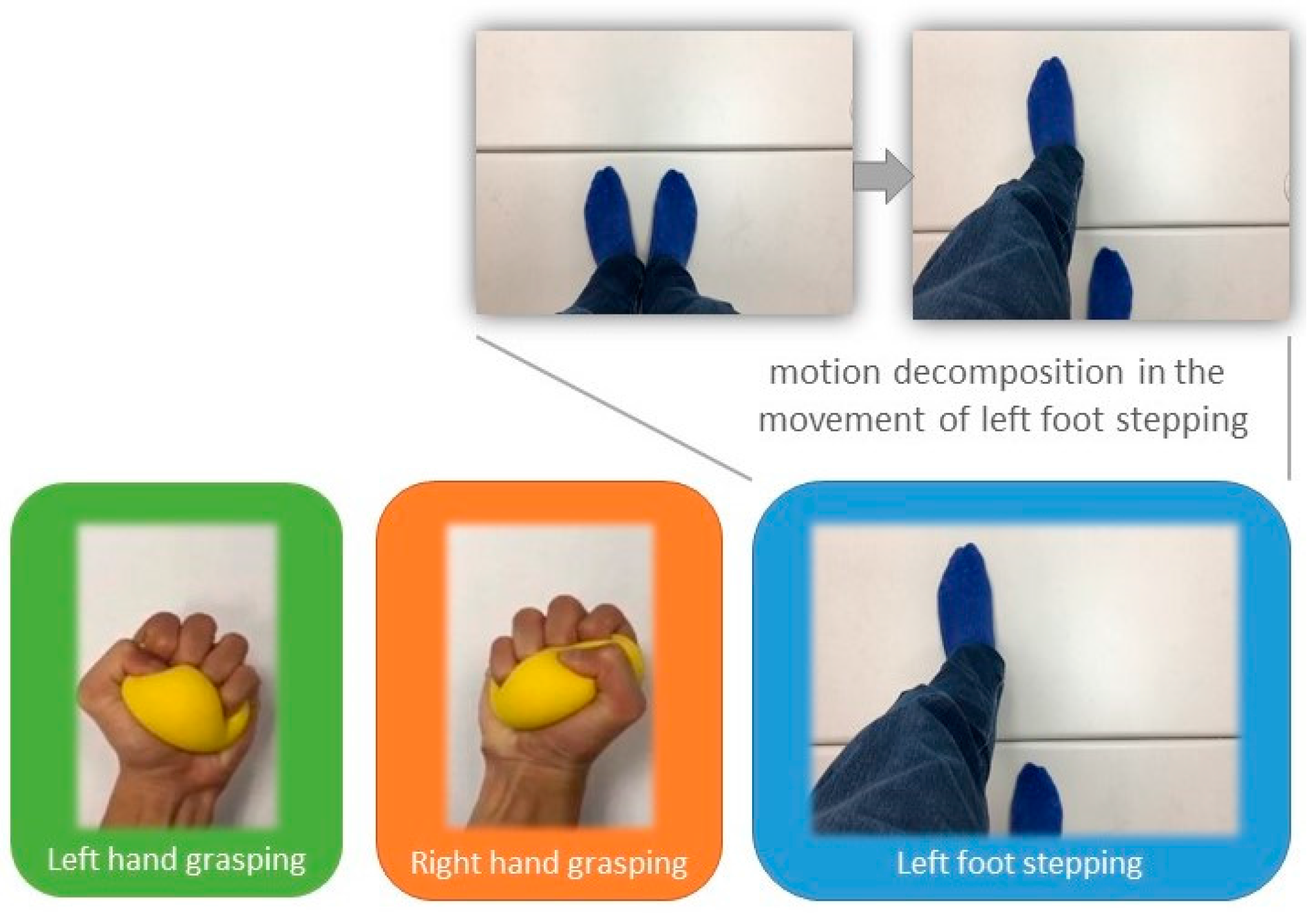
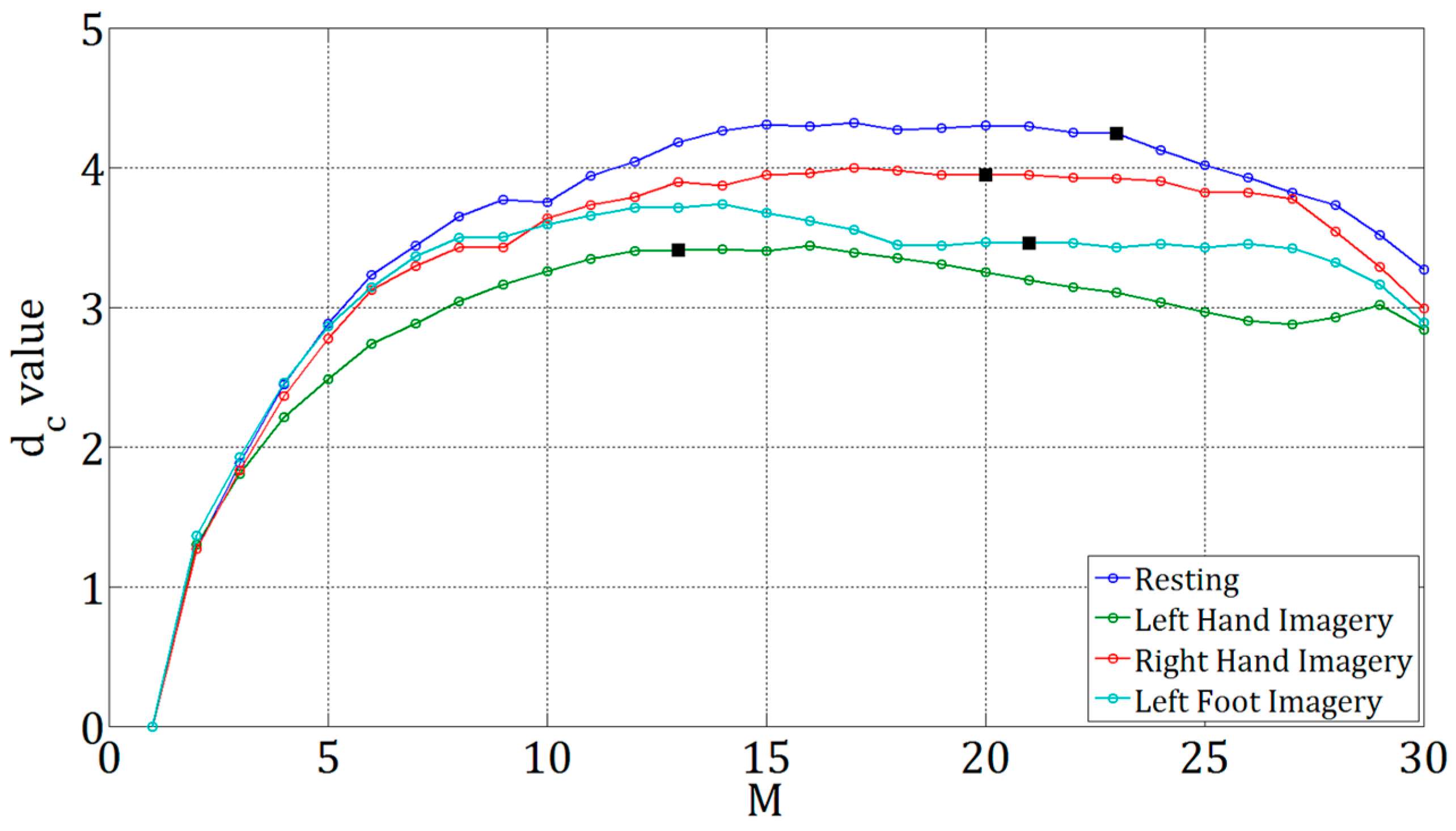
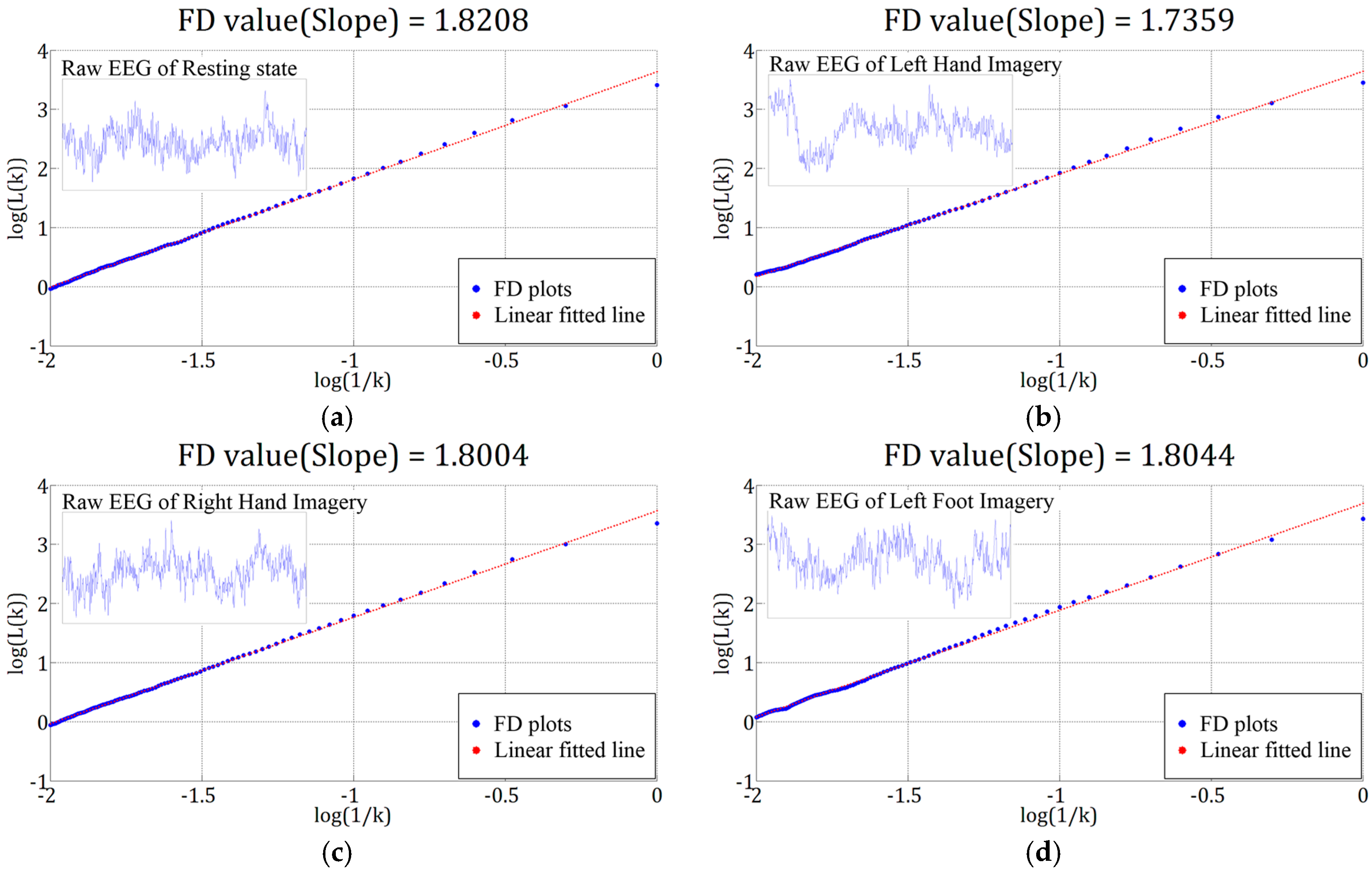
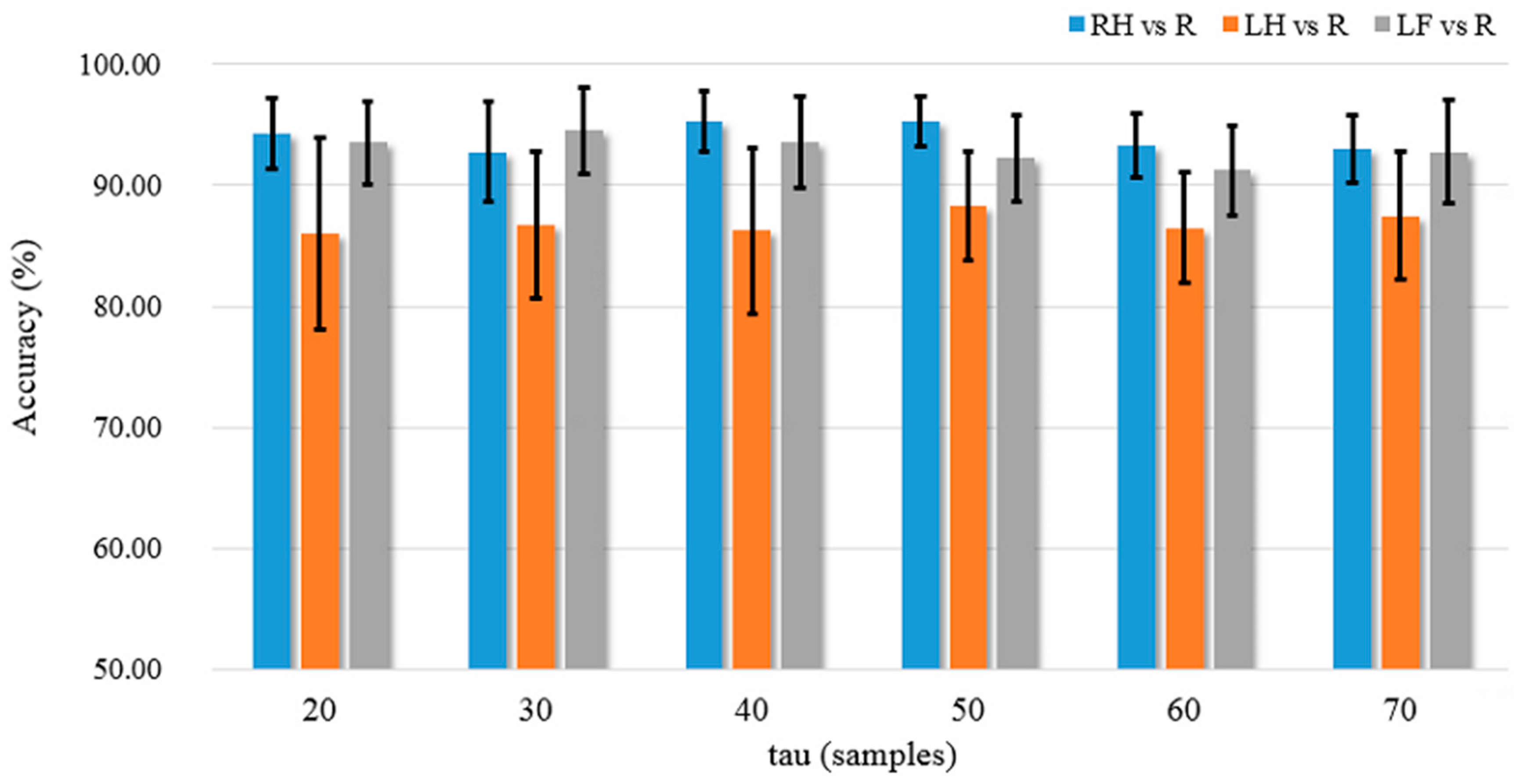
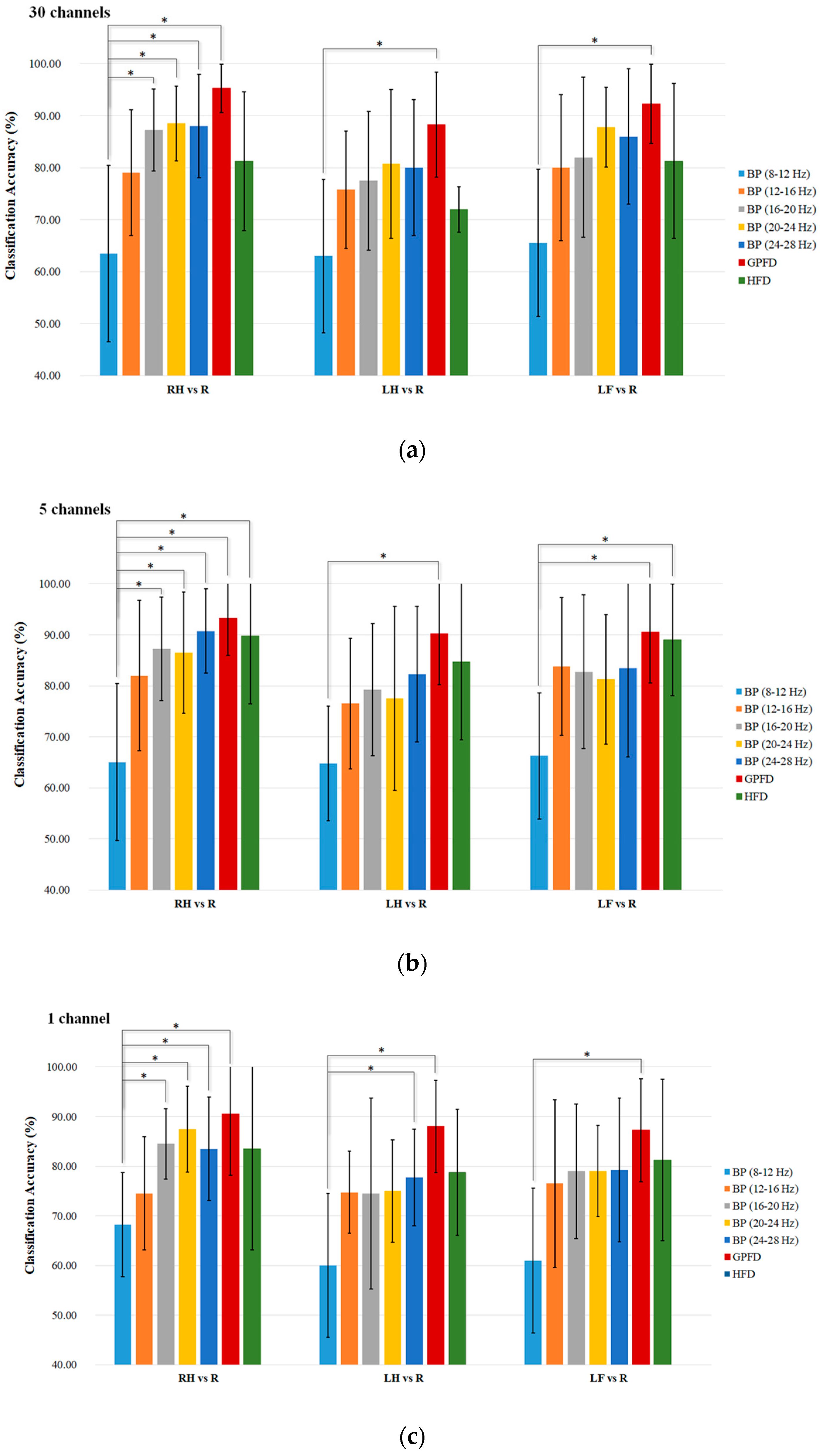
3.1. Results
The average
K-NN classification accuracies over the five patient participants among different feature extraction methods are listed in
Figure 7a–c, where the results shown in the three subplots are based on all 30 EEG channels (without channel selection), the top five channels, and the best channel, respectively. We used the Wilcoxon rank sum test to statistically examine the difference of classification accuracy between different feature extraction methods, because the one-sample Kolmogorov Smirnov test rejected the null hypothesis of normal distribution of the data (
p < 0.05).
Table 2,
Table 3 and
Table 4 list the top five channels and the corresponding Fisher values in RH-R, LH-R, and LF-R classification conditions. Different subbands in beta band may have different levels of beta rebound induced by a motor imagery [
25,
50]. Therefore, we divided the mu and beta bands into five subbands, and computed the BP of the five subbands for the purpose of comparison.
Among the BP features, the mu band performs the worst (only 60–70%) for all tasks and channel configurations. Overall, the high beta band of 20–28 Hz performs the best. Taking the RH-R classification task as an example, the BP of 24–28 Hz achieves high accuracy of 88% in 30-channel configuration (
Figure 7a), and accuracy of 90.75% in the configuration with five optimal channels (
Figure 7b). When using one channel, the BP of 20–24 Hz can still give high RH-R classification accuracy of 87.5%. In contrast, the GPFD method provides a higher accuracy than all the BPs and HFD, and the averaged classification accuracy of GPFD is significantly higher than that of the mu-band BP in all tasks and all channel configurations (
p < 0.05). Also, GPFD is still capable of maintaining a high accuracy even if the number of channels is largely reduced from 30 to 5, and even from 5 to 1. Taking RH-R classification as an example, the accuracies achieved by GPFD in 30, 5, and 1 channel configurations are 95.25%, 93.25%, and 90.50%, respectively. The performance drop is very limited, and the accuracy remains to be above 90% even if only one single channel (i.e., top channel) is used (
Figure 7c).
The above results have shown that the top five channels are able to achieve high accuracy. However, it is unclear whether the channels with the smallest Fisher scores are really noisy. Therefore, we further conducted an experiment to examine whether the Fisher’s criterion-based channel selection can remove noisy/redundant channels.
Table 5 shows the comparison of average
K-NN classification accuracies between the top-five-channel (listed in
Table 2,
Table 3 and
Table 4) and the worst-five-channel configurations, where the worst five EEG channels are the ones having the lowest Fisher score values among the 30 channels. The results show that most accuracies of the worst-five-channel configurations are close to chance level, and the accuracies of the top five channels are much higher. Taking RH-R classification as an example, the BP of 20–24 Hz only gives accuracy of 62.50% by the worst-five-channel configuration, while its accuracy achieves 86.50% when using the top five channels. Similarly, the difference of RH-R classification accuracy between the two configurations achieves almost 30% (93.25% − 65.50% = 27.75%) when the GPFD feature was used. The comparisons indicate that some brain regions are close to useless while some are very helpful for the discrimination between resting and motor imagery.
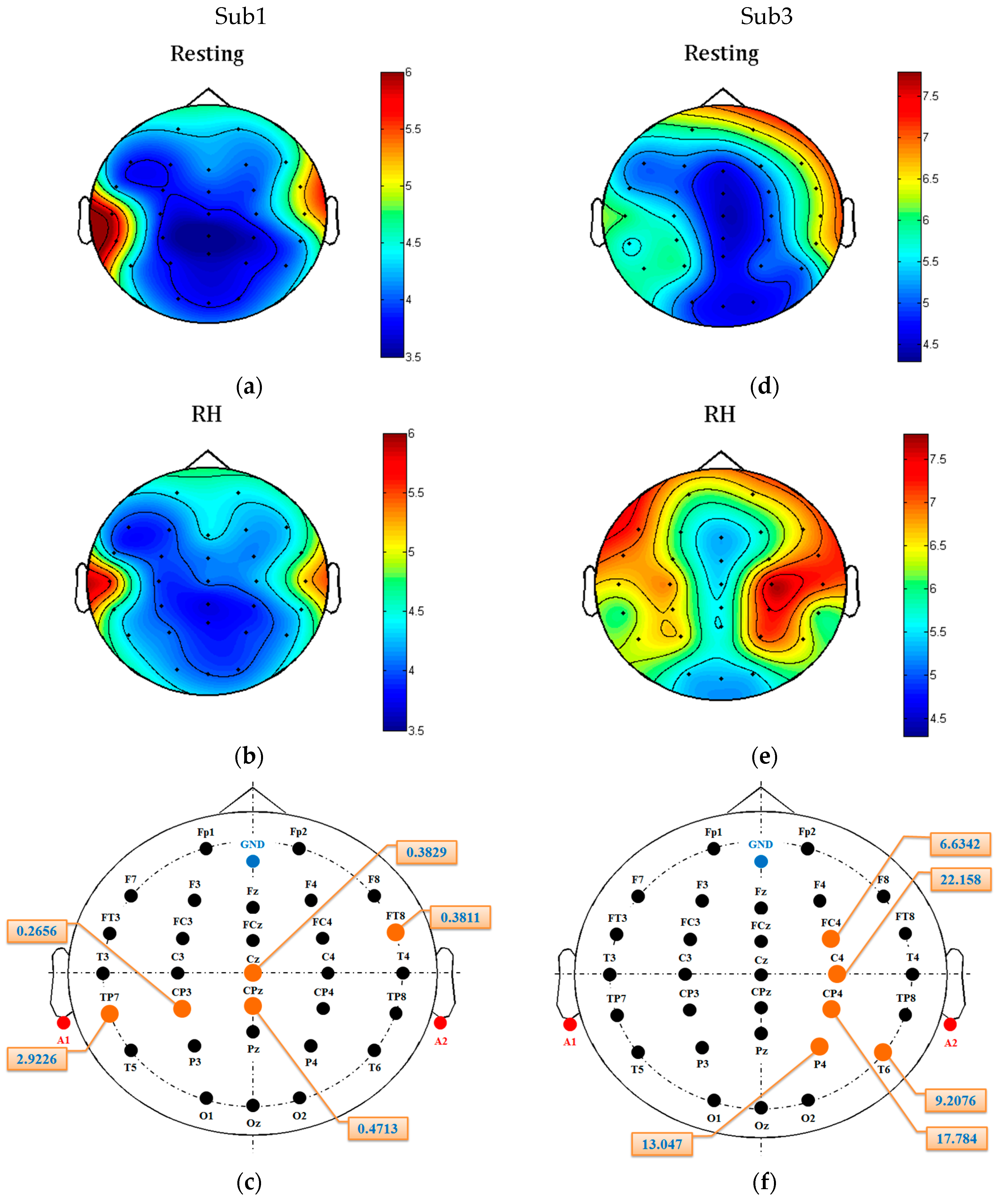
Figure 8 shows two patients’ GPFD topoplots of resting and RH imagery states. For patient Sub1, no significant changes can be observed in some areas, such as frontal and occipital. For patient Sub3, significant difference between the two states is observed from the area around C4, while some other regions such as occipital and the right prefrontal (FP2) show almost no difference.
Figure 8c and f show that the top-five-channel configurations for the two patients are different even if the features used are the same (i.e., GPFD). The above results and observations demonstrate that (1) channel selection is necessary, (2) best EEG channel configuration is patient-dependent, and (3) the best patient-dependent five-channel configuration obtained by the Fisher’s criterion is able to achieve high classification accuracy.
The results listed in
Figure 7 have shown that the GPFD method is superior to other methods. However, the classification accuracies in
Figure 7 are based on the simple classifier
K-NN. We conducted another experiment to see if the GPFD-based motor imagery classification performance in ALS can be improved by using the more sophisticated classifier LDA. According to
Table 6, LDA performs better than
K-NN in most cases. However, the improvement is very limited. The maximum improvement is only 2.25% (from 88.25% of
K-NN to 90.50% of LDA), occurring in the LH-R classification using 30 channels. One possible reason is that when a simple
K-NN classifier is used, the resulting classification accuracy based on GPFD feature is already very high (88.25%). Therefore, the space of the accuracy that can be improved by other more advanced classifiers like LDA is limited (only 100% − 88.25% = 11.75%), which might explain why LDA outperforms
K-NN by only 2.5%. However, the error reduction rate is high, because 11.75% − 9.50%/11.75% = 21.28%. From the error-reduction point of view, LDA improved a lot.
3.2. Discussion
No previous studies have been focused on the FD-based motor imagery classification for patients with ALS. Nevertheless, our results have shown the success of the use of FD (particularly the GPFD) as the feature for classification. As mentioned in
Section 1, the previous works on motor imagery classification studies involving ALS patients ([
9,
11]) were all based on the SMR feature, i.e., the mu and beta BPs of EEG signals recorded at the sensorimotor area. To compare with the previous approach, we calculated the features (BPs of five subbands within the alpha and beta bands, GPFD, HFD) of the EEG signals from five sensorimotor channels (C3, Cz, C4, CP3, CP4). The comparison of accuracies between the sensorimotor area and the top-five-channel configuration (listed in
Table 2,
Table 3 and
Table 4) is shown in
Figure 9, where
K-NN is used as the classifier, and the accuracies in the top five channels are the same as those in
Figure 7b. According to
Figure 9, it is clear that the BPs from the sensorimotor area do not provide satisfactory average classification accuracy (<80% in all cases). Our results are consistent with the ones of earlier study [
9] and the latest study [
11], where the accuracies are all below 80%. In [
9], the five patients’ classification accuracies of hand motor imagery vs. resting are inside of the range of 70–80%, and the accuracy is lower than 60% for a patient without receiving SMR training. In [
11], the average accuracy is around 60%. The results of our study and the previous studies [
9,
11] show that the BPs from the sensorimotor area are not robust features for the motor imagery classification in ALS. On the other hand, our RH-R and LF-R classification results show that the classification accuracy of high beta BPs (20–24 Hz and 24–28 Hz) from the sensorimotor area is higher than 70%, which is also much higher than the accuracies of around 60% reported in [
9,
11] in which the EEG features are also the BPs from the sensorimotor area. Such difference could be due to the fact that the classification in our study was processed offline while these studies [
9,
11] calculated classification using online feedbacks of motor imagery. Since offline analysis operates in a synchronous (cue-based) mode, the offline accuracy is often higher than the accuracy of online testing (i.e., asynchronous or self-paced mode).
The results in
Figure 9 also reveal that the top-five-channel configuration is more robust than the five channels within the sensorimotor area. For all features (BP, GPFD, HFD) and all classification tasks (RH-R, LH-R, LF-R), the top-five-channel configuration achieves a better average classification accuracy than the five channels within the sensorimotor area. For the BP feature of 12–16 Hz, the LH-R classification accuracy of top five channels is significantly higher than the accuracy of the five sensorimotor channels (
p < 0.05). For the BP feature of 24–28 Hz, the top-five-channel configuration also achieves a significantly higher accuracy in RH-R classification than the five sensorimotor channels (
p < 0.05). Moreover, we can see from
Table 2,
Table 3 and
Table 4 that most of top five channels do not fall within the sensorimotor area. Taking the patient Sub1 as an example (BP of 20–24 Hz, RH-R classification), the patient’s top five channels (TP7, T4, FP2, T3, O2) does not contain any from the sensorimotor area. All these comparison results suggest that the searching of best EEG recording sites for ALS patients should not be restricted within the sensorimotor area.
In this study, we have compared the performances between two FD methods (GPFD and HFD) with BPs of different subbands. The mean classification accuracies by the two FD methods were all different for all tasks, which was due to the fact that the two methods estimate the FD by different approaches. Moreover, GPFD achieved higher mean accuracies than HFD for all classification tasks and all channel configurations. One possible reason is that Higuchi’s method is very sensitive to noise [
51]. Such high noise sensitivity could limit the discriminating ability of the FD features estimated by Higuchi’s method, because in this study the inputs were signal-trial raw EG signals which had low signal-to-noise ratio.
We have compared the GPFD and FD with BP features of different subbands. In BCI studies, common spatial pattern (CSP) [
19,
50,
52,
53] and autoregressive model (AR) [
54] have been also widely used. CSP transforms a filtered multi-channel EEG
(
, where
denotes the number of channels) to new EEG signal
(
) by a transform
:
, where the signal
needs to be spectrally filtered in advance [
52]. The training phase of CSP is to obtain the matrix
(see the steps summarized in [
50]). The normalized log powers of the first
p and the last
p rows of the new signal matrix
are optimal for the discrimination between two classes. Therefore, a CSP feature vector consists of 2
p features, where
p is a free parameter of CSP method. AR is a time-domain feature extraction method which involves only one parameter, the order of the model. Given an order value, the ARM finds the best fit to the real EEG signal using the least-square method. Supposing that the order is set as
, then for each channel’s EEG signal there are
AR coefficients extracted. The AR coefficients from each of the
channels are concatenated to a feature vector of
dimension. The searching values for
include 2, 3, 4, 5, 6, and 7. In our experiment, the optimal CSP parameter and the AM parameter were all determined by the LOO-CV procedure determined in
Section 2.7. According to
Figure 7, GPFD is superior to other features. Therefore, here we only compared the GPFD with CSP and AR features, and the accuracies of other features (BP and HFD) are already shown in
Figure 7. The classification results based on
K-NN classifier are listed in
Table 7. According to
Table 7, the best RH-R classification accuracy in the three channel configurations (30, top five, and top one) are 95.25%, 93.25%, and 90.50%, respectively, which are all obtained by GPFD. However, beta-CSP, i.e., raw EEG signals were band-pass filtered by a 3-order Butterworth filter (13–30 Hz) and then sent into CSP for feature extraction, performs better than mu-CSP in two conditions (30 and top five channels). Also, beta-CSP is better than GPFD for LH-R classification when 30 channel were used. Overall, CSP and GPFD are comparable to each other in the condition of 30 channels. However, the results show that GPFD was much better than CSP in top-five-channel condition, especially for LF-R classification, where GPFD achieved a high accuracy of 90.50% while the accuracies of both mu-CSP and beta-CSP were all below 80%. On the other hand, CSP is based on the decomposition of the signal parameterized by a matrix that projects the signals in the original recording space to the ones in the surrogate sensor space. However, this decomposition relies on at least two EEG channels; otherwise the spatial covariance matrix of CSP cannot be decomposed (see [
55] for detailed formulation). In other words, at least two channels are required to record EEG signals when using the CSP method, which is its limitation in use. Therefore, the CSP method cannot be applied in top-one-channel condition, as shown in
Table 7 where N/A mean “not available”. This is one advantage of GPFD over CSP, and GPFD can still achieve high accuracy (90.50% in RH-R classification) even if only one channel was used. AR and mu-CSP gives similar accuracies. However, beta-CSP outperforms AR greatly. The comparison results listed in
Table 7 indicate that GPFD is more suitable in motor imagery classification for ALS patients, especially when there are only few channels used for EEG recording. Although the classifier design is beyond the scope of our study, it is expected that the classification accuracy can be further improved by using advanced classifiers, the convolutional neural networks (CNN) [
56] for example.
The successful implementation of GPFD feature extraction and Fisher’s criterion-based channel selection strategy has several implications for further development of motor imagery BCI systems for patients with ALS. First, the current study tested the accuracies for three different classification tasks (RH vs. R, LH vs. R, and LF vs. R). Each of the binary classifications can be the basis for further developing a two-state asynchronous BCI [
57] by which the ALS patients can respond to their family members’ questions (express “yes” for example) by performing a motor imagery, which can increase their quality of life. Such simple function is useful for late-stage ALS patients whose speaking function are severely impaired (for example the patient Sub5 in this study), and is extremely useful for ALS patients who are in the completely locked-in state. Currently, our methods were tested on the EEGs from the late-stage ALS patients who are still able to communicate (but very slow). It is unknown whether it is possible to successfully transfer the methods (including the EEG data collection paradigm) to completely locked-in ALS patients. However, a recent study has evidenced that cognitively intact patients ALS patients produced motor imagery decoding accuracies better than cognitively impaired patients [
9]. Moreover, in our data collection stage (
Figure 3), a voice instruction is presented before every motor imagery period. Thus, the locked-in patients may be able to follow the instruction to perform the instructed motor imagery tasks even if they are unable to watch the computer screen. Therefore, it is believed that there exists such possibility as long as their cognition process is still normal (can hear, and follow the voice instructions). Nevertheless, it should be further justified in the future. Second, this study introduced the Fisher’s separability measure to automatically select a set of discriminating channels which are patient-dependent. This can be applied to effectively and efficiently calibrate the best EEG recording sites for different users/patients before training the motor imagery classification model. Although the results have demonstrated that the best channel configuration is capable of achieving high accuracy, the accuracy is not necessarily optimal in practice. In other words, the best channel configuration is not necessarily the best. The main reason is that the Fisher’s separability criterion calculates the Fisher scores of the features (or channels) separately, and does not consider the joint class separability of multiple features [
42]. In other words, some features that do not have relatively high Fisher scores may provide high or even higher classification accuracy when these features are used together as the input to the classifier. To deal with this drawback, some advanced methods could be applied, such as the joint mutual information [
58] and the evolutionary computation algorithms like particle swarm optimization (PSO) [
59]. Last, our results have shown that high accuracy can be achieved by the use of low-density electrodes (5 or 1) with GPFD feature. However, the results of the methods were based on a commercialized EEG amplifier, which is very expensive. To improve the motor imagery BCI’s applicability, there is a need to develop a low-cost and portable EEG acquisition and preprocessing module so that the BCI system based on the proposed methods can really be used to help patients with ALS.