Carbon Nanotube-Based Chemiresistive Sensors
Abstract
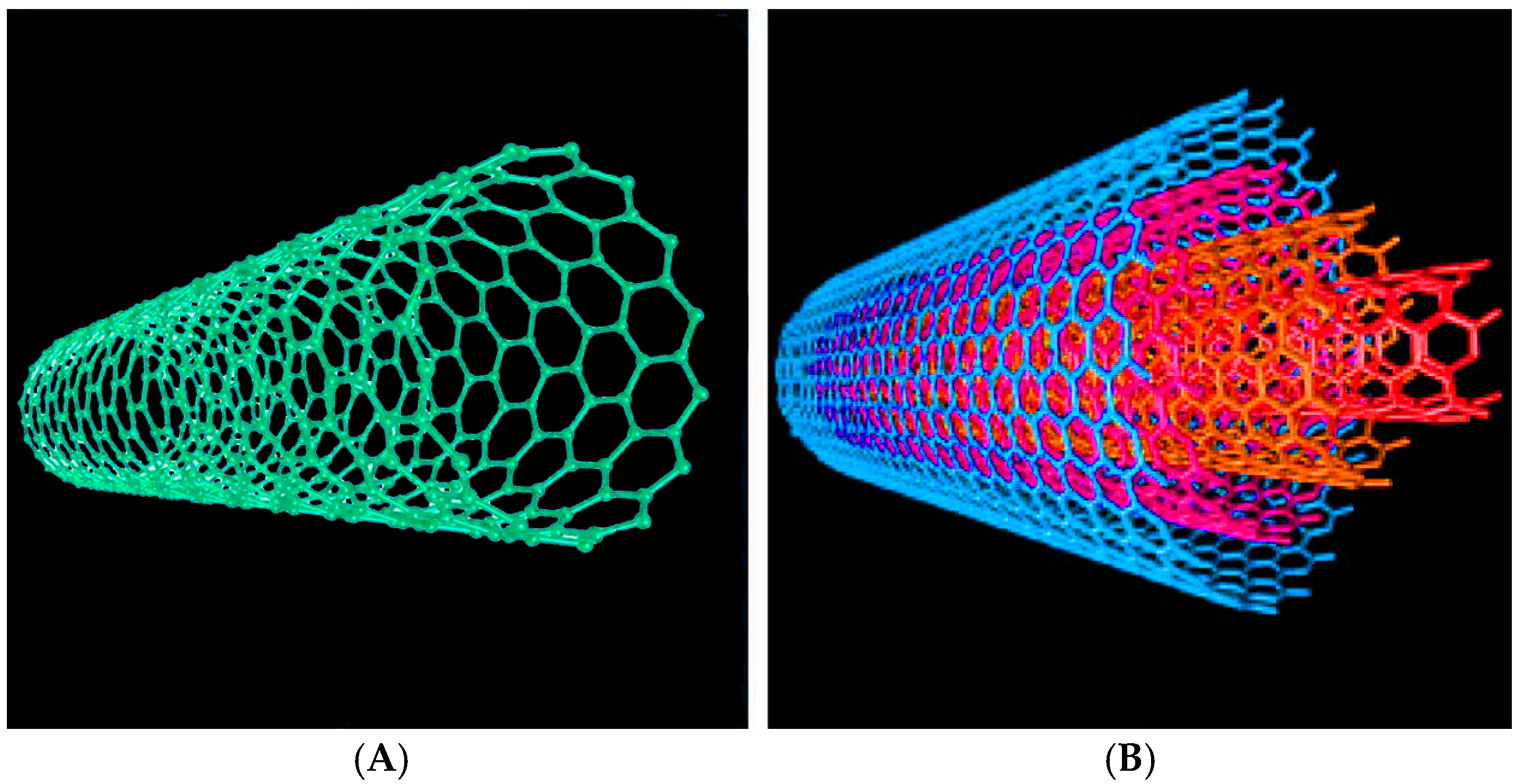
:1. Introduction
2. Functionalization of CNTs for Chemiresistive Sensors
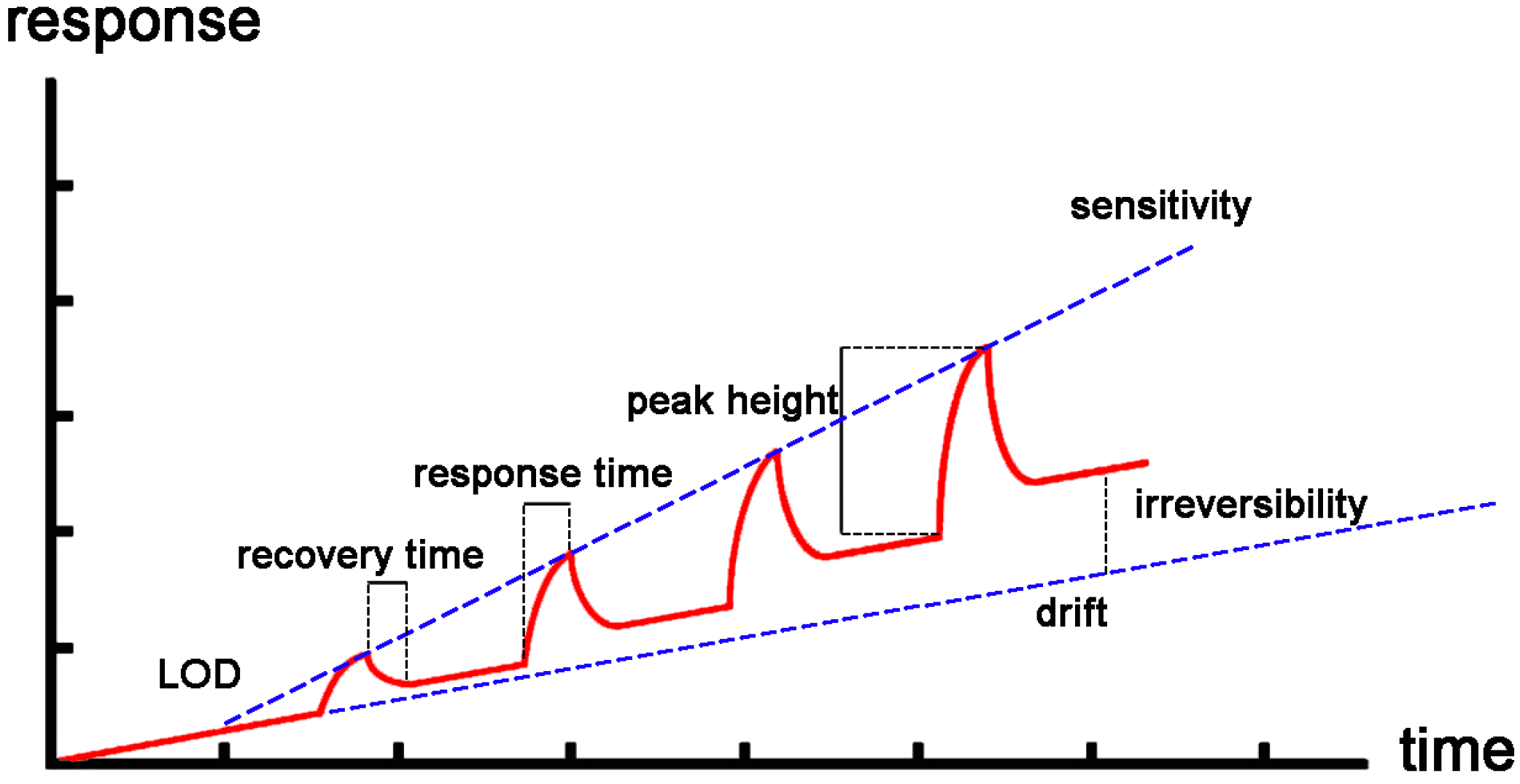
3. Main Performance Parameter of CNT Sensors
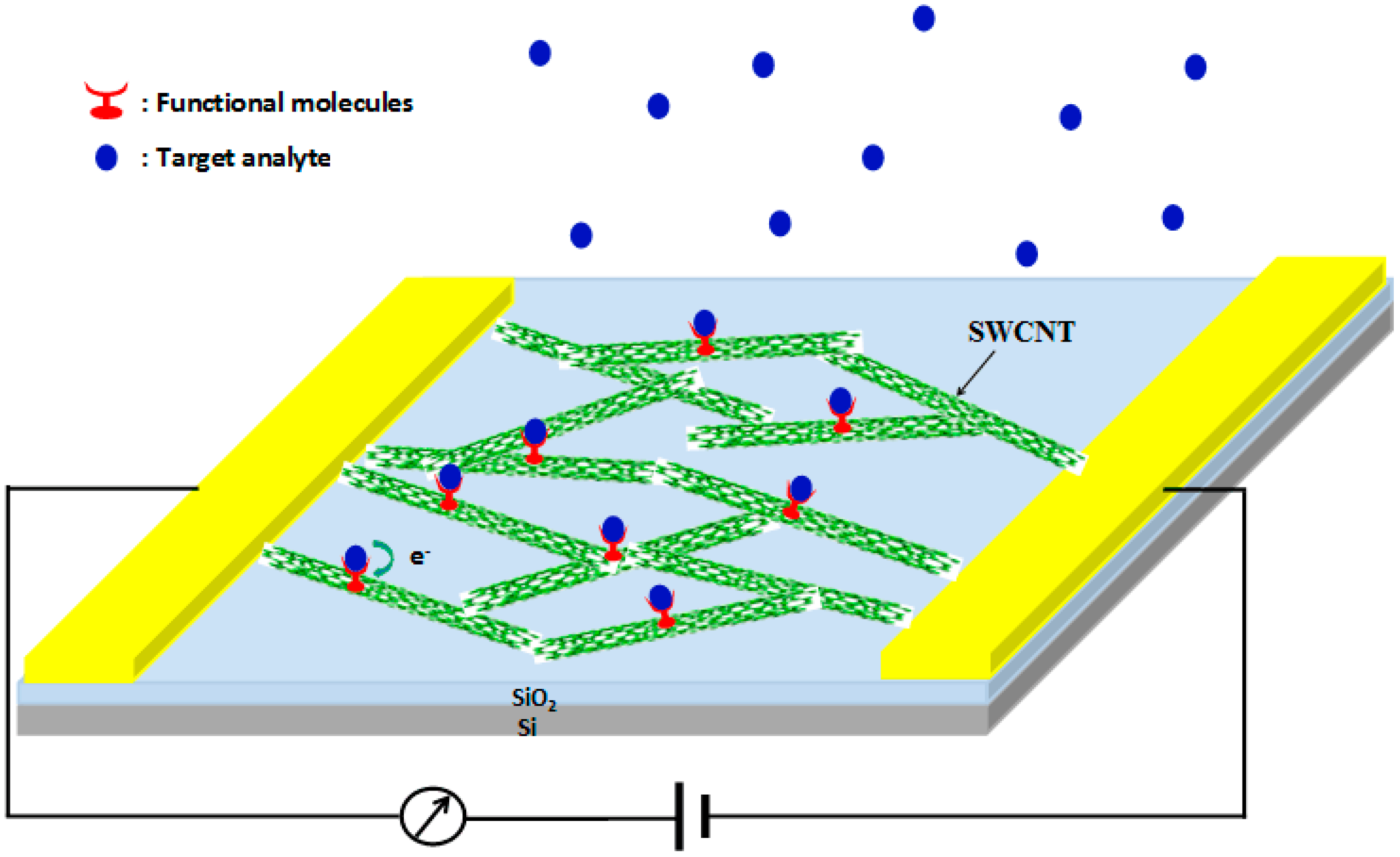
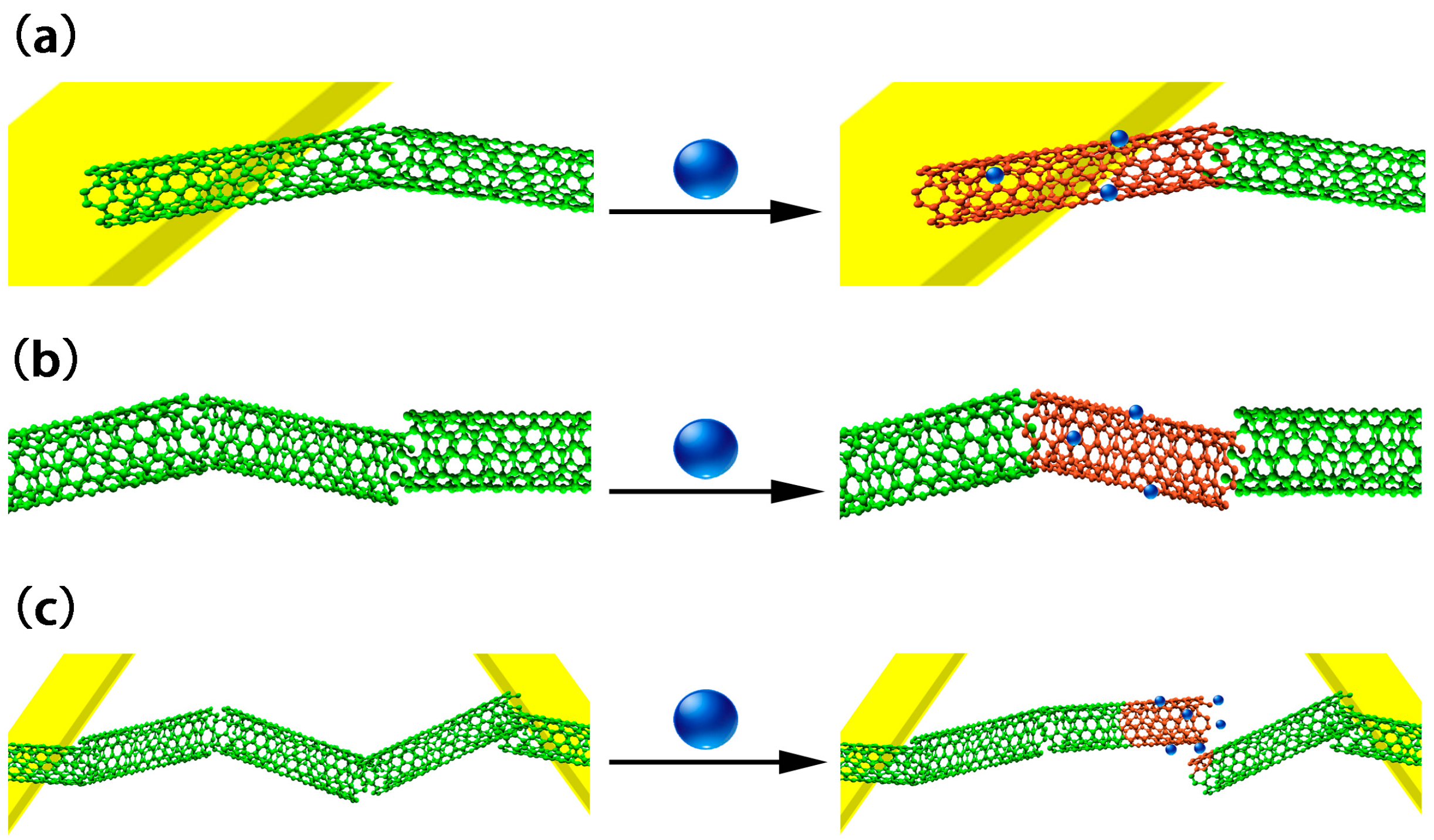
4. Sensing Mechanism of Chemiresistive Sensors
5. Nitrogen Dioxide (NO2) Chemiresistive Sensors
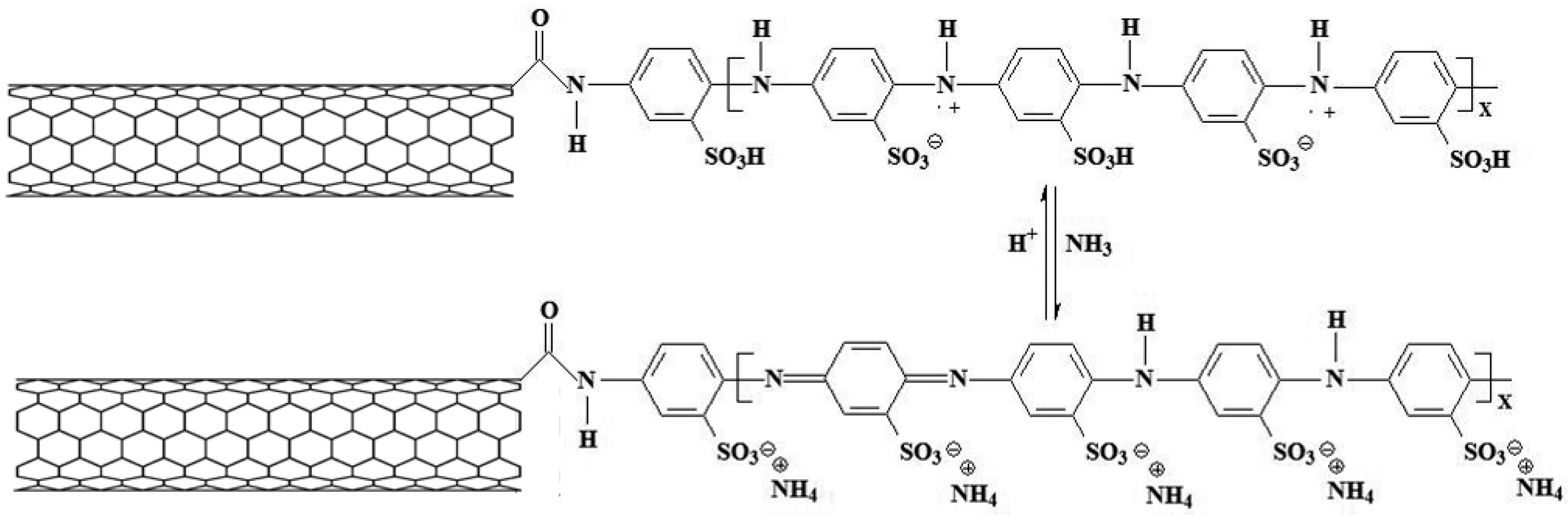
6. Ammonia (NH3) Chemiresistive Sensors
7. CNT-Based Hydrogen (H2) Chemiresistive Sensors
8. CNT-Based Greenhouse Gases Chemiresistive Sensors
9. CNT-Based Volatile Organic Compound (VOC) Chemiresistive Sensors
10. CNT-Basedchemiresistive Sensors for Military and Defense Application
11. CNT-Based Biological Chemiresistive Sensors
12. Conclusion and Outlook
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon nanotube gas and vapor sensors. Angew. Chem. Int. Ed. Engl. 2008, 47, 6550–6570. [Google Scholar] [CrossRef] [PubMed]
- Contés-de Jesús, E.; Li, J.; Cabrera, R.C. Latest Advances in Modified/Functionalized Carbon Nanotube- Based Gas Sensors. Nanomater. Nanotechnol. 2013. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Pingarrón, J.M.; Riu, J.; Rius, F.X. Electrochemical sensing based on carbon nanotubes. Trac Trend Anal. Chem. 2010, 29, 939–953. [Google Scholar] [CrossRef]
- Goldoni, A.; Petaccia, L.; Lizzit, S.; Larciprete, R. Sensing gases with carbon nanotubes: A review of the actual situation. J. Phys. Condens. Matter 2010, 22, 013001. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Kumar, A. Carbon nanotube (CNT) gas sensors for emissions from fossil fuel burning. Sens. Actuators B Chem. 2014, 203, 349–362. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Mubeen, S.; Myung, N.V.; Deshusses, M.A. Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19, 332001. [Google Scholar] [CrossRef] [PubMed]
- Merkoçi, A.; Pumera, M.; Llopis, X.; Pérez, B.; del Valle, M.; Alegret, S. New materials for electrochemical sensing VI: Carbon nanotubes. Trac Trend Anal. Chem. 2005, 24, 826–838. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Fu, K.; Lin, Y.; Huang, W. Functionalized Carbon Nanotubes: Properties and Applications. Accounts Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, T.; Yuan, S.; Tang, S. Functionalization of multi-walled carbon nanotubes with phenylenediamine for enhanced CO2 adsorption. Adsorption 2016, 23, 73–85. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. Functionalization of Carbon Nanotubes. In Functional Molecular Nanostructures; Schlüter, A.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 193–237. [Google Scholar]
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. Engl. 2002, 4, 1853–1859. [Google Scholar] [CrossRef]
- Feng, W.; Yi, W.; Wu, H.; Ozaki, M.; Yoshino, K. Enhancement of third-order optical nonlinearities by conjugated polymer-bonded carbon nanotubes. J. Appl. Phys. 2005, 98, 034301. [Google Scholar] [CrossRef]
- Shao, L.; Mu, C.; Du, H.; Czech, Z.; Du, H.; Bai, Y. Covalent marriage of multi-walled carbon nanotubes (MWNTs) and β-cyclodextrin (β-CD) by silicon coupling reagents. Appl. Surf. Sci. 2011, 258, 1682–1688. [Google Scholar] [CrossRef]
- Paul, S.; Lee, Y.-S.; Choi, J.-A.; Kang, Y.-C.; Kim, D.-W. Synthesis and Electrochemical Characterization of Polypyrrole/Multi-walled Carbon Nanotube Composite Electrodes for Supercapacitor Applications. B Korean Chem. Soc. 2010, 31, 1228–1232. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Mahouche Chergui, S.; Ledebt, A.; Mammeri, F.; Herbst, F.; Carbonnier, B.; Ben Romdhane, H.; Delamar, M.; Chehimi, M.M. Hairy Carbon Nanotube@Nano-Pd Heterostructures: Design, Characterization, and Application in Suzuki C−C Coupling Reaction. Langmuir ACS J. Surf. Colloids 2010, 26, 16115–16121. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Qin, D.; Ford, W.T.; Resasco, D.E.; Herrera, J.E. Functionalization of Single-Walled Carbon Nanotubes with Polystyrene via Grafting to and Grafting from Methods. Macromolecules 2004, 37, 752–757. [Google Scholar] [CrossRef]
- Hamwi, A.; Alvergnat, H.; Bonnamy, S.; Béguin, F. Fluorination of carbon nanotubes. Carbon 1997, 35, 723–728. [Google Scholar] [CrossRef]
- Kong, H.; Gao, C.; Yan, D. Controlled Functionalization of Multiwalled Carbon Nanotubes by in Situ Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2004, 126, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jeong, H.S.; Kim, B.M. Studies on the functionalization of MWNTs and their application as a recyclable catalyst for CC bond coupling reactions. Catal. Commun. 2014, 46, 71–74. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, C.; Li, H.; Du, Z. Fabrication of silica nanoparticles on the surface of functionalized multi-walled carbon nanotubes. Carbon 2011, 49, 126–132. [Google Scholar] [CrossRef]
- Fennell, J.F., Jr.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbaek, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. Engl. 2016, 55, 1266–1281. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah; Asmaly, H.A.; Saleh, T.A.; Laoui, T.; Gupta, V.K.; Atieh, M.A. Enhanced adsorption of phenols from liquids by aluminum oxide/carbon nanotubes: Comprehensive study from synthesis to surface properties. J. Mol. Liq. 2015, 206, 176–182. [Google Scholar]
- Liu, L.; Ma, W.; Zhang, Z. Macroscopic Carbon Nanotube Assemblies: Preparation, Properties, and Potential Applications. Small 2011, 7, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, K.; Burghard, M. Electrochemically functionalized carbon nanotubes for device applications. J. Mater. Chem. 2008, 18, 3071. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, S.S.; Kumar, S.; Agrawal, S.; Singh, M.; Vijay, Y.K. Characterization of gas sensing behavior of multi walled carbon nanotube polyaniline composite films. Int. J. Hydrogen Energy 2009, 34, 8444–8450. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Buongiorno Nardelli, M.; Lu, W.; Bernholc, J. Carbon Nanotube−Metal Cluster Composites: A New Road to Chemical Sensors? Nano. Lett. 2005, 5, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.S.; Liu, L.V.; Wang, Y.A. Adsorption of Small Gas Molecules onto Pt-Doped Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2008, 112, 7401–7411. [Google Scholar] [CrossRef]
- Larciprete, R.; Lizzit, S.; Petaccia, L.; Goldoni, A. NO2 decomposition on Rh clusters supported on single-walled carbon nanotubes. Appl.Phys. Lett. 2006, 88, 243111. [Google Scholar] [CrossRef]
- Penza, M.; Rossi, R.; Alvisi, M.; Signore, M.A.; Cassano, G.; Dimaio, D.; Pentassuglia, R.; Piscopiello, E.; Serra, E.; Falconieri, M. Characterization of metal-modified and vertically-aligned carbon nanotube films for functionally enhanced gas sensor applications. Thin Solid Films 2009, 517, 6211–6216. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Bannov, A.G.; Jasek, O.; Manakhov, A.; Marik, M.; Necas, D.; Zajickova, L. High-Performance Ammonia Gas Sensors Based on Plasma Treated Carbon Nanostructures. IEEE Sens. J. 2017, 17, 1964–1970. [Google Scholar] [CrossRef]
- Piloto, C.; Mirri, F.; Bengio, E.A.; Notarianni, M.; Gupta, B.; Shafiei, M.; Pasquali, M.; Motta, N. Room temperature gas sensing properties of ultrathin carbon nanotube films by surfactant-free dip coating. Sens. Actuators B Chem. 2016, 227, 128–134. [Google Scholar] [CrossRef]
- Dilonardo, E.; Penza, M.; Alvisi, M.; Di Franco, C.; Rossi, R.; Palmisano, F.; Torsi, L.; Cioffi, N. Electrophoretic deposition of Au NPs on MWCNT-based gas sensor for tailored gas detection with enhanced sensing properties. Sens. Actuaorst B Chem. 2016, 223, 417–428. [Google Scholar] [CrossRef]
- Yoon, B.; Liu, S.F.; Swager, T.M. Surface-Anchored Poly (4-vinylpyridine)-Single-Walled Carbon Nanotube-Metal Composites for Gas Detection. Chem. Mater. 2016, 28, 5916–5924. [Google Scholar] [CrossRef]
- Datta, K.; Ghosh, P.; More, M.A.; Shirsat, M.D.; Mulchandani, A. Controlled functionalization of single-walled carbon nanotubes for enhanced ammonia sensing: A comparative study. J. Phys. D Appl. Phys. 2012, 45, 355305. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.T.; Yu, H.; Chen, J.X.; Gao, B.B.; Lang, J.P. One Unique 1D Silver(I)-Bromide-Thiol Coordination Polymer Used for Highly Efficient Chemiresistive Sensing of Ammonia and Amines in Water. Inorg. Chem. 2016, 55, 9417–9423. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Wang, S.; Gu, W.; Zhuang, J.; Jin, H.; Zhang, W.; Zhang, T.; Wang, J. Ultrasensitive room-temperature detection of NO2 with tellurium nanotube based chemiresistive sensor. Sens. Actuators B Chem. 2014, 196, 321–327. [Google Scholar] [CrossRef]
- Valentini, L.; Armentano, I.; Kenny, J.M.; Cantalini, C.; Lozzi, L.; Santucci, S. Sensors for sub-ppm NO2 gas detection based on carbon nanotube thin films. Appl. Phys. Lett. 2003, 82, 961–963. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, P.; Saini, R.; Bedi, R.K.; Mahajan, A. Kinetic response study in chemiresistive gas sensor based on carbon nanotube surface functionalized with substituted phthalocyanines. AIP Conf. Proc. 2016, 1728, 020493. [Google Scholar]
- Gaikwad, S.; Bodkhe, G.; Deshmukh, M.; Patil, H.; Rushi, A.; Shirsat, M.D.; Koinkar, P.; Kim, Y.-H.; Mulchandani, A. Chemiresistive sensor based on polythiophene-modified single-walled carbon nanotubes for detection of NO2. Mod. Phys. Lett. B 2015, 29, 1540046. [Google Scholar] [CrossRef]
- Wang, S.G.; Zhang, Q.; Yang, D.J.; Sellin, P.J.; Zhong, G.F. Multi-walled carbon nanotube-based gas sensors for NH3 detection. Diam. Relat. Mater. 2004, 13, 1327–1332. [Google Scholar] [CrossRef]
- Mirica, K.A.; Weis, J.G.; Schnorr, J.M.; Esser, B.; Swager, T.M. Mechanical Drawing of Gas Sensors on Paper. Angew. Chem. Int. Ed. Engl. 2012, 51, 10740–10745. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, F.; Tognolini, S.; Borghetti, P.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. Enhancing the sensitivity of chemiresistor gas sensors based on pristine carbon nanotubes to detect low-ppb ammonia concentrations in the environment. Analyst 2013, 138, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Jia, Y.; Meng, F.; Li, M.; Liu, J. Gas sensors for ammonia detection based on polyaniline-coated multi-wall carbon nanotubes. Mat. Sci. Eng. B 2009, 163, 76–81. [Google Scholar] [CrossRef]
- Nguyen, L.; Phan, P.; Duong, H.; Nguyen, C.; Nguyen, L. Enhancement of NH3 Gas Sensitivity at Room Temperature by Carbon Nanotube-Based Sensor Coated with Co Nanoparticles. Sensors 2013, 13, 1754. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Scardaci, V.; Kim, H.-Y.; Hallam, T.; Nolan, H.; Bolf, B.E.; Maltbie, G.S.; Abbott, J.E.; Duesberg, G.S. Highly sensitive, transparent, and flexible gas sensors based on gold nanoparticle decorated carbon nanotubes. Sens. Actuators B. Chem. 2013, 188, 571–575. [Google Scholar] [CrossRef]
- Liu, S.F.; Petty, A.R.; Sazama, G.T.; Swager, T.M. Single-walled carbon nanotube/metalloporphyrin composites for the chemiresistive detection of amines and meat spoilage. Angew. Chem. Int. Ed. Engl. 2015, 54, 6554–6557. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, E.; Davis, M.; Burch, T.; Itkis, M.E.; Zhao, B.; Sunshine, S.; Haddon, R.C. Chemically Functionalized Single-Walled Carbon Nanotubes as Ammonia Sensors. J. Phys. Chem. B 2004, 108, 19717–19720. [Google Scholar] [CrossRef]
- Zhang, T.; Nix, M.B.; Yoo, B.-Y.; Deshusses, M.A.; Myung, N.V. Electrochemically Functionalized Single-Walled Carbon Nanotube Gas Sensor. Electroanalysis 2006, 18, 1153–1158. [Google Scholar] [CrossRef]
- Lone, M.Y.; Kumar, A.; Husain, S.; Zulfequar, M.; Harsh; Husain, M. Growth of single wall carbon nanotubes using PECVD technique: An efficient chemiresistor gas sensor. Phys. E Low Dimens. Syst. Nanostruct. 2017, 87, 261–265. [Google Scholar] [CrossRef]
- Sayago, I.; Terrado, E.; Aleixandre, M.; Horrillo, M.C.; Fernández, M.J.; Lozano, J.; Lafuente, E.; Maser, W.K.; Benito, A.M.; Martinez, M.T.; Gutiérrez, J.; Muñoz, E. Novel selective sensors based on carbon nanotube films for hydrogen detection. Sens. Actuators B Chem. 2007, 122, 75–80. [Google Scholar] [CrossRef]
- Randeniya, L.K.; Martin, P.J.; Bendavid, A. Detection of hydrogen using multi-walled carbon-nanotube yarns coated with nanocrystalline Pd and Pd/Pt layered structures. Carbon 2012, 50, 1786–1792. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. Hybrid carbon nanostructured ensembles as chemiresistive hydrogen gas sensors. Carbon 2011, 49, 227–236. [Google Scholar] [CrossRef]
- Li, W.; Hoa, N.D.; Kim, D. High-performance carbon nanotube hydrogen sensor. Sens. Actuators B Chem. 2010, 149, 184–188. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Han, J.; Ng, H.T.; Binder, C.; Partridge, C.; Meyyappan, M. Room temperature methane detection using palladium loaded single-walled carbon nanotube sensors. Chem. Phys. Lett. 2004, 391, 344–348. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Wang, X.; Zhu, Z.; Ma, H.; Zhang, T.; Jin, J. Poly(ionic liquid)-wrapped single-walled carbon nanotubes for sub-ppb detection of CO2. Chem. Commun. 2012, 48, 8222–8224. [Google Scholar] [CrossRef] [PubMed]
- Sainato, M.; Humayun, M.T.; Gundel, L.; Solomon, P.; Stan, L.; Divan, R.; Paprotny, I. Parts per million CH4 chemoresistor sensors based on multi wall carbon nanotubes/metal-oxide nanoparticles. Proceedings of 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Chiou, J.C.; Wu, C.C.; Huang, Y.C.; Chang, S.C.; Lin, T.M. Effects of Operating Temperature on Droplet Casting of Flexible Polymer/Multi-Walled Carbon Nanotube Composite Gas Sensors. Sensors 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gu, H.; Swager, T.M. Carbon Nanotube/Polythiophene Chemiresistive Sensors for Chemical Warfare Agents. J. Am. Chem. Soc. 2008, 130, 5392–5393. [Google Scholar] [CrossRef] [PubMed]
- Rushi, A.D.; Gaikwad, S.; Deshmukh, M.; Patil, H.; Bodkhe, G.; Shirsat, M.D. Functionalized carbon nanotubes: Facile development of gas sensor platform. AIP Conf. Proc. 2016, 1728, 020164. [Google Scholar]
- Sarkar, T.; Srinives, S.; Sarkar, S.; Haddon, R.C.; Mulchandani, A. Single-Walled Carbon Nanotube–Poly(porphyrin) Hybrid for Volatile Organic Compounds Detection. J. Phys. Chem. C 2014, 118, 1602–1610. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Sarkar, T.; Kakoullis, J.; Myung, N.V.; Konnanath, B.; Spanias, A.; Mulchandani, A. Porphyrin-Functionalized Single-Walled Carbon Nanotube Chemiresistive Sensor Arrays for VOCs. J. Phys. Chem. C 2012, 116, 3845–3850. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Moh, L.C.H.; Swager, T.M. Single-Walled Carbon Nanotube–Metalloporphyrin Chemiresistive Gas Sensor Arrays for Volatile Organic Compounds. Chem. Mater. 2015, 27, 3560–3563. [Google Scholar] [CrossRef]
- Shi, D.; Wei, L.; Wang, J.; Zhao, J.; Chen, C.; Xu, D.; Geng, H.; Zhang, Y. Solid organic acid tetrafluorohydroquinone functionalized single-walled carbon nanotube chemiresistive sensors for highly sensitive and selective formaldehyde detection. Sens. Actuators B Chem. 2013, 177, 370–375. [Google Scholar] [CrossRef]
- Xie, H.; Sheng, C.; Chen, X.; Wang, X.; Li, Z.; Zhou, J. Multi-wall carbon nanotube gas sensors modified with amino-group to detect low concentration of formaldehyde. Sens. Actuators B Chem. 2012, 168, 34–38. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, D.; Zhang, Y.; Liu, C. Boron-Doped Carbon Nanotubes Serving as a Novel Chemical Sensor for Formaldehyde. J. Phys. Chem. B 2006, 110, 18267–18271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Zhang, J.; Chen, J.L.; Zhu, Z.Q.; Liu, Q.J. Improvement of response to formaldehyde at Ag–LaFeO3 based gas sensors through incorporation of SWCNTs. Sens. Actuators B Chem. 2014, 195, 509–514. [Google Scholar] [CrossRef]
- Yijiang, L.; Meyyappan, M.; Jing, L. A carbon-nanotube-based sensor array for formaldehyde detection. Nanotechnology 2011, 22, 055502. [Google Scholar]
- Frazier, K.M.; Swager, T.M. Robust Cyclohexanone Selective Chemiresistors Based on Single-Walled Carbon Nanotubes. Anal. Chem. 2013, 85, 7154–7158. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon Nanotube Sensors for Gas and Organic Vapor Detection. Nano. Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Wei, L.; Lu, D.; Wang, J.; Wei, H.; Zhao, J.; Geng, H.; Zhang, Y. Highly sensitive detection of trinitrotoluene in water by chemiresistive sensor based on noncovalently amino functionalized single-walled carbon nanotube. Sens. Actuators B Chem. 2014, 190, 529–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Bunes, B.R.; Wu, N.; Gross, D.E.; Moore, J.S.; Zang, L. Oligomer-Coated Carbon Nanotube Chemiresistive Sensors for Selective Detection of Nitroaromatic Explosives. ACS Appl. Mater. Inter. 2015, 7, 7471–7475. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Shi, D.; Ye, P.; Dai, Z.; Chen, H.; Chen, C.; Wang, J.; Zhang, L.; Xu, D.; Wang, Z.; Zhang, Y. Hole doping and surface functionalization of single-walled carbon nanotube chemiresistive sensors for ultrasensitive and highly selective organophosphor vapor detection. Nanotechnology 2011, 22, 425501. [Google Scholar]
- Balasubramanian, K.; Burghard, M. Biosensors based on carbon nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Landry, M.P.; Vander Ende, E.; Lima, B.M.A.; Reuel, N.F.; Zhang, J.; Nelson, J.; Mu, B.; Hilmer, A.; Strano, M. Neurotransmitter Detection Using Corona Phase Molecular Recognition on Fluorescent Single-Walled Carbon Nanotube Sensors. J. Am. Chem. Soc. 2014, 136, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Baghayeri, M.; Amiri, A.; Farhadi, S. Development of non-enzymatic glucose sensor based on efficient loading Ag nanoparticles on functionalized carbon nanotubes. Sens. Actuators B Chem. 2016, 225, 354–362. [Google Scholar] [CrossRef]
- Garcia-Aljaro, C.; Cella, L.N.; Shirale, D.J.; Park, M.; Munoz, F.J.; Yates, M.V.; Mulchandani, A. Carbon nanotubes-based chemiresistive biosensors for detection of microorganisms. Biosens. Bioelectron. 2010, 26, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Cella, L.N.; Chen, W.; Myung, N.V.; Mulchandani, A. Single-Walled Carbon Nanotube-Based Chemiresistive Affinity Biosensors for Small Molecules: Ultrasensitive Glucose Detection. J. Am. Chem. Soc. 2010, 132, 5024–5026. [Google Scholar] [CrossRef] [PubMed]
- Puri, N.; Niazi, A.; Biradar, A.M.; Mulchandani, A.; Rajesh. Conducting polymer functionalized single-walled carbon nanotube based chemiresistive biosensor for the detection of human cardiac myoglobin. Appl. Phys. Lett. 2014, 105, 153701. [Google Scholar] [CrossRef]
- Vikash, S.; Nitin, K.P.; Ashok, M.; Rajesh. Platinum nanoparticles-single-walled carbon nanotubes hybrid based chemiresistive sensor array for myoglobin detection. Mater. Res. Express 2016, 3, 035006. [Google Scholar]
- Sharma, V.; Puri, N.K.; Rajesh. Hybrid of Platinum Nanoparticles and 1-Dimentional Single-walled Carbon Nanotubes Based Chemiresistive Biosensor. Sens. Transducers 2016, 197, 34–38. [Google Scholar]
- Durga Prakash, M.; Vanjari, S.R.; Sharma, C.S.; Singh, S.G. Ultrasensitive, Label Free, Chemiresistive Nanobiosensor Using Multiwalled Carbon Nanotubes Embedded Electrospun SU-8 Nanofibers. Sensors 2016, 16, 1354. [Google Scholar] [CrossRef] [PubMed]
| Target Analytes | CNT Material/Method | LOD | Response Time | Rreversibility | Reference |
|---|---|---|---|---|---|
| NO2 | Pristine | 10 ppb | a few minutes | reversible | [43] |
| polythiophene-SWNTs | 10 ppb | low ~20 s | N/S | [45] | |
| NH3 | pristine | N/S | ~180 s | reversible | [46] |
| Au nanoparticles-decorated SWCNT | 255 ppb | ~20 s | N/S | [51] | |
| PANI-coated MWNT | 0.2 ppm | 10 s–120 s | N/S | [49] | |
| SWNT-PABS | 5 ppm | ~1 min | reversible | [53] | |
| PANI-SWNT network | 50 ppb | sub-ppm | reversible | [54] |
| Target Analytes | CNT Material/Method | LOD | Reference |
|---|---|---|---|
| H2 | Pt-Pd-MWCNT | 400 ppm | [57] |
| Pd-MWCNT | 2000 ppm | [57] | |
| CO2 | poly(ionic liquid) (PIL)-wrapped SWNTs | 500 ppt | [61] |
| CH4 | MWCNTs/ZnO composite | 10 ppm | [62] |
| Target Analytes | CNT Material/Method | Detection Limit | Response Time | Reversibility | Reference |
|---|---|---|---|---|---|
| nitrotoluene | pristine | 262 ppb | n/s | reversible | [75] |
| SWNTs-poly(tetraphenylporphyrin) hybird | 9 ppm | ~8 min | reversible | [66] | |
| TNT | PMA-SWCNT network | 10 ppt | <1 min | n/s | [76] |
| formaldhyde | TFQ-functionalized SWNT | ppb level | < 1 min | n/s | [69] |
| amino-functionalized MWCNTs | 20 ppb | 7–10 s | reversible | [70] | |
| SWCNT-Ag-LaFeO3 structure | 0.2 ppm | 6 s | reversible | [72] | |
| cyclohexanone | trifunctional selectors functionalized SWCNTs | 5 ppm | < 30 s | reversible | [74] |
| DMMP | SWNT-TFQ network | 20 ppt | < 2 min | n/s | [78] |
| Target Analytes | CNT Material/Method | LOD/Concentration Range | Reference |
|---|---|---|---|
| glucose | polysaccharide immobilized SWNTs | 50 nM for ConA cyclodextrin, 3.7 mM for dextran solution | [83] |
| myoglobin | poly(pyrrole-co-pyrrolepropylic acid) deposited SWCNT | 1.0 ng mL−1 to 1000 ng mL−1 | [84] |
| PtNP-SWNT | 0.1–1000 ng mL−1 | [85] | |
| MWCTs embedded electrospun SU-8 nanofibers | LOD:6 fg/mL Range: 20 fg/mL to 70 fg/mL | [87] | |
| c TnI | PtNP-SWNT | 0.001 ng mL−1 to 10 ng mL−1 | [86] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. https://doi.org/10.3390/s17040882
Tang R, Shi Y, Hou Z, Wei L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors. 2017; 17(4):882. https://doi.org/10.3390/s17040882
Chicago/Turabian StyleTang, Ruixian, Yongji Shi, Zhongyu Hou, and Liangming Wei. 2017. "Carbon Nanotube-Based Chemiresistive Sensors" Sensors 17, no. 4: 882. https://doi.org/10.3390/s17040882





