Study of ZnS Nanostructures Based Electrochemical and Photoelectrochemical Biosensors for Uric Acid Detection †
Abstract
:1. Introduction
2. Experimental Details
2.1. Reagents
2.2. Synthesis of ZnS Nanostructures and Characterization
2.3. Fabrication of Biosensors and Measurements
3. Results and Discussion
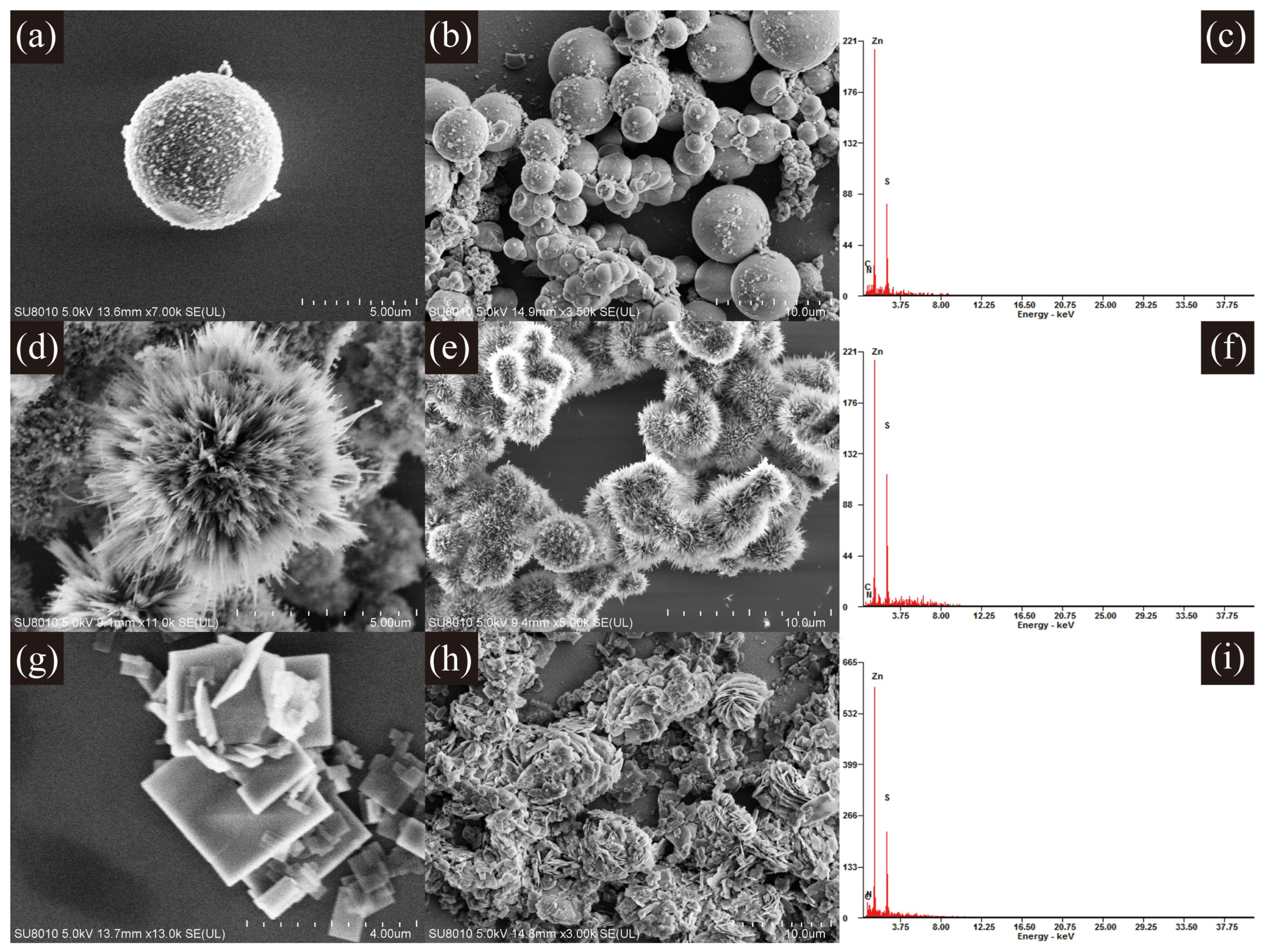
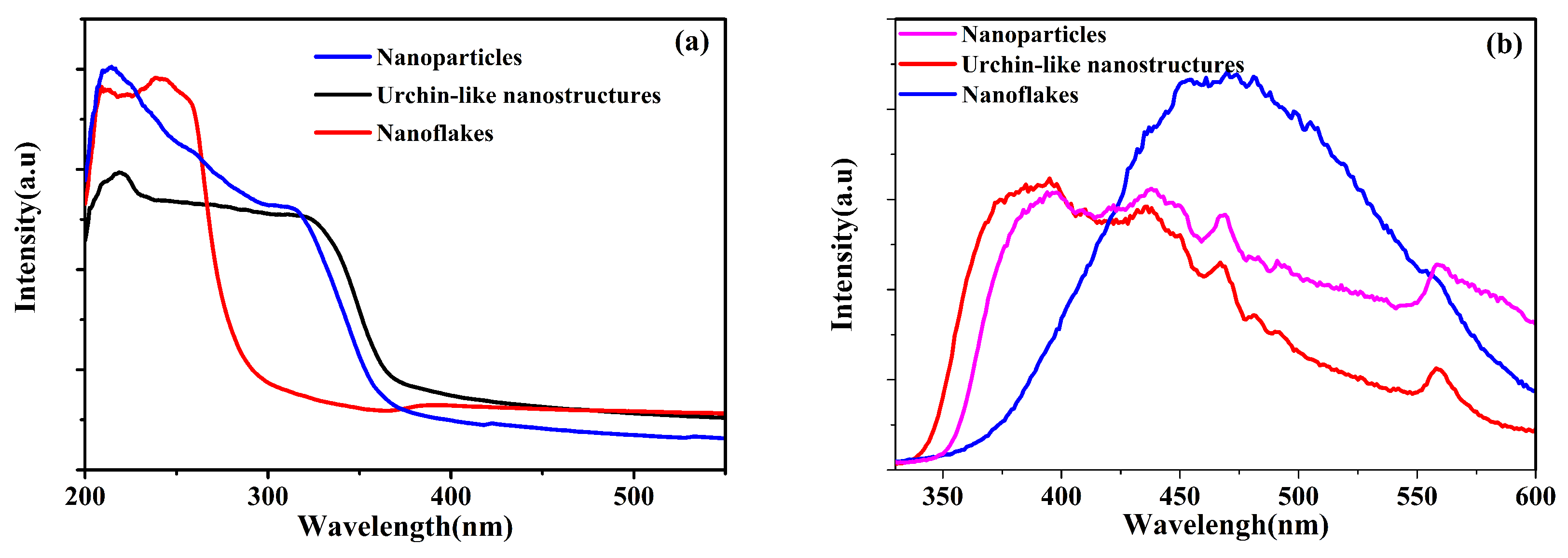
3.1. Characterization of ZnS Nanostructures
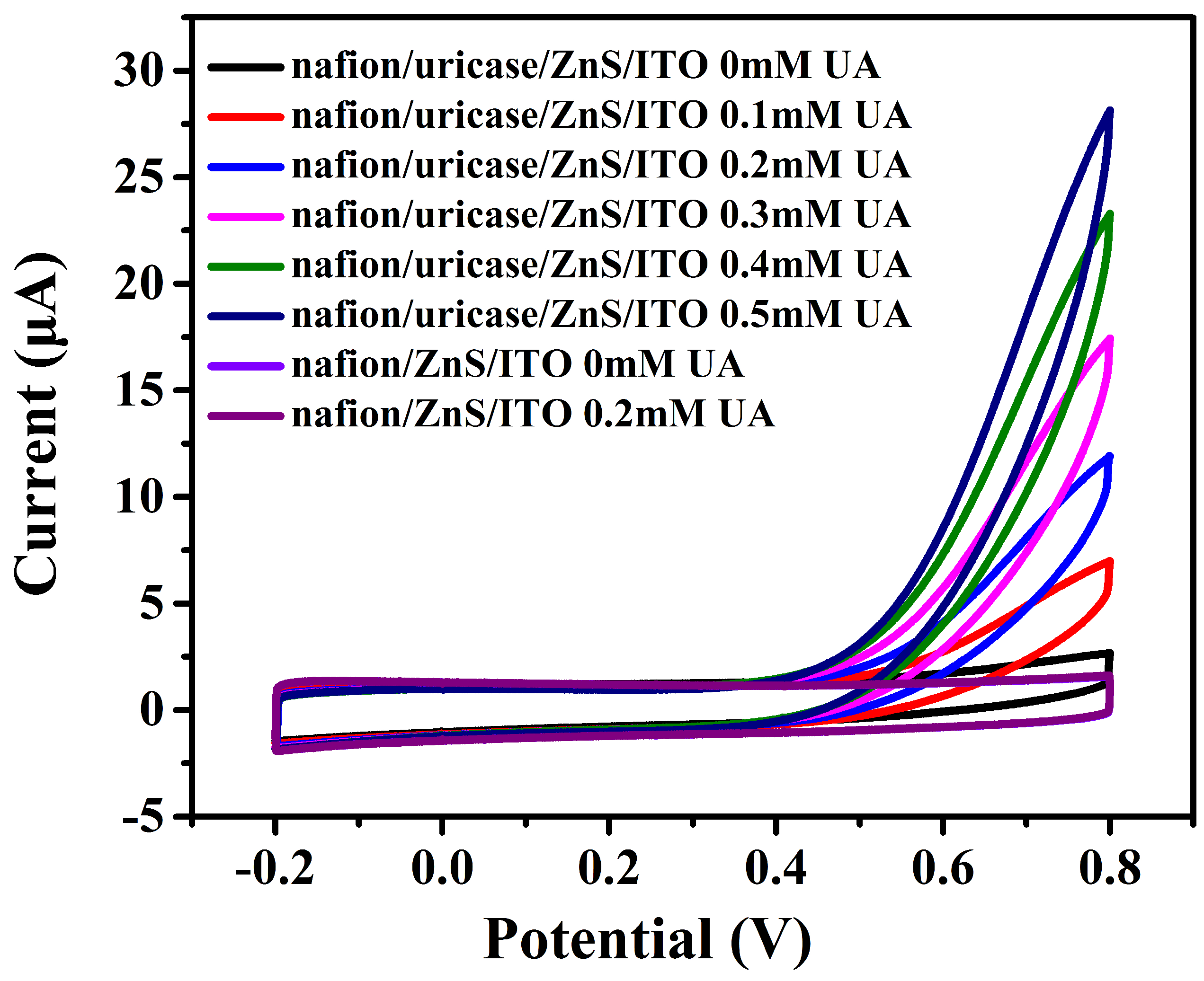
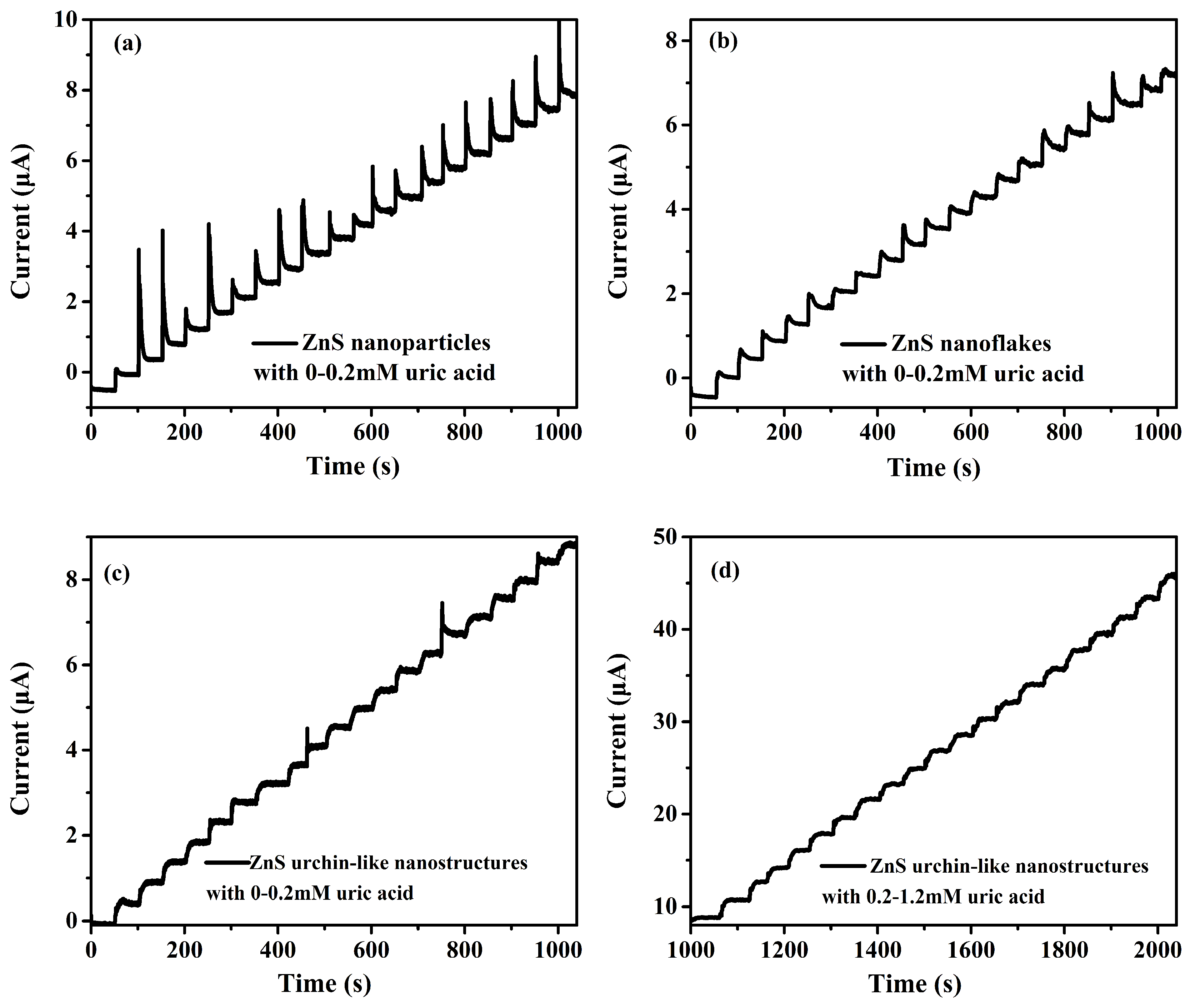
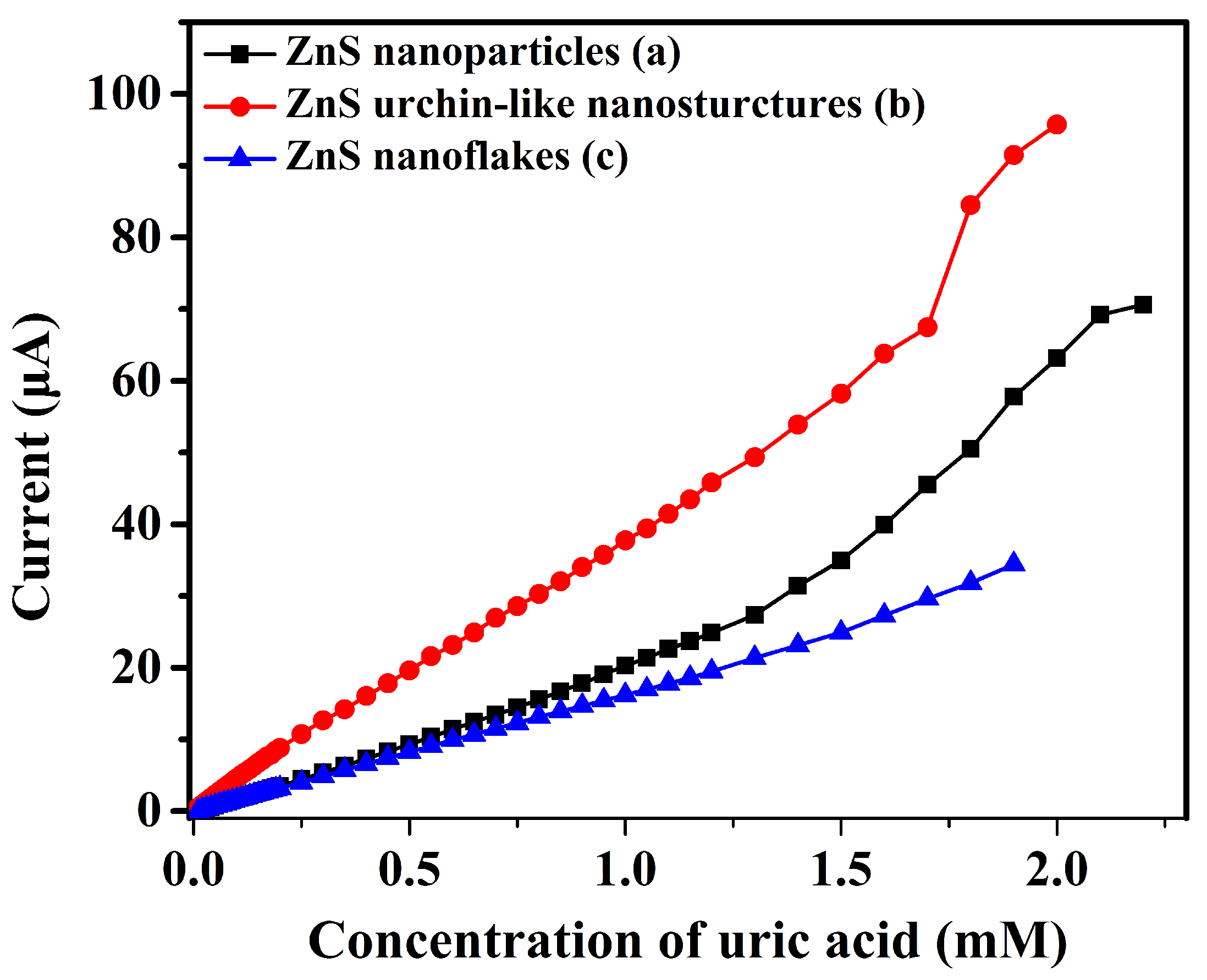
3.2. Electrochemical Characterizations and Measurements
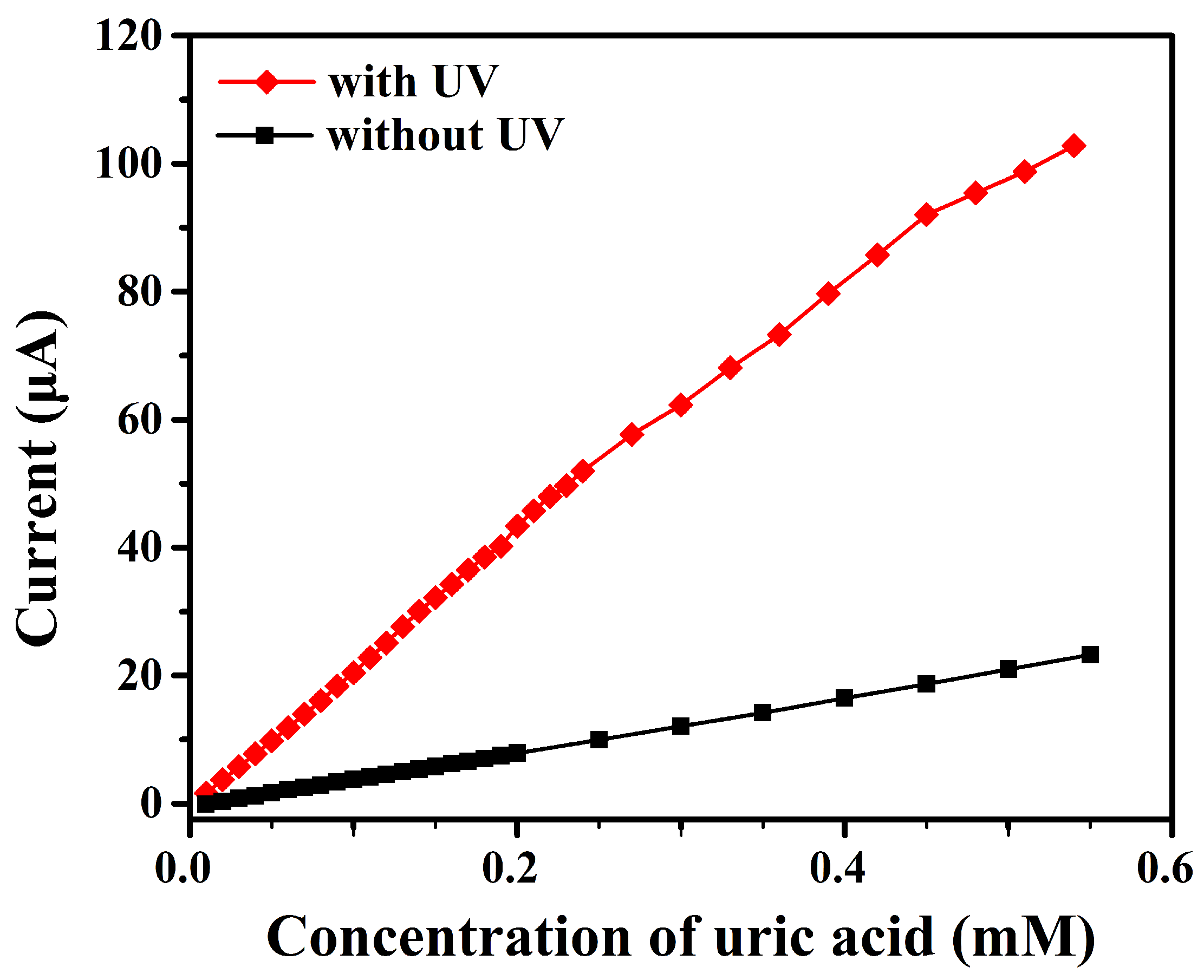
3.3. Photoelectrochemical Measurements
3.4. Stability and Selectivity Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alderman, M.H.; Cohen, H.; Madhavan, S.; Kivlighn, S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 1999, 34, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Akyilmaz, E.; Sezgintürk, M.K.; Dinçkaya, E. A biosensor based on urate oxidase–peroxidase coupled enzyme system for uric acid determination in urine. Talanta 2003, 61, 73–79. [Google Scholar] [CrossRef]
- Alderman, M.; Aiyer, K.J. Uric acid: Role in cardiovascular disease and effects of losartan. Curr. Med. Res. Opin. 2004, 20, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kar, S.; Santra, S.; Jompol, Y.; Arif, M.; Khondaker, S.I. Solvothermal Synthesis of High-Aspect Ratio Alloy Semiconductor Nanowires: Cd1−xZnxS, a Case Study. J. Phys. Chem. C 2009, 113, 3617–3624. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Jang, N.K.; Khang, G.; Hahn, Y.B. Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens. Actuators B Chem. 2015, 206, 146–151. [Google Scholar] [CrossRef]
- Zhang, F.; Li, C.; Li, X.; Wang, X.; Wan, Q.; Xian, Y.; Jin, L.; Yamamoto, K. ZnS quantum dots derived a reagentless uric acid biosensor. Talanta 2006, 68, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Jindal, K.; Tomar, M.; Gupta, V. Nitrogen-doped zinc oxide thin films biosensor for determination of uric acid. Analyst 2013, 138, 4353–4362. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, T.; Kumar, D.; Tanwar, V.K.; Sharma, V.; Singh, N.; Biradar, A.M. An amperometric uric acid biosensor based on Bis [sulfosuccinimidyl] suberate crosslinker/3-aminopropyltriethoxysilane surface modified ITO glass electrode. Thin Solid Films 2010, 519, 1128–1134. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, X.; Kang, Z.; Lin, P.; Fang, X.; Lei, Y.; Ma, S.; Zhang, Y. Highly sensitive uric acid biosensor based on individual zinc oxide micro/nanowires. Microchim. Acta 2013, 180, 759–766. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Kianipour, S.; Babaei, A.; Nasimi, A.A.; Shabanian, M. A new sensor based on glassy carbon electrode modified with nanocomposite for simultaneous determination of acetaminophen, ascorbic acid and uric acid. J. Saudi Chem. Soc. 2016, 20, S480–S487. [Google Scholar] [CrossRef]
- Cui, R.; Wang, X.; Zhang, G.; Wang, C. Simultaneous determination of dopamine, ascorbic acid, and uric acid using helical carbon nanotubes modified electrode. Sens. Actuators B Chem. 2012, 161, 1139–1143. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Wang, A.; Cai, W.; Deng, B.; Zhang, Z. An Electrochemical Sensor Based on Reduced Graphene Oxide and ZnO Nanorods-Modified Glassy Carbon Electrode for Uric Acid Detection. Arabian J. Sci. Eng. 2016, 41, 135–141. [Google Scholar] [CrossRef]
- Chauhan, N.; Pundir, C.S. An amperometric uric acid biosensor based on multiwalled carbon nanotube-gold nanoparticle composite. Anal. Biochem. 2011, 413, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, H.; Xiao, X.; Hark, S. The epitaxial growth of ZnS nanowire arrays and their applications in UV-light detection. J. Mater. Chem. 2012, 22, 1199–1205. [Google Scholar] [CrossRef]
- Fang, X.; Bando, Y.; Gautam, U.K.; Zhai, T.; Zeng, H.; Xu, X.; Liao, M.; Golberg, D. ZnO and ZnS nanostructures: Ultraviolet-light emitters, lasers, and sensors. Crit. Rev. Solid State Mater. Sci. 2009, 34, 190–223. [Google Scholar] [CrossRef]
- Aboulaich, A.; Balan, L.; Ghanbaja, J.; Medjahdi, G.; Merlin, C.; Schneider, R. Aqueous route to biocompatible ZnSe: Mn/ZnO core/shell quantum dots using 1-thioglycerol as stabilizer. Chem. Mater. 2011, 23, 3706–3713. [Google Scholar] [CrossRef]
- Wu, P.; Miao, L.; Wang, H.; Shao, X.G.; Yan, X.P. A Multidimensional Sensing Device for the Discrimination of Proteins Based on Manganese-Doped ZnS Quantum Dots. Angew. Chem. Int. Ed. 2011, 50, 8118–8121. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Niu, W.; Wu, X.; Li, L.; Yang, J.; Shuang, S.; Dong, C. Fluorescence enhancement detection of uric acid based on water-soluble 3-mercaptopropionic acid-capped core/shell ZnS:Cu/ZnS. RSC Adv. 2014, 4, 25183–25188. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Ai, S.; Sun, Z.; Wan, Q.; Zhu, Z.; Xian, Y.; Jin, L.; Yamamoto, K. Immobilization of uricase on ZnO nanorods for a reagentless uric acid biosensor. Anal. Chim. Acta 2004, 519, 155–160. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, N.; Wei, X.; Jiang, Z.; Kuo, W.C.H. Synthesis of ZnS urchin-like nanostructures for electrochemical determination of uric acid. In Proceedings of the 2016 IEEESENSORS, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Baş, D.; Boyacı, S.H. Quantitative photoelectrochemical detection of biotin conjugated CdSe/ZnS quantum dots on the avidin immobilized ITO electrodes. Electroanalysis 2009, 21, 1829–1834. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, W.; Yang, H.; Zhang, L.; Ma, J.; Zhao, H.; Sun, M.; Yang, L. Photoelectrochemical performance of multiple semiconductors (CdS/CdSe/ZnS) cosensitized TiO2 photoelectrodes. J. Phys. Chem. C 2012, 116, 2615–2621. [Google Scholar] [CrossRef]
- Wang, G.L.; Liu, K.L.; Dong, Y.M.; Li, Z.J.; Zhang, C. In Situ formation of p–n junction: A novel principle for photoelectrochemical sensor and its application for mercury (II) ion detection. Anal. Chim. Acta 2014, 827, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.; Yan, P.; Yan, D.; Fan, X.; Wang, M.; Qu, D.; Liu, J. Hydrothermal synthesis of single-crystal ZnS nanowires. Appl. Phys. A 2006, 84, 409–412. [Google Scholar] [CrossRef]
- Deng, Z.X.; Wang, C.; Sun, X.M.; Li, Y.D. Structure-directing coordination template effect of ethylenediamine in formations of ZnS and ZnSe nanocrystallites via solvothermal route. Inorg. Chem. 2002, 41, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yoshimura, M. Shape and Phase Control of ZnS Nanocrystals: Template Fabrication of Wurtzite ZnS Single Crystal Nanosheets and ZnO Flake-like Dendrites from a Lamellar Molecular Precursor ZnS·(NH2CH2CH2NH2) 0.5. Adv. Mater. 2002, 14, 296–300. [Google Scholar] [CrossRef]
- Mitsui, T.; Yamamoto, N.; Tadokoro, T.; Ohta, S. Cathodoluminescence image of defects and luminescence centers in ZnS/GaAs(100). J. Appl. Phys. 1996, 80, 6972–6979. [Google Scholar] [CrossRef]
- Yue, G.; Yan, P.; Yan, D.; Liu, J.; Qu, D.; Yang, Q.; Fan, X. Synthesis of two-dimensional micron-sized single-crystalline ZnS thin nanosheets and their photoluminescence properties. J. Cryst. Growth 2006, 293, 428–432. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, N.; Wei, X.; Sharma, M.; Wang, J.; Jiang, Z. Mercaptopropionic acid capped ZnS:Mn/ZnS core/shell quantum dots as fluorescence probe for folic acid detection. In Proceedings of the 2015 IEEE 10th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Xi’an, China, 7–11 April 2015; pp. 306–309. [Google Scholar]
- Torimoto, T.; Obayashi, A.; Kuwabata, S.; Yasuda, H.; Mori, H.; Yoneyama, H. Preparation of size-quantized ZnS thin films using electrochemical atomic layer epitaxy and their photoelectrochemical properties. Langmuir 2000, 16, 5820–5824. [Google Scholar] [CrossRef]
- Du, J.; Yu, X.; Wu, Y.; Di, J. ZnS nanoparticles electrodeposited onto ITO electrode as a platform for fabrication of enzyme-based biosensors of glucose. Mater. Sci. Eng. C 2013, 33, 2031–2036. [Google Scholar] [CrossRef] [PubMed]



| Materials | Method | Sensitivity | LOD | Linear Range | Reference |
|---|---|---|---|---|---|
| ZnS nanoparticles | electrochemical | 43.18 | 1.79 | 0.01−1.5 | This work |
| ZnS nanoflakes | electrochemical | 34.28 | 1.51 | 0.01−2.0 | This work |
| ZnS urchin-like nanostructures | electrochemical | 76.12 | 0.7 | 0.01−1.7 | This work |
| ZnS urchin-like nanostructures | photoelectrochemcial | 413.98 | 0.045 | 0.01−0.54 | This work |
| ZnO nanosheets | electrochemical | 129.81 | 0.019 | 0.05−2.0 | [5] |
| ZnS quantum dots | photoluminescence | - | 2 | 0.005−2 | [6] |
| ZnO thin films | electrochemical | 1100 | - | 0.05−1.0 | [7] |
| BS3/APTES | electrochemical | 46.26 | 8.4 | 0.058−0.71 | [8] |
| ZnO micro/nano wires | electrochemical | 89.74 | 25.6 | 0.1−0.59 | [9] |
| RGO-ZnO/GCE | electrochemical | 109.75 | 0.312 | 0.001−0.8 | [10] |
| RGO-ZnO/GCE | electrochemical | 161.2 | 0.46 | 0.001−0.08 | [11] |
| HCNTs/GCE | electrochemical | - | 1.5 | 0.0067−0.065 | [12] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wei, X.; Peng, N.; Wang, J.; Jiang, Z. Study of ZnS Nanostructures Based Electrochemical and Photoelectrochemical Biosensors for Uric Acid Detection. Sensors 2017, 17, 1235. https://doi.org/10.3390/s17061235
Zhao Y, Wei X, Peng N, Wang J, Jiang Z. Study of ZnS Nanostructures Based Electrochemical and Photoelectrochemical Biosensors for Uric Acid Detection. Sensors. 2017; 17(6):1235. https://doi.org/10.3390/s17061235
Chicago/Turabian StyleZhao, Yao, Xueyong Wei, Niancai Peng, Jiuhong Wang, and Zhuangde Jiang. 2017. "Study of ZnS Nanostructures Based Electrochemical and Photoelectrochemical Biosensors for Uric Acid Detection" Sensors 17, no. 6: 1235. https://doi.org/10.3390/s17061235






