Biosensor Based on Tyrosinase Immobilized on Graphene-Decorated Gold Nanoparticle/Chitosan for Phenolic Detection in Aqueous
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Electrodes and Apparatus
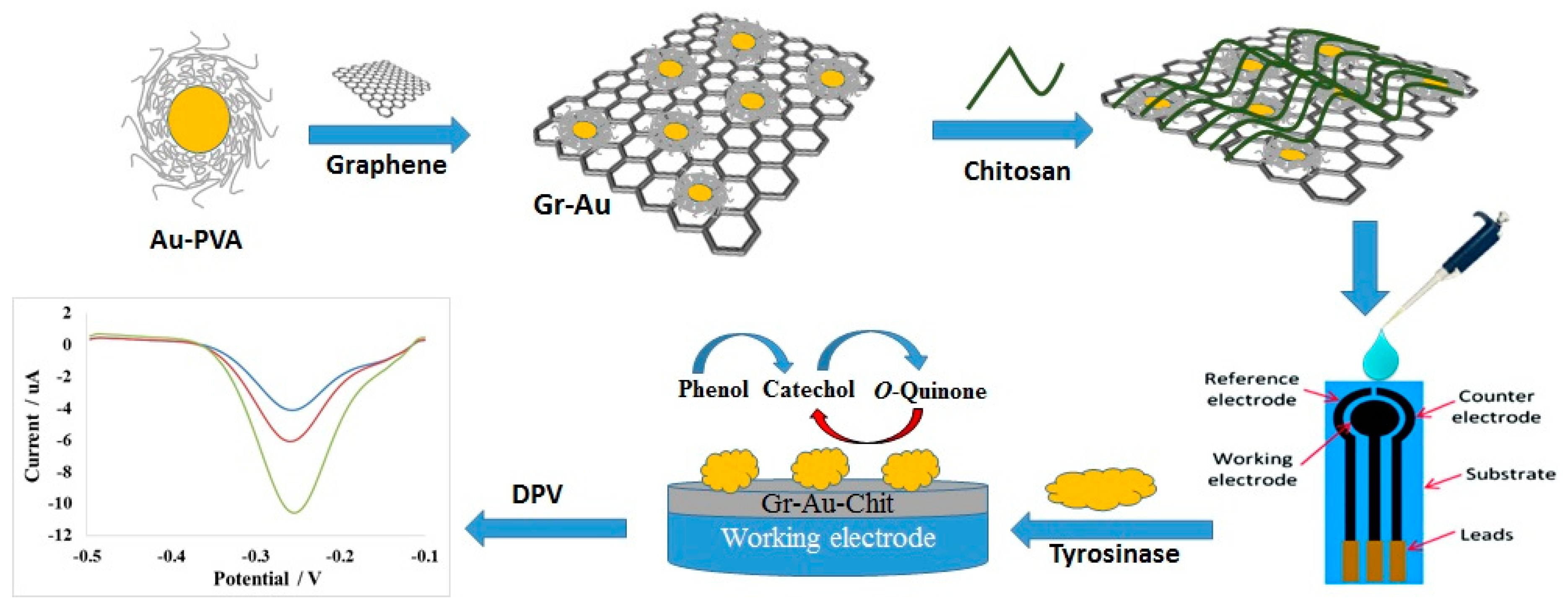
2.3. Preparation of Graphene-Gold-Chitosan/Tyrosinase Bionanocomposite
2.4. HPLC Analysis
3. Results and Discussion
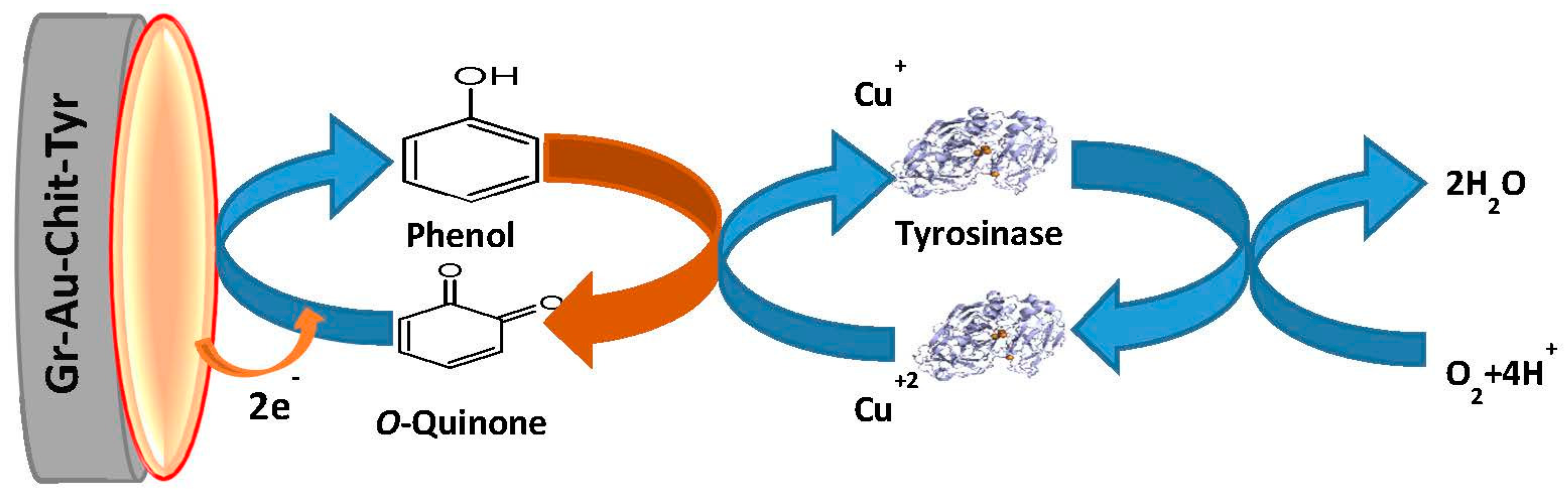
3.1. Proposed Mechanism
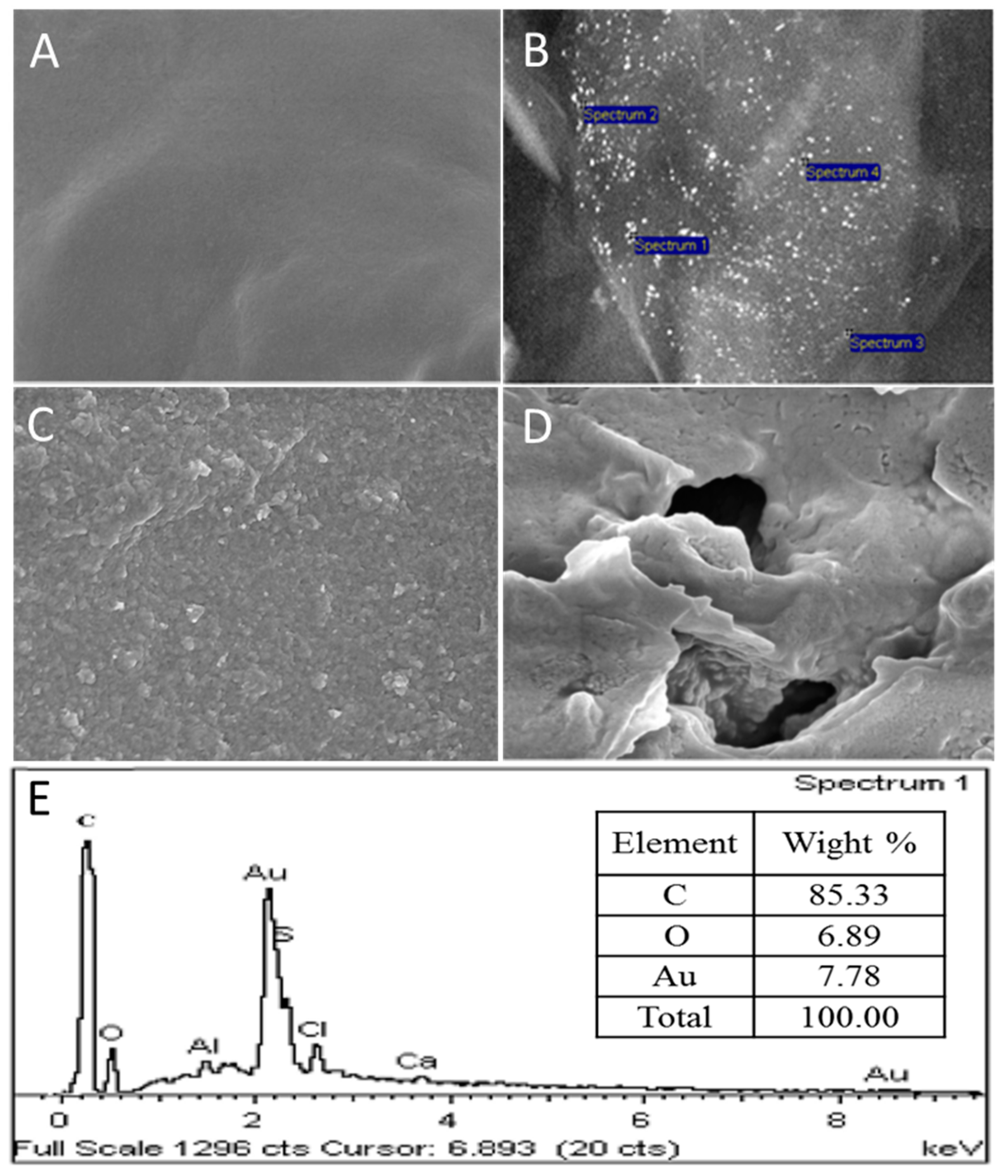
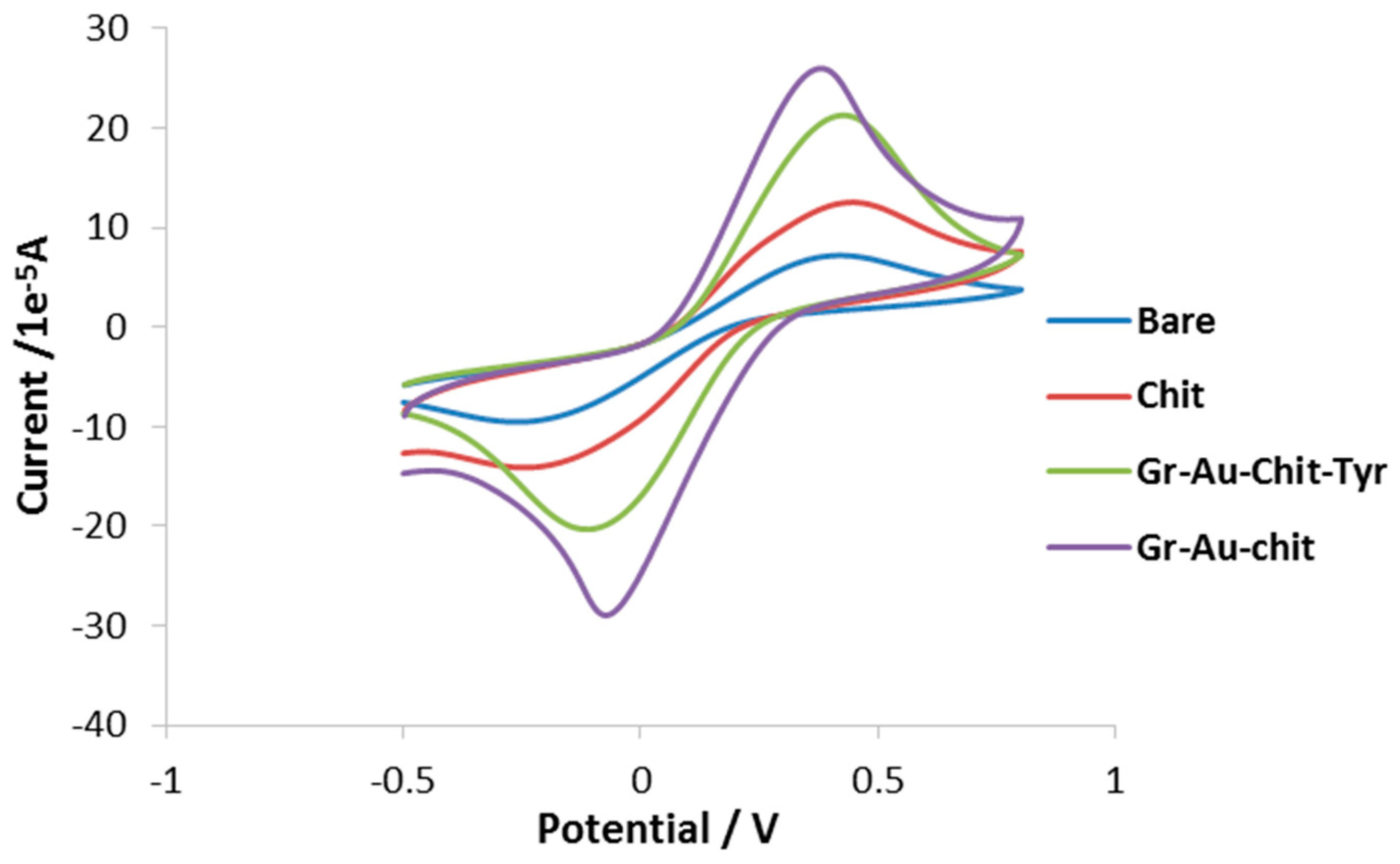
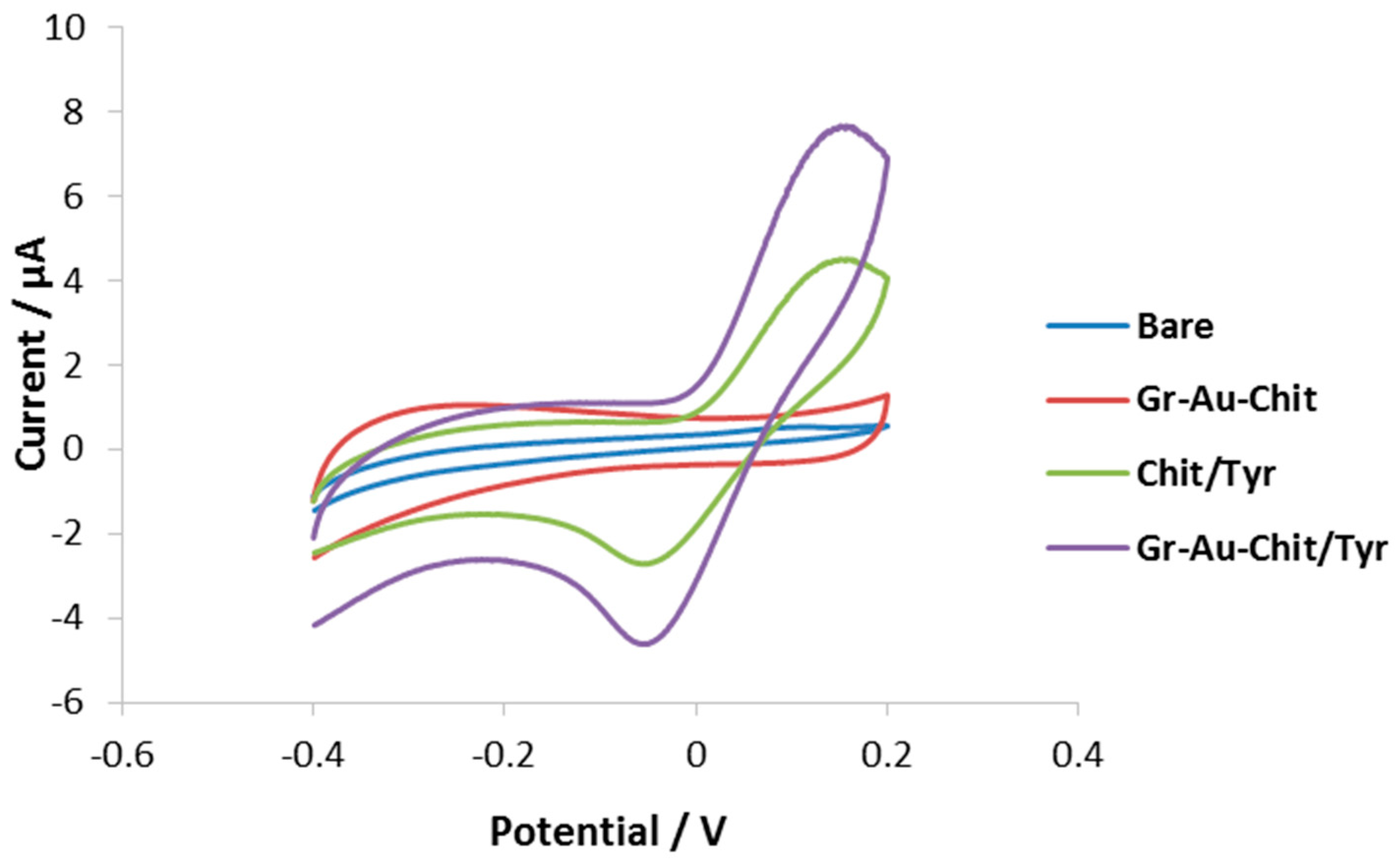
3.2. Characterization of the Modified Electrode
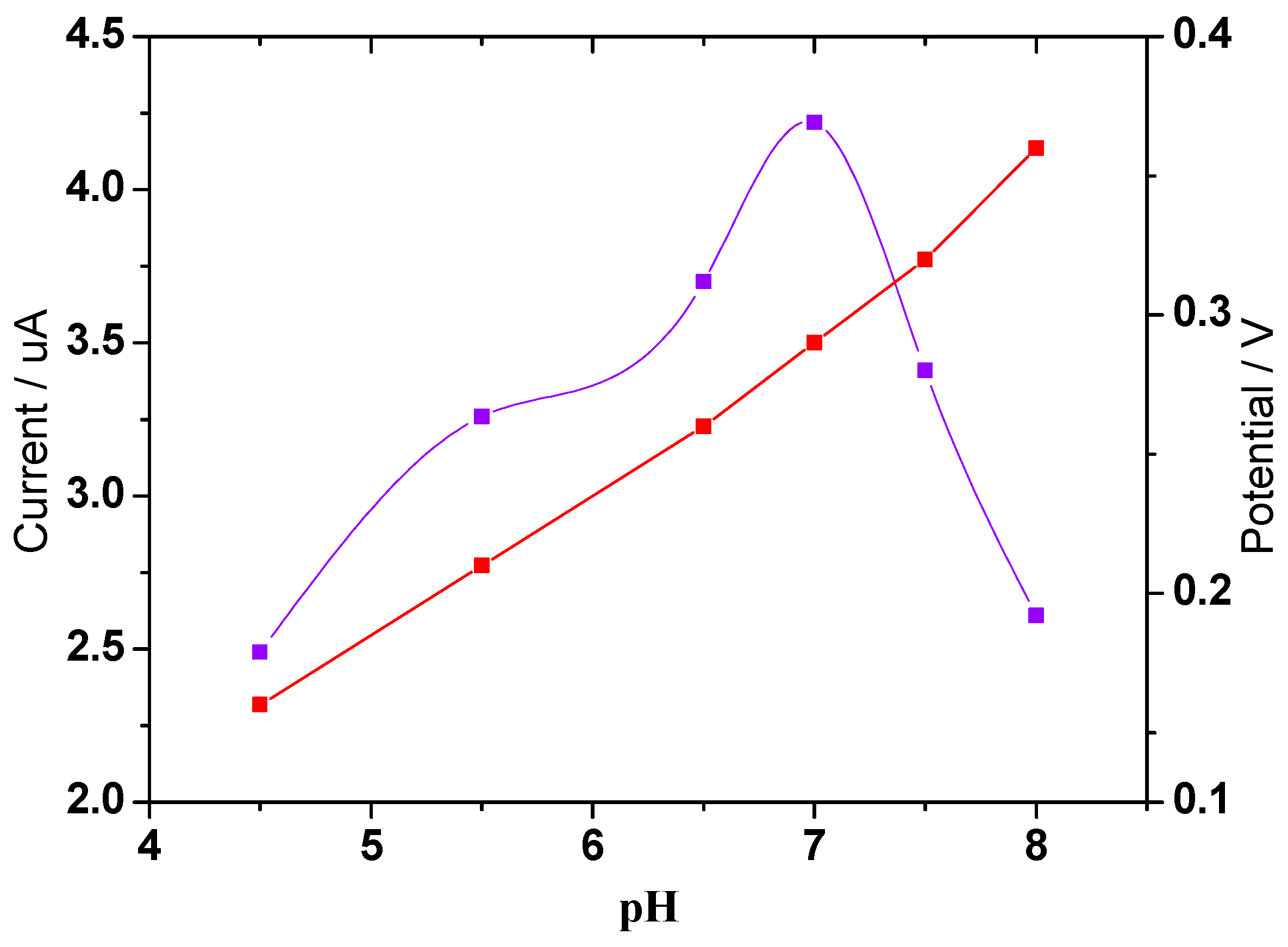
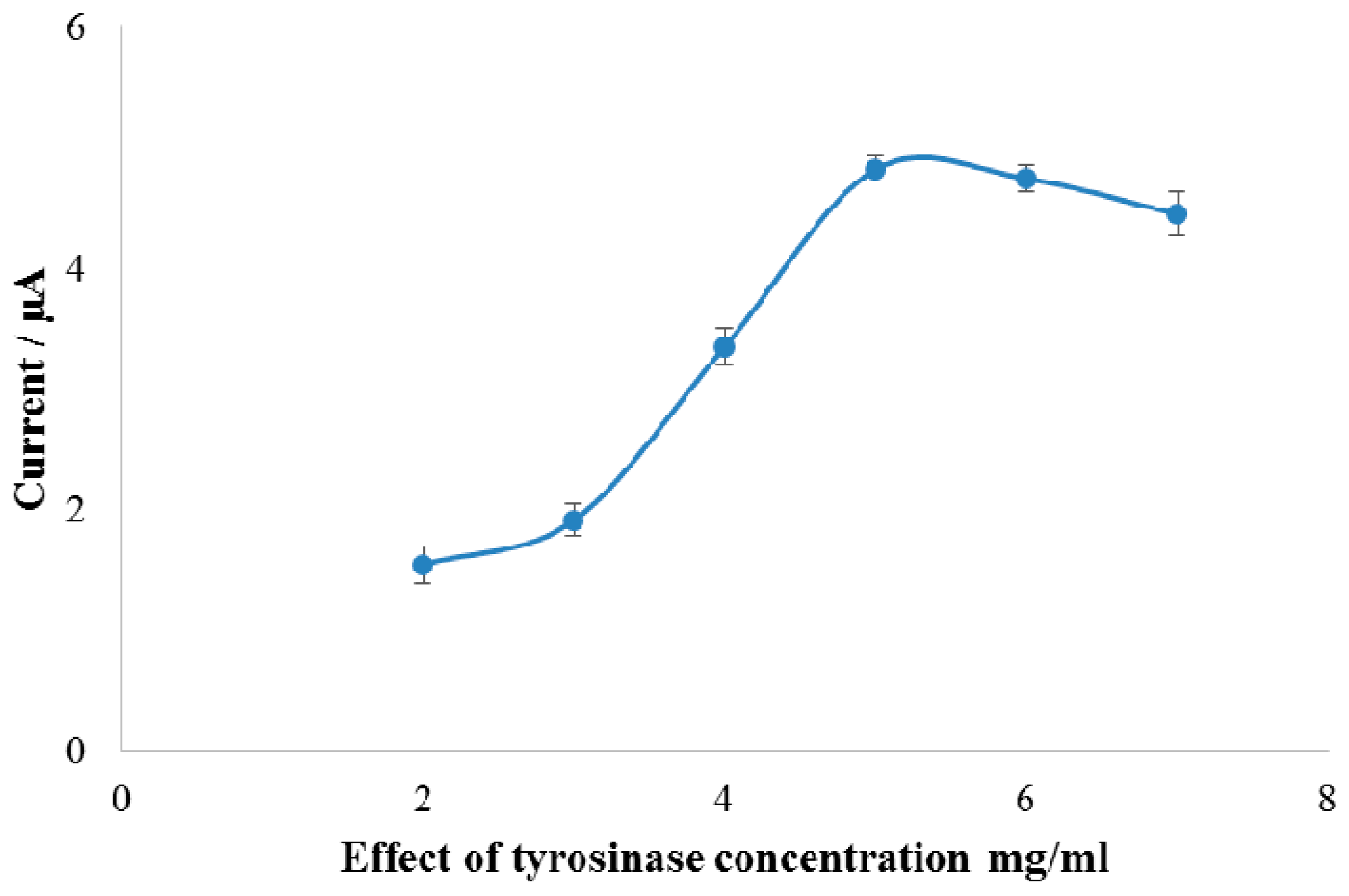
3.3. Optimization of the Experimental Parameters
3.4. Analytical Performance of the Developed Biosensor
3.5. Effect of Interfering Ions and Analytical Application of the Biosensor
3.6. Analysis of Real Sample
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Negash, N.; Alemu, H.; Tessema, M. Determination of Phenol and Chlorophenols at Single-Wall Carbon Nanotubes/Poly (3,4-ethylenedioxythiophene ) modified Glassy Carbon Electrode Using Flow Injection Amperometry. Am. J. Anal. Chem. 2014, 5, 188–198. [Google Scholar] [CrossRef]
- Freire, R.S.; Dur, N. Electrochemical Biosensor-Based Devices for Continuous Phenols Monitoring in Environmental Matrices. J. Braz. Chem. Soc. 2002, 13, 456–462. [Google Scholar] [CrossRef]
- Dong, X.; Huang, W.; Chen, P. In Situ Synthesis of Reduced Graphene Oxide and Gold Nanocomposites for Nanoelectronics and Biosensing. Nanoscale Res. Lett. 2011, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nurul Karim, M.; Lee, H.J. Amperometric phenol biosensor based on covalent immobilization of tyrosinase on Au nanoparticle modified screen printed carbon electrodes. Talanta 2013, 116, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.R.; Becker, J.Y.; Cembrano, J. Improved selective electrocatalytic oxidation of phenols by tyrosinase-based carbon paste electrode biosensor. Electrochim. Acta 2000, 45, 4373–4379. [Google Scholar] [CrossRef]
- Chaubey, A.; Pande, K.K.; Singh, V.S.; Malhotra, B.D. Co-immobilization of lactate oxidase and lactate dehydrogenase on conducting polyaniline films. Anal. Chim. Acta 2000, 407, 97–103. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Ju, H. Mediator-free phenol sensor based on titania sol Á gel encapsulation matrix for immobilization of tyrosinase byavapor deposition method. Biosens. Bioelectron. 2003, 19, 509–514. [Google Scholar] [CrossRef]
- Ve, C.; Tran-minh, C. Amperometric tyrosinase based biosensor using an electrogenerated polythiophene film as an entrapment support. Talanta 2003, 59, 535–544. [Google Scholar]
- Hashemnia, S.; Khayatzadeh, S.; Hashemnia, M. Electrochemical detection of phenolic compounds using composite film of multiwall carbon nanotube/surfactant/tyrosinase on a carbon paste electrode. J. Solid State Electrochem. 2012, 16, 473–479. [Google Scholar] [CrossRef]
- Kim, G.; Shim, J.; Kang, M.; Moon, S. Optimized coverage of gold nanoparticles at tyrosinase electrode for measurement of a pesticide in various water samples. J. Hazard. Mater. 2008, 156, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ye, J.; Zhang, J.; Huang, X.; Yu, C. A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J. Electroanal. Chem. 2011, 650, 209–213. [Google Scholar] [CrossRef]
- Sun, X.; Hu, S.; Li, L.; Xiang, J.; Sun, W. Sensitive electrochemical detection of hydroquinone with carbon ionogel electrode based on BMIMPF6. J. Electroanal. Chem. 2011, 651, 94–99. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.H.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Yin, Z.; Wu, S.; Mandler, D.; Zhang, H. Fabrication of Nanoelectrode Ensembles by Electrodepositon of Au Nanoparticles on Single-Layer Graphene Oxide Sheets. J. R. Soc. Chem. RSC 2012, 4, 2728–2733. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K. Design, Synthesis, and Characterization of Graphene—Nanoparticle Hybrid Materials for Bioapplications. Chem. Rev. 2014, 115, 2483–2531. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, Y.; Wang, X.; Xu, L.; Xiang, J.; Sun, W. Electrochemical detection of hydroquinone with a gold nanoparticle and graphene modified carbon ionic liquid electrode. Sens. Actuators B Chem. 2012, 168, 27–33. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, H.; Li, Y.; Li, C. Electrochemical simultaneous determination of hydroquinone and p-nitrophenol based on host—Guest molecular recognition capability of dual β-cyclodextrin functionalized Au@graphene nanohybrids. Sens. Actuators B. Chem. 2015, 207, 1–8. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, R.; Liu, C.; Yang, S.; Lu, Z.; Luo, S. Electrochemical detection of 4-nitrophenol based on a glassy carbon electrode modified with a reduced graphene oxide/Au nanoparticle composite. Anal. Methods 2013, 5, 5508. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, L.; Zhang, X.; Huan, S.; Shen, G.; Yu, R. Analytica Chimica Acta A novel tyrosinase biosensor based on hydroxyapatite—Chitosan nanocomposite for the detection of phenolic compounds. Anal. Chim. Acta 2010, 665, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Reza, K.K.; Ali, M.A.; Srivastava, S.; Agrawal, V.V.; Biradar, A.M. Tyrosinase conjugated reduced graphene oxide based biointerface for bisphenol A sensor. Biosens. Bioelectron. 2015, 74, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Piao, Y.; Choi, K.S.; Seo, T.S. Fabrication of free-standing graphene composite films as electrochemical biosensors. Carbon 2012, 50, 123–133. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.; Liang, Y.; Liu, W. A Graphene-Based Electrochemical Sensor for Rapid Determination of Phenols in Water. Sensors 2013, 13, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Fu, X.; Wu, L.; Qin, Y.; Song, Y. A Novel Tyrosinase Biosensor based on Graphene and Co3O4 Nanocomposite Materials for Rapid Determining Catechol. Int. J. Electrochem. Sci. 2016, 11, 250–258. [Google Scholar]
- Izham, M.; Brett, G.L.; Tiruvalam, R.; Forde, M.M.; Sharples, K.; Thetford, A.; et al. Involvement of Surface-Bound Radicals in the Oxidation of Toluene Using Supported Au-Pd Nanoparticles. Angew. Chim. 2012, 51, 5981–5985. [Google Scholar]
- Dimitratos, N.; Villa, A.; Bianchi, C.L.; Prati, L.; Makkee, M. Gold on titania: Effect of preparation method in the liquid phase oxidation. Appl. Catal. A Gen. 2006, 311, 185–192. [Google Scholar] [CrossRef]
- Parlak, O.; Tiwari, A.; Turner, A.P.; Tiwari, A. Biosensors and Bioelectronics Template-directed hierarchical self-assembly of graphene based hybrid structure for electrochemical biosensing. Biosens. Bioelectron. 2013, 49, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, G.; Liu, Y.; Tang, J.; Tang, W. Immobilizing haemoglobin on gold/grapheme—Chitosan nanocomposite as efficient hydrogen peroxide biosensor. Sens. Actuators B Chem. 2014, 197, 164–171. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Muthoosamy, K.; Zhou, M.; Ashokkumar, M.; Huang, N.M.; Manickam, S. Sonochemical and sustainable synthesis of graphene-gold (G-Au) nanocomposites for enzymeless and selective electrochemical detection of nitric oxide. Biosens. Bioelectron. 2017, 87, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Dai, Y.; Liu, Q.; Xie, Y.; Xu, X. The FT-IR spectrometric analysis of the changes of polyphenol oxidase II secondary structure. J. Mol. Struct. 2003, 644, 139–144. [Google Scholar] [CrossRef]
- Gu, B.X.; Xu, C.X.; Zhu, G.P.; Liu, S.Q.; Chen, L.Y.; Li, X.S. Tyrosinase immobilization on ZnO nanorods for phenol detection. J. Phys. Chem. B 2009, 113, 377–381. [Google Scholar]
- Singh, J.; Khanra, P.; Kuila, T.; Srivastava, M.; Das, A.K.; Hoon, N.; Jung, B.J.; Kim, D.Y.; Lee, S.H.; Lee, D.W.; et al. Preparation of sulfonated poly (ether-ether-ketone) functionalized ternary graphene/AuNPs/chitosan nanocomposite for efficient glucose biosensor. Process Biochem. 2013, 48, 1724–1735. [Google Scholar] [CrossRef]
- Ou, J.; Tao, Y.; Xue, J.; Kong, Y.; Dai, J.; Deng, L. Electrochemistry Communications Electrochemical enantiorecognition of tryptophan enantiomers based on graphene quantum dots—Chitosan composite film. Electrochem. Commun. 2015, 57, 5–9. [Google Scholar] [CrossRef]
- Li, B.J.; Qiu, J.; Xu, J.; Chen, H.; Xia, X. The Synergistic Effect of Prussian-Blue-Grafted Carbon Nanotube/Poly (4-vinylpyridine) Composites for Amperometric Sensing. Adv. Funct. Mater. 2007, 17, 1574–1580. [Google Scholar] [CrossRef]
- Han, R.; Cui, L.; Ai, S.; Yin, H. Amperometric biosensor based on tyrosinase immobilized in hydrotalcite-like compounds film for the determination of polyphenols. J. Solid State Electrochem. 2012, 16, 449–456. [Google Scholar] [CrossRef]
- Penu, R.; Obreja, A.C.; Patroi, D.; Diaconu, M.; Lucian, G. Graphene and gold nanoparticles based reagentless biodevice for phenolic endocrine disruptors monitoring. Microchem. J. 2015, 121, 130–135. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Nastajute, G.; Snitka, V.; Kausaite, A.; German, N. Spectrophotometric evaluation of gold nanoparticles as red-ox mediator for glucose oxidase. Sens. Actuators B 2009, 137, 483–489. [Google Scholar] [CrossRef]
- Mazeiko, V.; Kausaite-minkstimiene, A.; Ramanaviciene, A.; Balevicius, Z.; Ramanavicius, A. Gold nanoparticle and conducting polymer-polyaniline-based nanocomposites for glucose biosensor design. Sens. Actuators B 2013, 189, 187–193. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Cadevall, M.; Guix, M.; Ros, J.; Merkoc, A. Biosensors and Bioelectronics Bismuth nanoparticles for phenolic compounds biosensing application. Biosens. Bioelectron. 2013, 40, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Takashima, W.; Kaneto, K. Amperometric phenol biosensor based on covalent immobilization of tyrosinase onto an electrochemically prepared novel copolymer poly (N-3-aminopropyl pyrrole-co-pyrrole) film. Sens. Actuators B 2004, 102, 271–277. [Google Scholar]
- Cheng, Y.; Liu, Y.; Huang, J.; Li, K.; Xian, Y.; Zhang, W.; Jin, L. Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles-coated carbon nanotubes nanocomposite for rapid detection of coliforms. Electrochim. Acta 2009, 54, 2588–2594. [Google Scholar] [CrossRef]
- Maria-solano, M.A.; Ortiz-ruiz, C.V.; Munoz-munoza, J.L.; Teruel-puche, J.A.; Berna, J.; Garcia-ruizd, P.A.; Garcia-canovasa, F. nzymatic Further insight into the pH effect on the catalysis of mushroom tyrosinase. J. Mol. Catal. 2016, 125, 6–15. [Google Scholar] [CrossRef]
- Sánchez, G.; Mayén, M.; Rodríguez, J.M. New Biosensor for Phenols Compounds Based on Gold Nanoparticle-Modified PVC/TTF-TCNQ Composite Electrode. Int. J. Electrochem. Sci. 2012, 7, 10952–10964. [Google Scholar]
- Wang, S.; Tan, Y.; Zhao, D.; Liu, G. Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles—Chitosan nanocomposite. Biosens. Bioelectron. 2008, 23, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Janegitz, B.C.; Medeiros, R.A.; Rocha-filho, R.C.; Fatibello-filho, O. Diamond & Related Materials Direct electrochemistry of tyrosinase and biosensing for phenol based on gold nanoparticles electrodeposited on a boron-doped diamond electrode. Diam. Relat. Mater. 2012, 25, 128–133. [Google Scholar]
- Liu, Z.; Liu, B.; Kong, J.; Deng, J. Probing Trace Phenols Based on Mediator-Free Alumina Sol—Gel-Derived Tyrosinase Biosensor. Anal. Chem. 2000, 72, 4707–4712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, M.; Chen, X.; Li, Y.; Wang, J. Electrochemical co-reduction synthesis of AuPt bimetallic nanoparticles-graphene nanocomposites for selective detection of dopamine in the presence of ascorbic acid and uric acid. Sensors 2015, 15, 16614–16631. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Huang, X.; Zhang, X.Y.; Hua, Z.L.; Wang, C.F.; Qin, Q.; Zhang, Q. TiO2-graphene nanoparticle based electrochemical sensor for the bimodal-response detection of 4-chlorophenol. RSC Adv. 2014, 4, 13461–13468. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Yang, H.; Yang, Y.; Shen, G.; Yu, R. A phenol biosensor based on immobilizing tyrosinase to modified core-shell magnetic nanoparticles supported at a carbon paste electrode. Anal. Chim. Acta 2005, 533, 3–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Lei, W.; Xia, X.; Xia, M.; Hao, Q. Electrochimica Acta Electrochemical determination of 4-nitrophenol at polycarbazole/N-doped graphene modified glassy carbon electrode. Electrochim. Acta 2014, 146, 568–576. [Google Scholar] [CrossRef]
- Sanz, V.C.; Mena, M.L.; Gonz, A.; Pingarr, J.M. Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes Application to the measurement of a bioelectrochemical polyphenols index in wines. Anal. Chim. Acta 2005, 528, 1–8. [Google Scholar] [CrossRef]
- Sethuraman, V.; Muthuraja, P.; Manisankar, P. Fabrication of an efficient polyaniline–polyphenol oxidase based biosensor for catechol. Anal. Methods 2013, 5, 6523. [Google Scholar]
- Tsai, Y.-C.; Chiu, C.-C. Amperometric biosensors based on multiwalled carbon nanotube-nafion-tyrosinase nanobiocomposites for the determination of phenolic compounds. Sens. Actuators B Chem. 2007, 125, 10–16. [Google Scholar] [CrossRef]
- Sethuraman, V.; Muthuraja, P.; Anandha Raj, J.; Manisankar, P. A highly sensitive electrochemical biosensor for catechol using conducting polymer reduced graphene oxide-metal oxide enzyme modified electrode. Biosens. Bioelectron. 2015, 84, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Kang, T.; Xue, R.; Ge, C. Biosensor based on a glassy carbon electrode modified with tyrosinase immobilized on multiwalled carbon nanotubes. Microchim. Acta 2011, 303–309. [Google Scholar] [CrossRef]
- Liu, X.; Yan, R.; Zhu, J.; Zhang, J.; Liu, X. Growing TiO2 nanotubes on graphene nanoplatelets and applying the nanonanocomposite as scaffold of electrochemical tyrosinase biosensor. Sens. Actuators B Chem. 2015, 209, 328–335. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Paul, C.; Caval, D.I.; Mihailciuc, C. Development of Amperometric Biosensors Based on Nanostructured Tyrosinase-Conducting Polymer Composite Electrodes. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [PubMed]


| Phenolic Compound | Linear Range (μM) | Sensitivity (μA μM−1) | LOD (μM) | Km (μM) |
|---|---|---|---|---|
| Phenol | 0.05–15 | 0.624 | 0.016 | 12.6 |
| Catechol | 0.05–11 | 0.483 | 0.02 | 13.1 |
| 4-chlorophenol | 0.05–13 | 0.431 | 0.024 | 16.1 |
| 4-nitrophenol | 0.05–19 | 0.334 | 0.031 | 17.8 |
| 2-4 Di chlorophenol | 0.05–15 | 0.213 | 0.041 | 24.2 |
| Sample | Added/μM | The Developed Method (μM) Mean ± SD (n = 3) | HPLC Method (μM) Mean ± SD (n = 3) | t-Test Value |
|---|---|---|---|---|
| Lake water | 1.0 | 0.98 ± 0.0416 | 0.99 ± 0.0333 | 0.53 |
| 1.5 | 1.46 ± 0.0503 | 1.53 ± 0.0727 | 1.42 | |
| 2.0 | 2.02 ± 0.0378 | 2.06 ± 0.0433 | 1.25 | |
| 2.5 | 2.39 ± 0.0305 | 2.43 ± 0.0338 | 1.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fartas, F.M.; Abdullah, J.; Yusof, N.A.; Sulaiman, Y.; Saiman, M.I. Biosensor Based on Tyrosinase Immobilized on Graphene-Decorated Gold Nanoparticle/Chitosan for Phenolic Detection in Aqueous. Sensors 2017, 17, 1132. https://doi.org/10.3390/s17051132
Fartas FM, Abdullah J, Yusof NA, Sulaiman Y, Saiman MI. Biosensor Based on Tyrosinase Immobilized on Graphene-Decorated Gold Nanoparticle/Chitosan for Phenolic Detection in Aqueous. Sensors. 2017; 17(5):1132. https://doi.org/10.3390/s17051132
Chicago/Turabian StyleFartas, Fuzi Mohamed, Jaafar Abdullah, Nor Azah Yusof, Yusran Sulaiman, and Mohd Izham Saiman. 2017. "Biosensor Based on Tyrosinase Immobilized on Graphene-Decorated Gold Nanoparticle/Chitosan for Phenolic Detection in Aqueous" Sensors 17, no. 5: 1132. https://doi.org/10.3390/s17051132






