Design and Fabrication of a Ratiometric Planar Optode for Simultaneous Imaging of pH and Oxygen
Abstract
:1. Introduction
2. Experimental
2.1. Materials Preparation
2.2. Synthesis of Lipophilic QDs
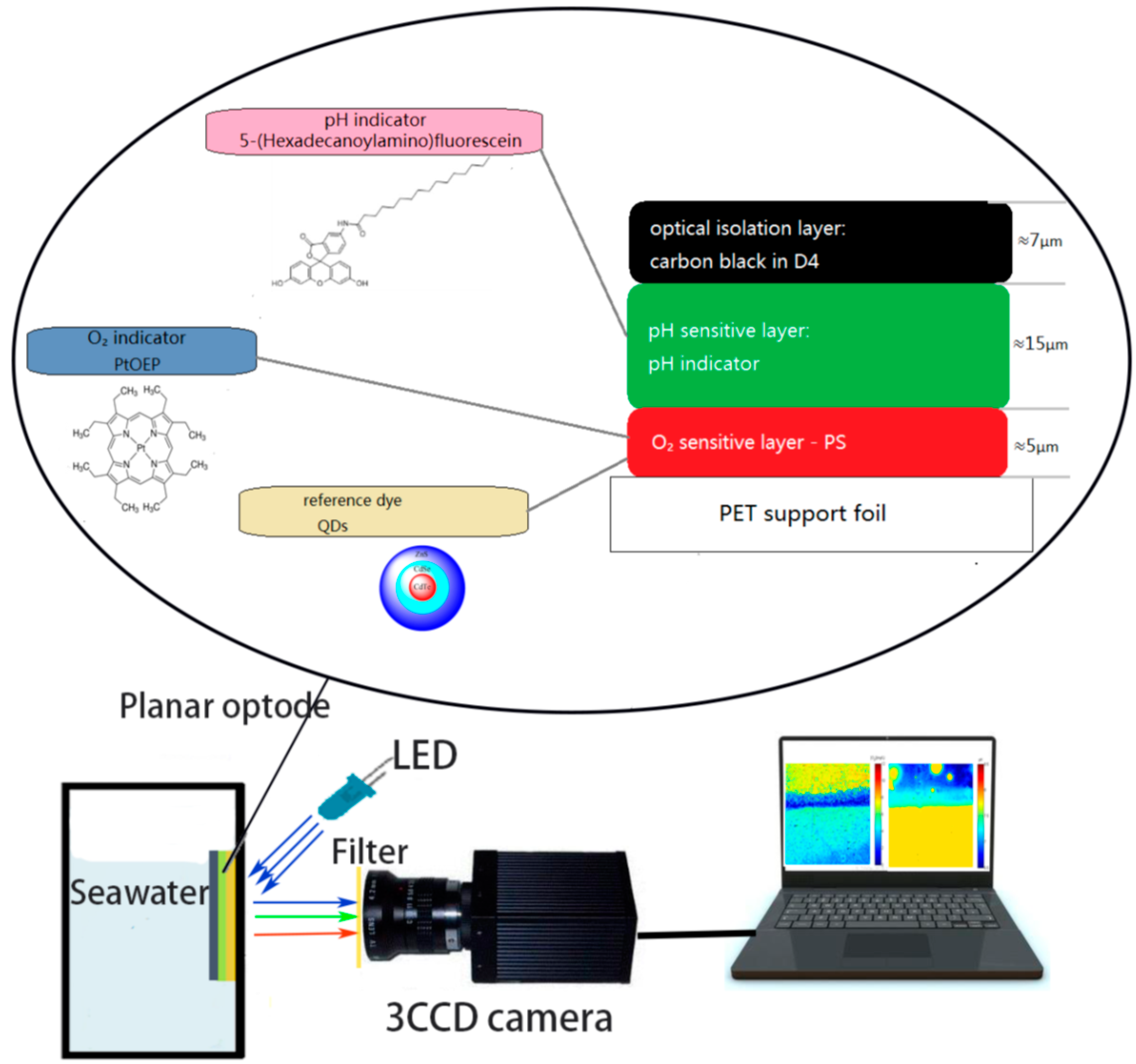
2.3. pH/Oxygen Planar Optode Fabrication
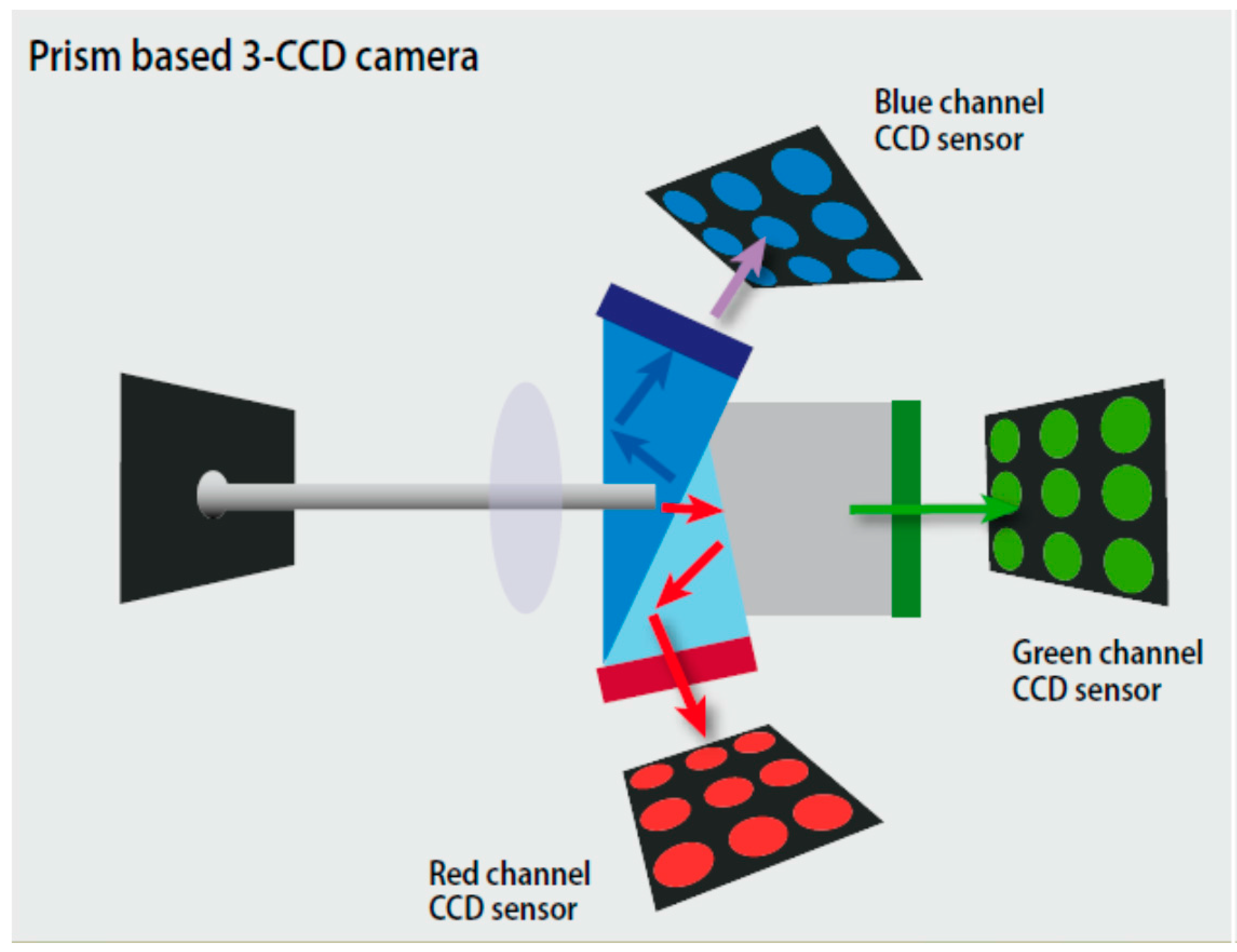
2.4. Instrument and Method
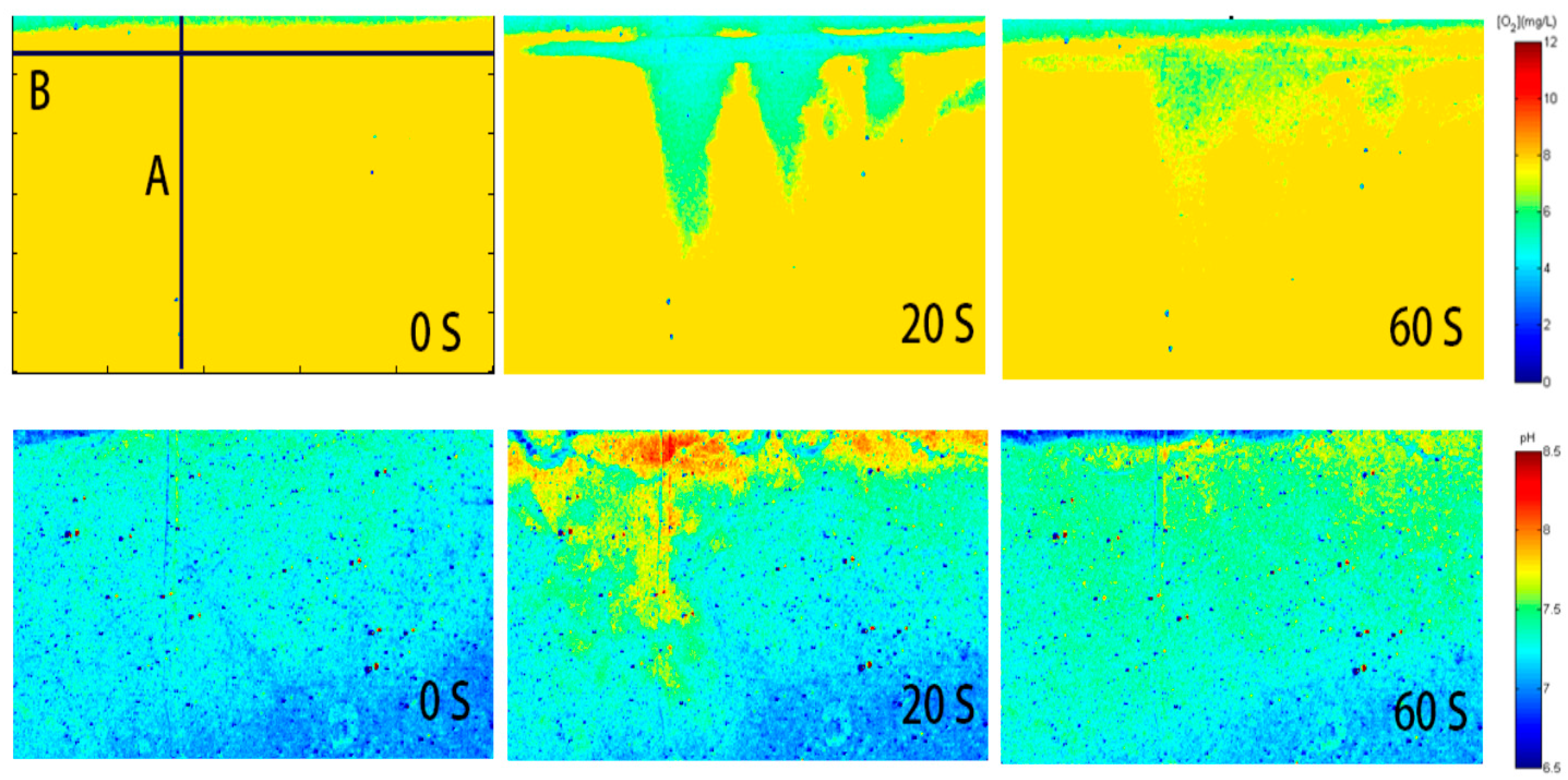
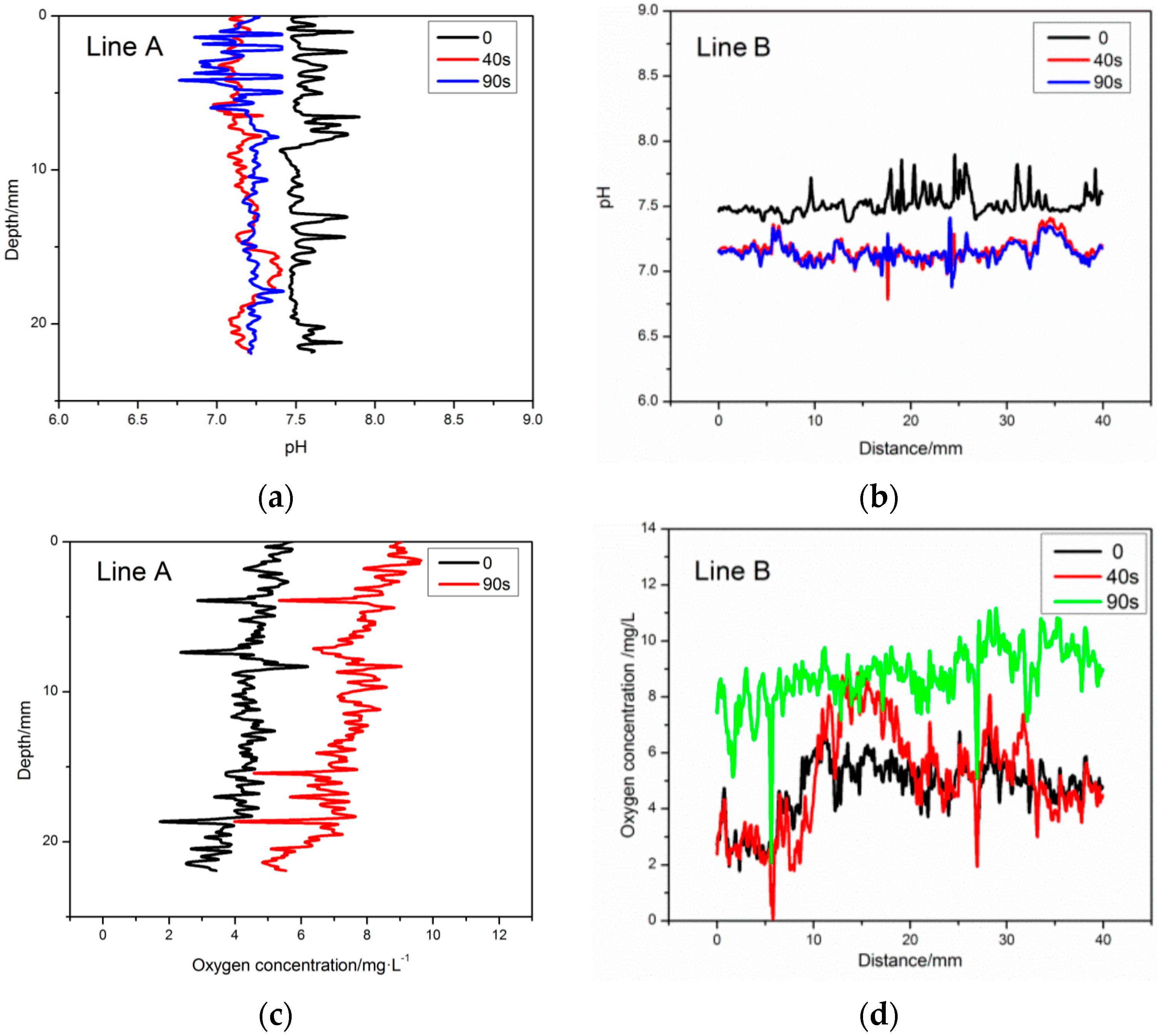
2.5. Simulated Rainfall Experiments
3. Results and Discussion
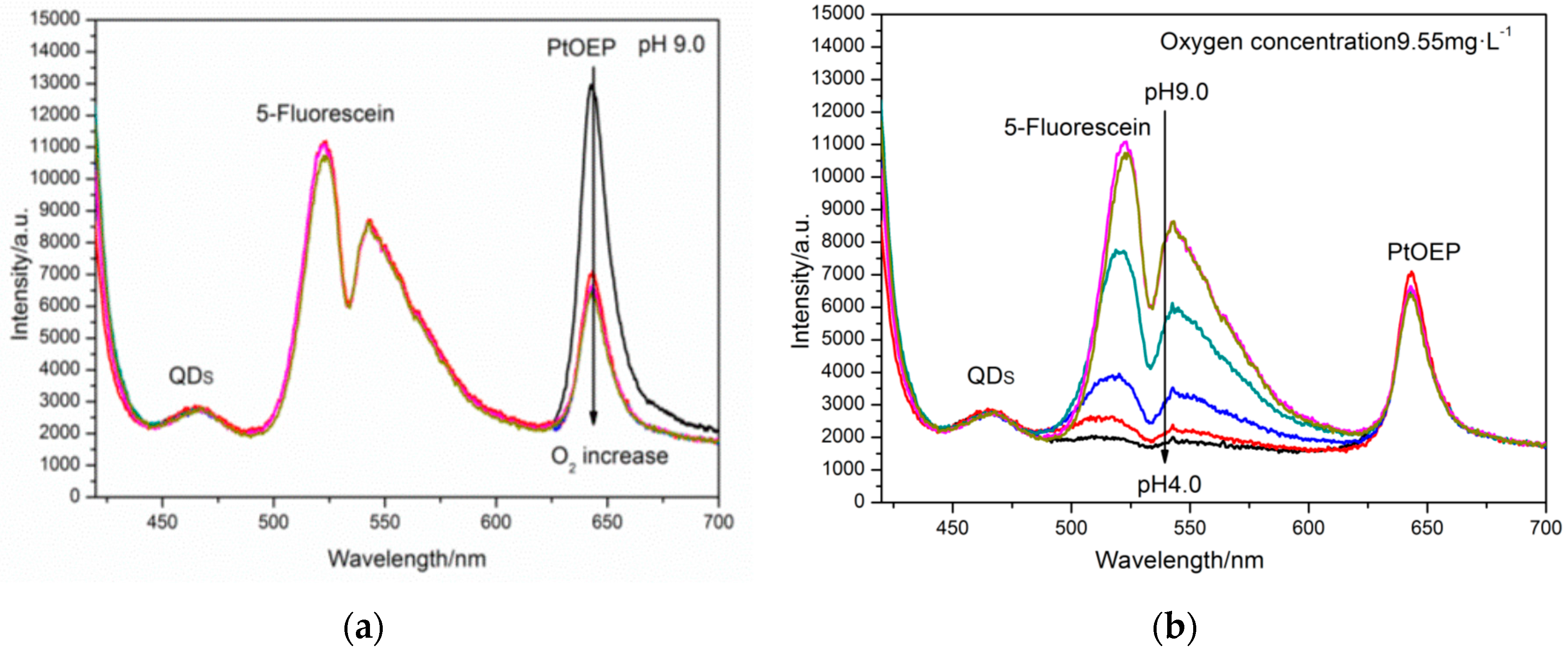
3.1. Optical Properties of Multiple pH/Oxygen Planar Optode
3.2. Calibration and Accuracy Evaluation of the pH/Oxygen Planar Optode
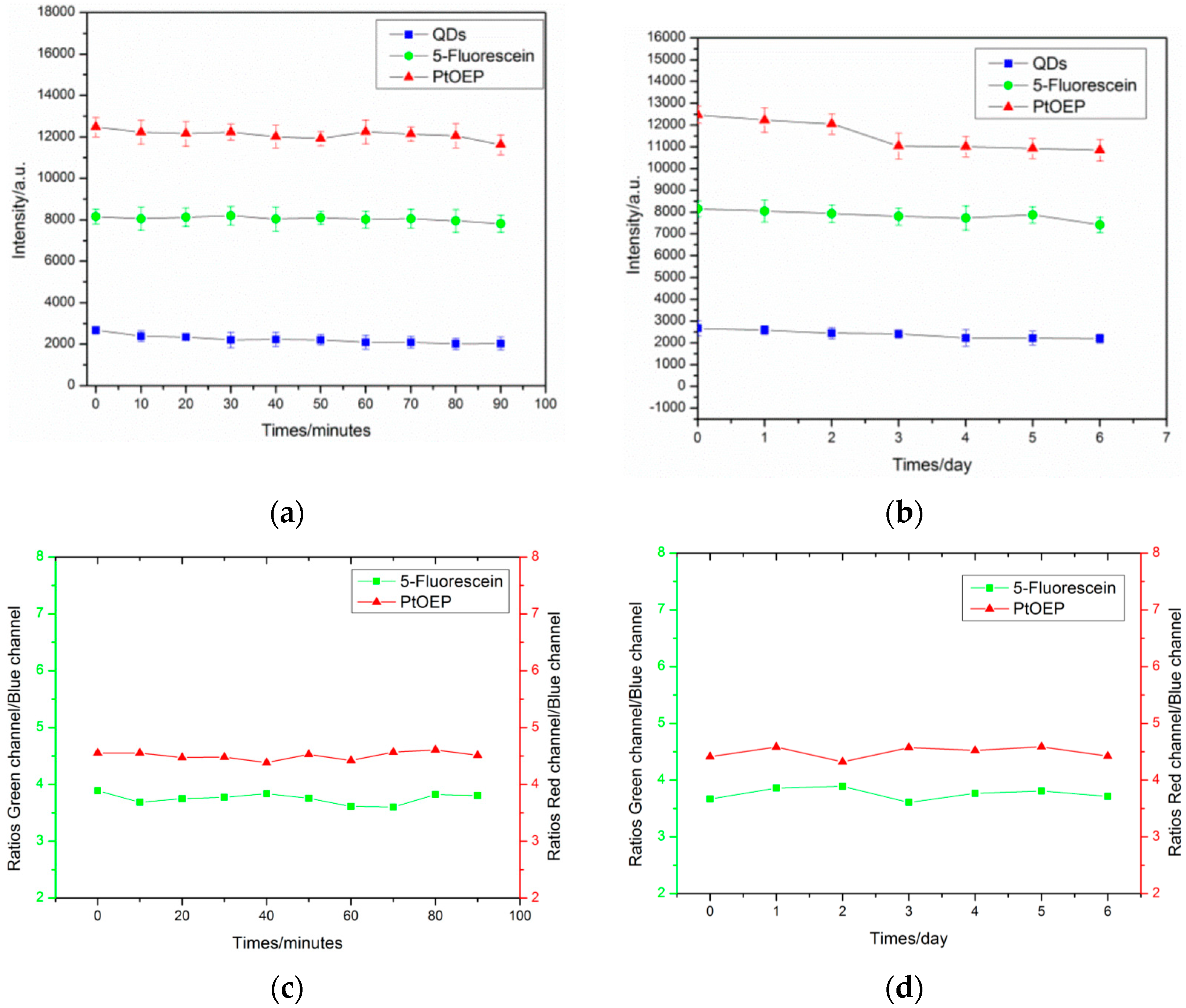
3.3. Long Term Stability and Photostability
3.4. Response Time of the pH/Oxygen Planar Optode
4. Application of the Planar Optode for Imaging oxygen and pH Simultaneously within Seawater
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DO | dissolved oxygen |
| PtOEP | Platinum(II) octaethylporphyrin |
| PS | polystyrene |
| 5-Fluorescein | 5-Hexadecanoylamino-fluorescein |
| D4 | Hydromed D4 |
| TOP | trioctylphosphine |
| OA | oleic acid |
| DC | direct current |
| ODE | octadecene |
| QDs | Quantumdots |
Appendix A. Matlab Script to Generate Colormap Images of Spatial Oxygen and pH
Im=imread('E:13.tif');
Imcut=imcrop(Im,[650,650,499,499]);
Imd=double(Imcut);
Imdr=Imd(:,:,1);
Imdg=Imd(:,:,2);
Imdb=Imd(:,:,3);
h=ones(3,3);
h(1,1) = 0;
h(1,3) = 0;
h(3,1) = 0;
h(1,3) =0;
Imdr = medfilt2(Imdr);
Imdg = medfilt2(Imdg);
Imdb = medfilt2(Imdb);
Imd2=zeros(m5,n5);
for i=1:m5
for j=1:n5
temp1=Imdr(i,j);
temp2=Imdb(i,j);
aver1=mean(temp1(:));
aver2=mean(temp2(:));
Imd2(i,j)=aver1/aver2; end
end
Idmd2=Imd2./I0;
for i=1:m5
for j=1:n5
if Imd2(i,j)>1
Imd2(i,j)=1;
end
end
end
do2=((1-A)./(Idmd2-A)-1)./K;
for i=1:m5
for j=1:n5
if do2(i,j)>14
do2(i,j)=14;
end
if do2(i,j)<=0
do2(i,j)=0;
end
end
end
imagesc(do2)
h=colorbar;
set(get(h,'Title'),'string','[O_2](mg/L)');
axis off
clear; clc;
Im=imread('E:12.tif');
Imcut=imcrop(Im,[590,420,500,500]);
Imd=double(Imcut);
m5=500
n5=500
Imdr=Imd(:,:,1);
Imdg=Imd(:,:,2);
Imdb=Imd(:,:,3);
h=ones(3,3);
h(1,1) = 0;
h(1,3) = 0;
h(3,1) = 0;
h(1,3) =0;
Imdr = medfilt2(Imdr);
Imdg = medfilt2(Imdg);
Imdb = medfilt2(Imdb);
Imd2=zeros(m5,n5);
for i=1:m5
for j=1:n5
temp1=Imdg(i,j);
temp2=Imdb(i,j);
aver1=mean(temp1(:));
aver2=mean(temp2(:));
Imd2(i,j)=temp1./temp2;
end
end
for i=1:m5
for j=1:n5
if Imd2(i,j)<0
Imd2(i,j)=0;
end
end
end
pH=7.13719+0.2310*log(-1.47284./(Imd2-2.67781)-1);
pH=real(pH)
imagesc(pH)
h=colorbar;
set(get(h,'Title'),'string','pH');
axis offReferences
- Glud, R.N.; Stahl, H.; Berg, P.; Wenzhofer, F.; Oguri, K.; Kitazato, H. In situ microscale variation in distribution and consumption of O2: A case study from a deep ocean margin sediment (Sagami Bay, Japan). Limnol. Oceanogr. 2009, 54, 723–734. [Google Scholar] [CrossRef]
- Glud, R.N.; Tengberg, A.; Kühl, M.; Hall, P.O.J.; Klimant, I. An in situ instrument for planar O2 optode measurements at benthic interfaces. Limnol. Oceanogr. 2001, 46, 2073–2080. [Google Scholar] [CrossRef]
- Larsen, M.; Borisov, S.M.; Grunwald, B.; Klimant, I.; Glud, R.N. A simple and inexpensive high resolution color ratiometric planar optode imaging approach: application to oxygen and pH sensing. Limnol. Oceanogr. Methods 2011, 9, 348–360. [Google Scholar] [CrossRef]
- Elgetti Brodersen, K.; Koren, K.; Lichtenberg, M.; Kühl, M. Nanoparticle-based measurements of pH and O2 dynamics in the rhizosphere of Zostera marina L.: Effects of temperature elevation and light-dark transitions. Plant Cell Environ. 2016, 39, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Brodersen, K.E.; Jakobsen, S.L.; Kühl, M. Optical sensor nanoparticles in artificial sediments–a new tool to visualize O2 dynamics around the rhizome and roots of seagrasses. Environ. Sci. Technol. 2015, 49, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Ingemann Jensen, S.; Kühl, M.; Glud, R.N.; Jørgensen, L.B.; Priemé, A. Oxic microzones and radial oxygen loss from roots of Zostera marina. Mar. Ecol.-Prog. Ser. Online 2005, 293, 49–58. [Google Scholar] [CrossRef]
- Rysgaard, S.; Glud, R.N.; Sejr, M.K.; Blicher, M.E.; Stahl, H.J. Denitrification activity and oxygen dynamics in Arctic sea ice. Polar Biol. 2008, 31, 527–537. [Google Scholar] [CrossRef]
- Glud, R.N.; Santegoeds, C.M.; De Beer, D.; Kohls, O.; Ramsing, N.B. Oxygen dynamics at the base of a biofilm studied with planar optodes. Aquat. Microb. Ecol. 1998, 14, 223–233. [Google Scholar] [CrossRef]
- Glud, R.N.; Kühl, M.; Kohls, O.; Ramsing, N.B. Heterogeneity of oxygen production and consumption in a photosynthetic microbial mat as studied by planar optodes. J. Phycol. 1999, 35, 270–279. [Google Scholar] [CrossRef]
- Behrens, J.W.; Stahl, H.J.; Steffensen, J.F.; Glud, R.N. Oxygen dynamics around buried lesser sandeels Ammodytes tobianus (Linnaeus 1785): mode of ventilation and oxygen requirements. J. Exp. Biol. 2007, 210, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Volkenborn, N.; Polerecky, L.; Wethey, D.S.; Woodin, S.A. Oscillatory porewater bioadvection in marine sediments induced by hydraulic activities of Arenicola marina. Limnol. Oceanogr. 2010, 55, 1231–1247. [Google Scholar] [CrossRef]
- Pischedda, L.; Poggiale, J.; Cuny, P.; Gilbert, F. Imaging oxygen distribution in marine sediments. The importance of bioturbation and sediment heterogeneity. Acta Biotheor. 2008, 56, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hulth, S.; Aller, R.C.; Engström, P.; Selander, E. A pH plate fluorosensor (optode) for early diagenetic studies of marine sediments. Limnol. Oceanogr. 2002, 47, 212–220. [Google Scholar] [CrossRef]
- Zhu, Q.; Aller, R.C.; Fan, Y. High-performance planar pH fluorosensor for two-dimensional pH measurements in marine sediment and water. Environ. Sci. Technol. 2005, 39, 8906–8911. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.H.; Breland, J.A. High precision multiwavelength pH determinations in seawater using cresol red. Deep Sea Res. Part A Oceanogr. Res. Pap. 1989, 36, 803–810. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, Z.; Pedersen, M.Ø.; Larsen, M. Rhizosphere O2 dynamics in young Zostera marina and Ruppia maritima. Mar. Ecol. 2015, 518, 95–105. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Glud, R.N.; Ramsing, N.B.; Gundersen, J.K.; Klimant, I. Planar optrodes: A new tool for fine scale measurements of two-dimensional O2 distribution in benthic communities. Mar. Ecol. Prog. Ser. 1996, 140, 217–226. [Google Scholar] [CrossRef]
- Rudolph-Mohr, N.; Vontobel, P.; Oswald, S.E. A multi-imaging approach to study the root-soil interface. Ann Bot 2014, 114, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Aller, R.C. Planar fluorescence sensors for two-dimensional measurements of H2S distributions and dynamics in sedimentary deposits. Mar. Chem. 2013, 157, 49–58. [Google Scholar] [CrossRef]
- Holst, G.; Kohls, O.; Klimant, I.; Konig, B.; Kuhl, M.; Richter, T. A modular luminescence lifetime imaging system for mapping oxygen distribution in biological samples. Sens. Actuators B Chem. 1998, 51, 163–170. [Google Scholar] [CrossRef]
- Holst, G.; Grunwald, B. Luminescence lifetime imaging with transparent oxygen optodes. Sens. Actuators B Chem. 2001, 74, 78–90. [Google Scholar] [CrossRef]
- Lu, H.; Jin, Y.; Tian, Y.; Zhang, W.; Holl, M.R.; Meldrum, D.R. New ratiometric optical oxygen and pH dual sensors with three emission colors for measuring photosynthetic activity in Cyanobacteria. J. Mater. Chem. 2011, 2011, 19293–19301. [Google Scholar] [CrossRef] [PubMed]
- Staal, M.; Prest, E.I.; Vrouwenvelder, J.S.; Rickelt, L.F.; Kuhl, M. A simple optode based method for imaging O2 distribution and dynamics in tap water biofilms. Water Res. 2011, 45, 5027–5037. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Kühl, M. A simple laminated paper-based sensor for temperature sensing and imaging. Sens. Actuators B 2015, 210, 124–128. [Google Scholar] [CrossRef]
- Wootton, C. A Practical Guide to Video and Audio Compression: From Sprockets and Rasters to Macroblocks; Taylor & Francis: Crowborough, UK, 2005. [Google Scholar]
- Wang, X.D.; Stolwijk, J.A.; Lang, T.; Sperber, M.; Meier, R.J.; Wegener, J.; Wolfbeis, O.S. Ultra-small, highly stable, and sensitive dual nanosensors for imaging intracellular oxygen and pH in cytosol. J. Am. Chem. Soc. 2012, 134, 17011–17014. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Vasylevska, A.S.; Krause, C.; Wolfbeis, O.S. Composite luminescent material for dual sensing of oxygen and temperature. Adv. Funct. Mater. 2006, 16, 1536–1542. [Google Scholar] [CrossRef]
- Moßhammer, M.; Strobl, M.; Kühl, M.; Klimant, I.; Borisov, S.M.; Koren, K. Design and Application of an Optical Sensor for Simultaneous Imaging of pH and Dissolved O2 with Low Cross-Talk. ACS Sens. 2016, 1, 681–687. [Google Scholar] [CrossRef]
- Danek, M.; Jensen, K.F.; Murray, C.B.; Bawendi, M.G. Synthesis of luminescent thin-film CdSe/ZnSe quantum dot composites using CdSe quantum dots passivated with an overlayer of ZnSe. Chem. Mater. 1996, 8, 173–180. [Google Scholar] [CrossRef]
- Wang, X.D.; Chen, X.; Xie, Z.X.; Wang, X.R. Reversible optical sensor strip for oxygen. Angew. Chem. 2008, 120, 7560–7563. [Google Scholar] [CrossRef]
- Han, M.; Gao, X.; Su, J.Z.; Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Uyeda, H.T.; Medintz, I.L.; Jaiswal, J.K.; Simon, S.M.; Mattoussi, H. Synthesis of compact multidentate ligands to prepare stable hydrophilic quantum dot fluorophores. J. Am. Chem. Soc. 2005, 127, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.; Zappa, C.J.; Meinen, C.S.; Christian, J.R.; Ho, D.T.; Dickson, A.G.; McGillis, W.R. Rain impacts on CO2 exchange in the western equatorial Pacific Ocean. Geophys. Res. Lett. 2010, 37, L23610. [Google Scholar] [CrossRef]
- Cunliffe, M.; Engel, A.; Frka, S.; Gasparovic, B.; Guitart, C.; Murrell, J.C.; Salter, M.; Stolle, C.; Upstill-Goddard, R.; Wurl, O. Sea surface microlayers: A unified physicochemical and biological perspective of the air-ocean interface. Prog. Oceanogr. 2013, 109, 104–116. [Google Scholar] [CrossRef]
- Bullen, C.R.; Mulvaney, P. Nucleation and growth kinetics of CdSe nanocrystals in octadecene. Nano Lett. 2004, 4, 2303–2307. [Google Scholar] [CrossRef]
- Stern, O.; Volmer, M. Über die abklingzeit der fluoreszenz. Phys. Z. 1919, 20, 183–188. [Google Scholar]
- Schroder, C.R.; Polerecky, L.; Klimant, I. Time-resolved pH/pO2 mapping with luminescent hybrid sensors. Anal. Chem. 2007, 79, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Okura, I. Photoluminescent determination of oxygen using metalloporphyrin-polymer sensing systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 91–100. [Google Scholar] [CrossRef]
- Tang, Y.; Tehan, E.C.; Tao, Z.Y.; Bright, F.V. Sol-gel-derived sensor materials that yield linear calibration plots, high sensitivity, and long-term stability. Anal. Chem. 2003, 75, 2407–2413. [Google Scholar] [CrossRef] [PubMed]


| Oxygen Electrodes | pH/Oxygen Planar Optode | |||
|---|---|---|---|---|
| Measured | Calculated | AE | RE | SD |
| 0.01 | 0.0096 | 0.0004 | 4.00% | 0.0038 |
| 4 | 3.884 | 0.116 | 2.90% | 0.1244 |
| 7 | 7.432 | 0.432 | 6.17% | 0.3102 |
| 9.34 | 9.974 | 0.634 | 6.79% | 0.7352 |
| 10.59 | 9.812 | 0.778 | 7.35% | 0.6321 |
| pH Electrodes | pH/Oxygen Planar Optode | |||
|---|---|---|---|---|
| Measured | Calculated | AE | RE | SD |
| 4.23 | 3.92 | 0.31 | 7.32% | 0.0462 |
| 4.89 | 4.63 | 0.26 | 5.32% | 0.1487 |
| 5.56 | 5.80 | 0.24 | 3.72% | 0.1292 |
| 6.45 | 6.27 | 0.18 | 2.79% | 0.3043 |
| 7.75 | 7.45 | 0.3 | 3.87% | 0.2945 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Yu, X.; Hao, Y. Design and Fabrication of a Ratiometric Planar Optode for Simultaneous Imaging of pH and Oxygen. Sensors 2017, 17, 1316. https://doi.org/10.3390/s17061316
Jiang Z, Yu X, Hao Y. Design and Fabrication of a Ratiometric Planar Optode for Simultaneous Imaging of pH and Oxygen. Sensors. 2017; 17(6):1316. https://doi.org/10.3390/s17061316
Chicago/Turabian StyleJiang, Zike, Xinsheng Yu, and Yingyan Hao. 2017. "Design and Fabrication of a Ratiometric Planar Optode for Simultaneous Imaging of pH and Oxygen" Sensors 17, no. 6: 1316. https://doi.org/10.3390/s17061316






