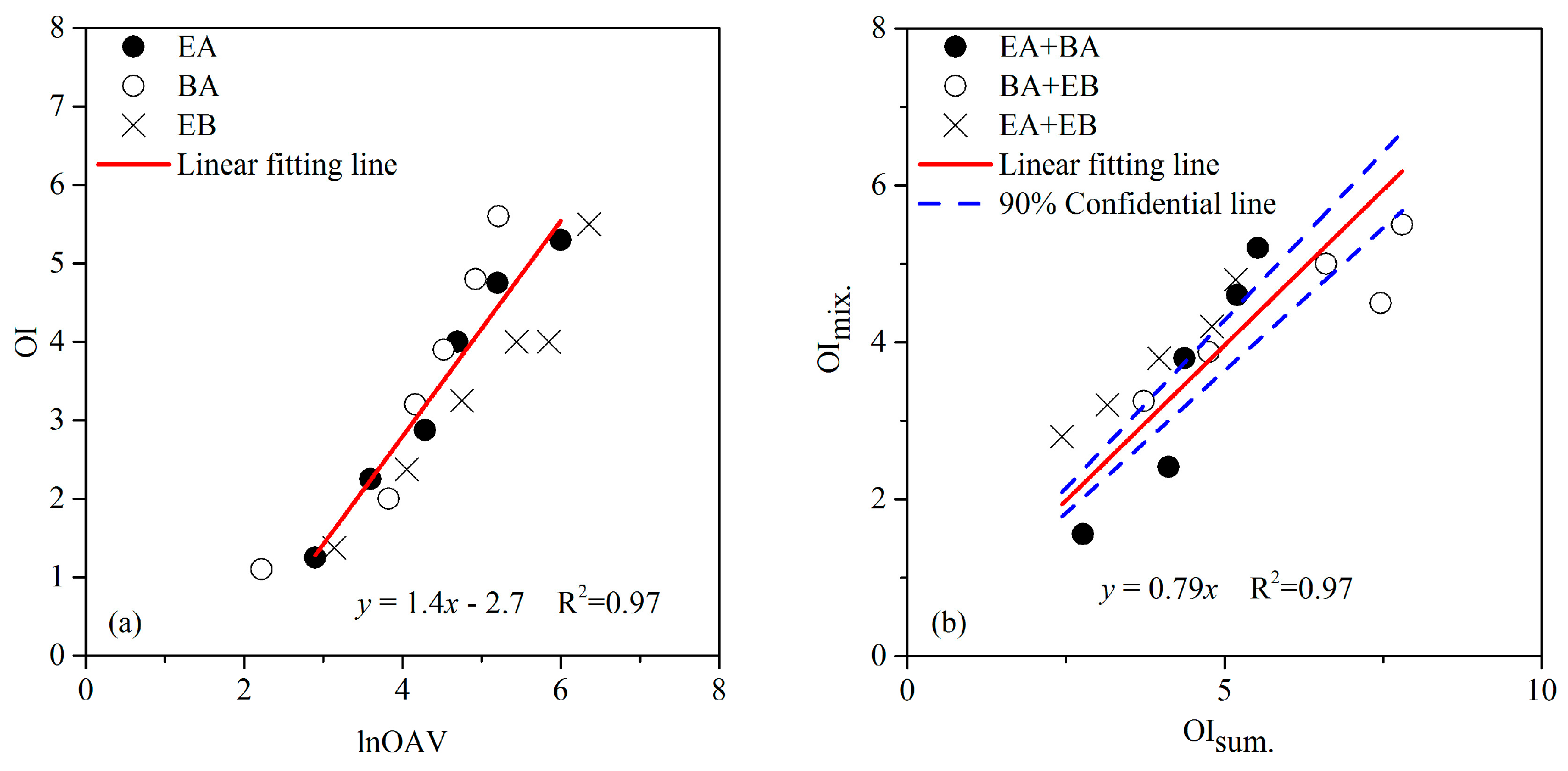
3.1. The MVM of Aldehydes
3.2. The MVM of Esters
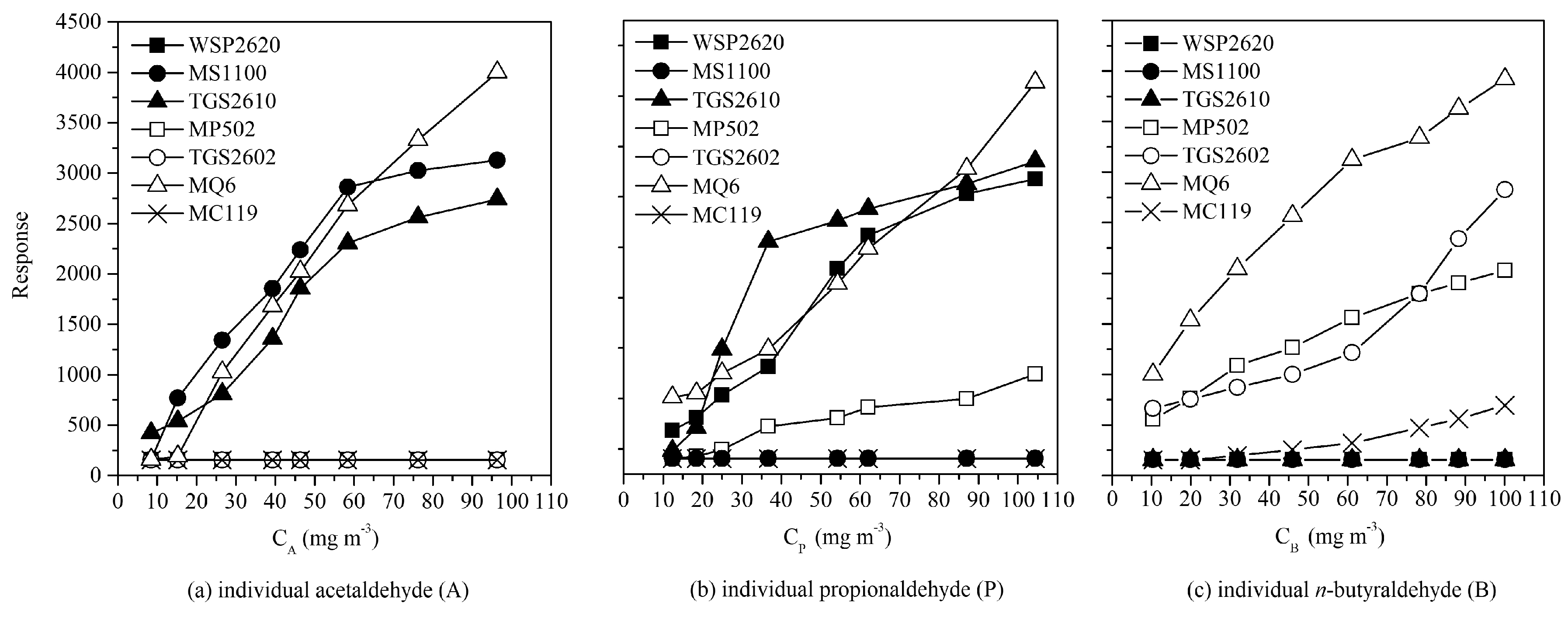
3.3. Sensor Array and BP Network Optimization
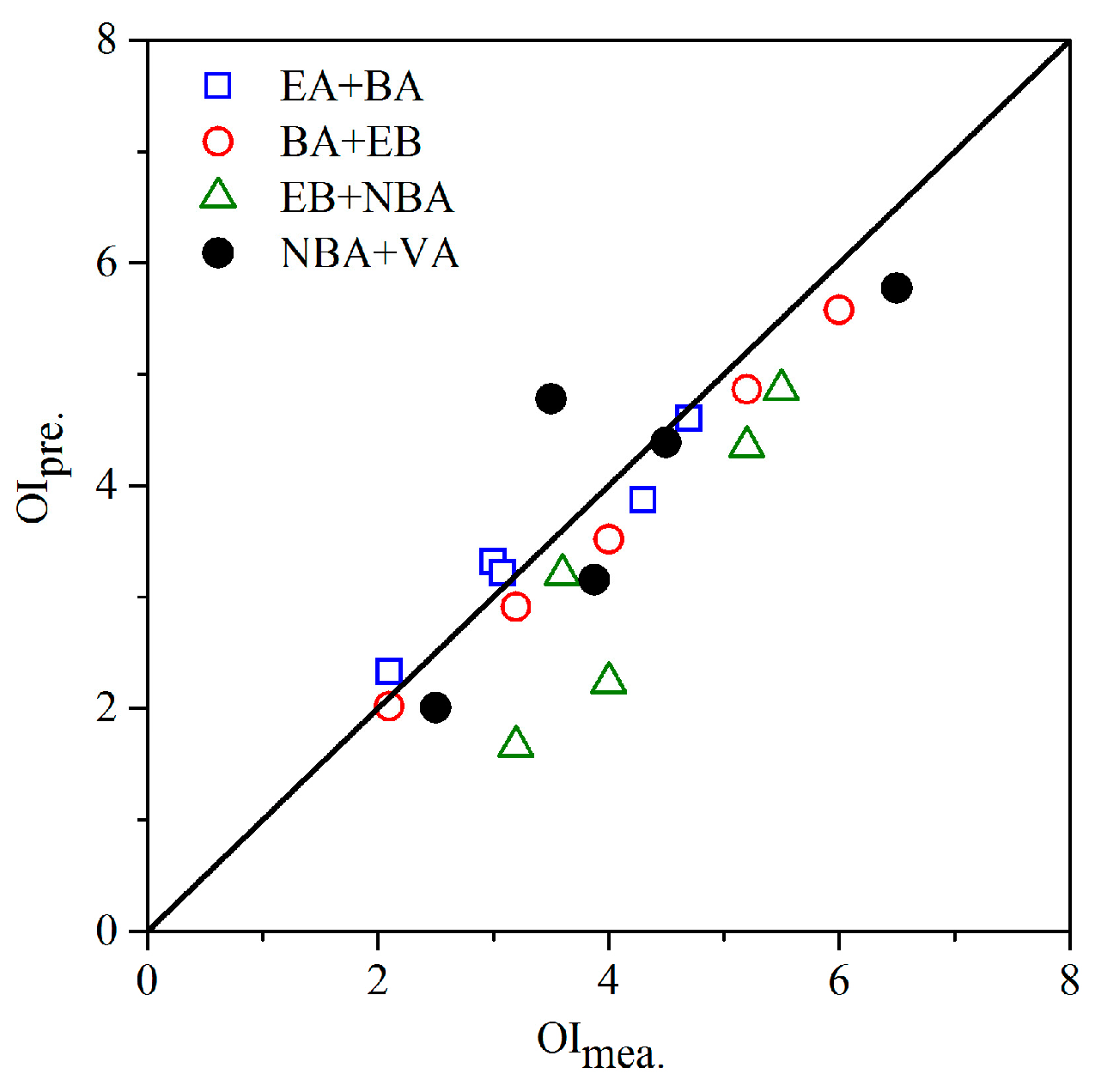
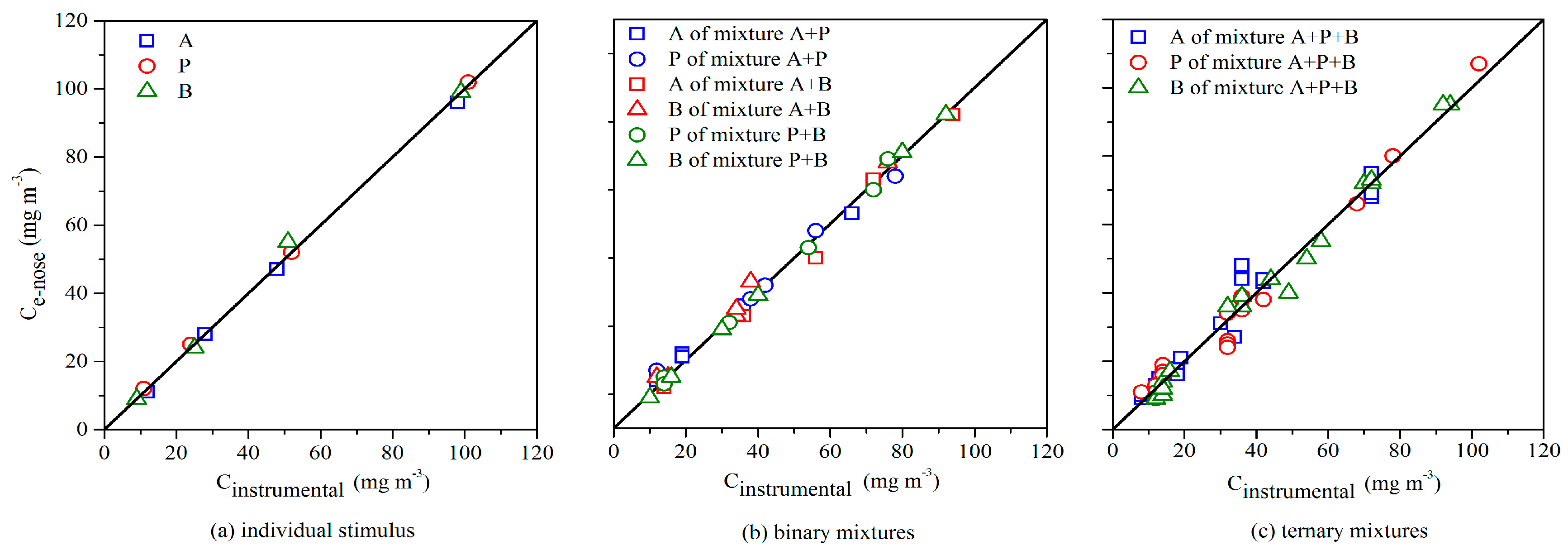
3.4. Odor Intensity Assessment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Y.J.; Lu, W.J.; Guo, H.W.; Ming, Z.Y.; Wang, C.; Xu, S.; Liu, Y.T.; Wang, H.T. Aromatic compound emissions from municipal solid waste landfill: Emission factors and their impact on air pollution. Atmos. Environ. 2016, 139, 205–213. [Google Scholar] [CrossRef]
- Lin, Z.J.; Norback, D.; Wang, T.T.; Zhang, X.; Shi, J.J.; Kan, H.D.; Zhao, Z.H. The first 2-year home environment in relation to the new onset and remission of asthmatic and allergic symptoms in 4246 preschool children. Sci. Total Environ. 2016, 553, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mao, I.F.; Chen, M.R.; Wang, L.; Chen, M.L.; Lai, S.C.; Tsai, C.J. Method development for determining the malodor source and pollution in industrial park. Sci. Total Environ. 2012, 437, 270–275. [Google Scholar] [CrossRef] [PubMed]
- De Blas, M.; Navazo, M.; Alonso, L.; Gangoiti, G.; Garcia, J.A.; de Camara, E.S.; Valdenebro, V.; Garcia-Ruiz, E.; Garcia-Borreguero, N. Continuous measurement of atmospheric reduced sulphur compounds as key tracers between odour complaints and source apportionment. Environ. Monit. Assess. 2017, 189, 102. [Google Scholar] [CrossRef] [PubMed]
- Lewkowska, P.; Dymerski, T.; Gebicki, J.; Namiesnik, J. The use of sensory analysis techniques to assess the quality of indoor air. Crit. Rev. Anal. Chem. 2017, 47, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Huang, H.B.; Xie, R.J.; Feng, Q.Y.; Fang, R.M.; Shu, Y.J.; Zhan, Y.J.; Ye, X.G.; Zhong, C. Enhanced degradation of gaseous benzene by a fenton reaction. Rsc Adv. 2017, 7, 71–76. [Google Scholar] [CrossRef]
- Zhi, R.; Zhao, L.; Zhang, D. A framework for the multi-level fusion of electronic nose and electronic tongue for tea quality assessment. Sensors 2017, 17, 1007. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, M. Bioinspired sensor systems. Sensors 2011, 11, 10180–10186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luo, D.; GholamHosseini, H.; Li, Z.; He, J. Identification of chinese herbal medicines with electronic nose technology: Applications and challenges. Sensors 2017, 17, 1073. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.; Benninga, M.A.; van Stein, R.M.; Brinkman, P.; Niemarkt, H.J.; de Boer, N.K.; de Meij, T.G. Effects of sampling conditions and environmental factors on fecal volatile organic compound analysis by an electronic nose device. Sensors 2016, 16, 1967. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic noses for environmental monitoring applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef] [PubMed]
- Giungato, P.; de Gennaro, G.; Barbieri, P.; Briguglio, S.; Amodio, M.; de Gennaro, L.; Lasigna, F. Improving recognition of odors in a waste management plant by using electronic noses with different technologies, gas chromatography-mass spectrometry/olfactometry and dynamic olfactometry. J. Clean Prod. 2016, 133, 1395–1402. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D.; Palleschi, G. How cutting-edge technologies impact the design of electrochemical (bio)sensors for environmental analysis: A review. Anal. Chim. Acta 2017, 959, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Buntat, Z.; Enzevaee, A.; Mirazimiabarghouei, S.J.; Bahadoran, M.; Shahidi, A.; Nikoukar, A. An analytical model and ann simulation for carbon nanotube based ammonium gas sensors. RSC Adv. 2014, 4, 36896–36904. [Google Scholar] [CrossRef]
- Gebicki, J.; Szulczynski, B.; Kaminski, M. Detection of authenticity of brand perfume using electronic nose prototypes. Meas. Sci. Technol. 2015, 26, 125103. [Google Scholar] [CrossRef]
- Wu, C.D.; Liu, J.M.; Yan, L.C.; Chen, H.Y.; Shao, H.Q.; Meng, T. Assessment of odor activity value coefficient and odor contribution based on binary interaction effects in waste disposal plant. Atmos. Environ. 2015, 103, 231–237. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, Y.H. Composition of key offensive odorants released from fresh food materials. Atmos. Environ. 2014, 89, 443–452. [Google Scholar] [CrossRef]
- Kim, K.H. Experimental demonstration of masking phenomena between competing odorants via an air dilution sensory test. Sensors 2010, 10, 7287–7302. [Google Scholar] [CrossRef] [PubMed]
- Amsellem, S.; Ohla, K. Perceived odor-taste congruence influences intensity and pleasantness differently. Chem. Senses 2016, 41, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cain, W.S.; Drexler, M. Scope and evaluation of odor counteraction and masking. Ann. N. Y. Acad. Sci. 1974, 237, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Rodriguez, O.; Rodrigues, A.E. Prediction model for the odor intensity of fragrance mixtures: A valuable tool for perfumed product design. Ind. Eng. Chem. Res. 2013, 52, 963–971. [Google Scholar] [CrossRef]
- Cain, W.S.; Schiet, F.T.; Olsson, M.J.; de Wijk, R.A. Comparison of models of odor interaction. Chem. Senses 1995, 20, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, J.M.; Fang, D.; Yan, L.C.; Wu, C.D. A novel electronic nose for simultaneous quantitative determination of concentrations and odor intensity analysis of benzene, toluene and ethylbenzene mixtures. RSC Adv. 2015, 5, 78686–78694. [Google Scholar] [CrossRef]
- Lindvall, T.; Svensson, L.T. A quantitative principle of perceived intensity summation in odor mixtures. J. Exp. Psychol. 1973, 100, 29–38. [Google Scholar] [PubMed]
- Yan, L.C.; Liu, J.M.; Fang, D. Use of a modified vector model for odor intensity prediction of odorant mixtures. Sensors 2015, 15, 5697–5709. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, A.; Maciejewska, M. Relationship between odour intensity assessed by human assessor and tgs sensor array response. Sens. Actuators B Chem. 2005, 106, 13–19. [Google Scholar] [CrossRef]
- Poivet, E.; Peterlin, Z.; Tahirova, N.; Xu, L.; Altomare, C.; Paria, A.; Zou, D.J.; Firestein, S. Applying medicinal chemistry strategies to understand odorant discrimination. Nat. Commun. 2016, 7, 11157. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y. Measurement of odor threshold by triangle odor bag method. In Odor Measurement Review; Japan Ministry of the Environment, Government of Japan: Tokyo, Japan, 2003; pp. 118–127. [Google Scholar]
- Odor Threshold Table for Chemicals. Available online: http://www.lbl.gov/ehs/chsp/html/odor_threshold.shtml (accessed on 30 June 2017).
- Flath, R.A.; Black, D.R.; Guadagni, D.G.; McFadden, W.H.; Schultz, T.H. Identification and organoleptic evaluation of compounds in delicious apple essence. J. Agric. Food Chem. 1967, 15, 29–38. [Google Scholar] [CrossRef]
- Abraham, M.H.; Sanchez-Moreno, R.; Cometto-Muniz, J.E.; Cain, W.S. An algorithm for 353 odor detection thresholds in humans. Chem. Senses 2012, 37, 207–218. [Google Scholar] [CrossRef] [PubMed]
- ASTM. E544-10 Standard Practices for Referencing Suprathreshold Odor Intensity; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Gutierrez, J.; Horrillo, M.C. Advances in artificial olfaction: Sensors and applications. Talanta 2014, 124, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H. The averaging effect of odorant mixing as determined by air dilution sensory tests: A case study on reduced sulfur compounds. Sensors 2011, 11, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Sanz, G.; Thomas-Danguin, T.; Hamdani, E.H.; Le Poupon, C.; Briand, L.; Pernollet, J.C.; Guichard, E.; Tromelin, A. Relationships between molecular structure and perceived odor quality of ligands for a human olfactory receptor. Chem. Senses 2008, 33, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Laska, M. Olfactory discrimination ability for aromatic odorants as a function of oxygen moiety. Chem. Senses 2002, 27, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bautze, V.; Bar, R.; Fissler, B.; Trapp, M.; Schmidt, D.; Beifuss, U.; Bufe, B.; Zufall, F.; Breer, H.; Strotmann, J. Mammalian-specific or 37 receptors are differentially activated by distinct odorous fatty aldehydes. Chem. Senses 2012, 37, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, M.; Jin, L. Data normalization to accelerate training for linear neural net to predict tropical cyclone tracks. Math. Probl. Eng. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Sun, Z.T.; Cheng, Z.W.; Wang, L.C.; Lou, Z.Y.; Zhu, N.W.; Zhou, X.J.; Feng, L.L. The typical MSW odorants identification and the spatial odorants distribution in a large-scale transfer station. Environ. Sci. Pollut. Res. Int. 2017, 24, 7705–7713. [Google Scholar] [CrossRef] [PubMed]

| Order | Odorant (Abbreviation) | CAS# | Chemical Structure | Reported Odor Threshold/ (mg/m3) | Measured Odor Threshold v/ (mg/m3) |
|---|---|---|---|---|---|
| 1 | Acetaldehyde (A) | 75-07-0 |  | 0.003 I/0.366 II | 0.039 |
| 2 | Propionaldehyde (P) | 123-38-6 |  | 0.002 I/0.376 II | 0.041 |
| 3 | n-Butyraldehyde (B) | 123-72-8 |  | 0.002 I/0.029 II | 0.052 |
| 4 | Ethyl acetate (EA) | 141-78-6 |  | 3.422 I/2.399 II | 0.276 |
| 5 | Butyl acetate (BA) | 123-86-4 |  | 0.083 I/0.034 II | 0.085 |
| 6 | Ethyl butyrate (EB) | 105-54-4 |  | 0.2 × 10−3 I/0.005 III | 0.053 |
| 7 | n-Butyl acrylate (NBA) | 141-32-2 |  | 0.017 II/0.003 IV | 0.038 |
| 8 | Vinyl acetate (VA) | 108-05-4 |  | 2.318 II/0.462 IV | 0.072 |
| Odor Intensity Levels | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | … | 8 | … | 12 | |
| 12-Point Scale | <10> | <20> | <40> | … | <1280> | … | <20480> |
| 8-Point Scale | <12> | <24> | <48> | … | <1550> | - | - |
| Subjects | Average Relative Error (ARE, %) | |||
|---|---|---|---|---|
| Individuals (n = 24) | Binary Mixtures (n = 75) | Ternary Mixtures (n = 51) | Mean Value | |
| a. Training functions | ||||
| trainlm | 5.0 | 10.0 | 19.4 | 11.5 |
| traingd | 6.0 | 10.1 | 21.0 | 12.4 |
| traingdm | 5.4 | 11.2 | 20.5 | 12.4 |
| traingdx | 6.0 | 10.2 | 26.2 | 14.1 |
| traingda | 8.0 | 12.1 | 21.7 | 13.9 |
| trainrp | 8.9 | 12.5 | 19.6 | 13.7 |
| b. Neuron quantity in the hidden layer | ||||
| 12 | 5.0 | 10.0 | 19.4 | 11.5 |
| 14 | 4.0 | 8.2 | 25.8 | 12.7 |
| 16 | 4.0 | 13.4 | 22.5 | 13.3 |
| 18 | 13.5 | 15.0 | 28.6 | 19.0 |
| 20 | 4.0 | 8.5 | 20.1 | 10.9 |
| 22 | 4.2 | 8.9 | 24.5 | 12.5 |
| c. Quantity of hidden layers | ||||
| 3 | 5.0 | 10.0 | 19.4 | 11.5 |
| 4 | 4.5 | 8.0 | 17.8 | 10.1 |
| 5 | 3.2 | 8.1 | 11.9 | 7.7 |
| 6 | 4.6 | 7.3 | 13.9 | 8.6 |
| 7 | 5.2 | 8.8 | 17.6 | 10.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Liu, J.; Jiang, S.; Wu, C.; Gao, K. The Regular Interaction Pattern among Odorants of the Same Type and Its Application in Odor Intensity Assessment. Sensors 2017, 17, 1624. https://doi.org/10.3390/s17071624
Yan L, Liu J, Jiang S, Wu C, Gao K. The Regular Interaction Pattern among Odorants of the Same Type and Its Application in Odor Intensity Assessment. Sensors. 2017; 17(7):1624. https://doi.org/10.3390/s17071624
Chicago/Turabian StyleYan, Luchun, Jiemin Liu, Shen Jiang, Chuandong Wu, and Kewei Gao. 2017. "The Regular Interaction Pattern among Odorants of the Same Type and Its Application in Odor Intensity Assessment" Sensors 17, no. 7: 1624. https://doi.org/10.3390/s17071624




