Quantitative Detection of NADH Using a Novel Enzyme-Assisted Method Based on Surface-Enhanced Raman Scattering
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Sample Preparation
2.3. SERS Measurements
3. Results and Discussion
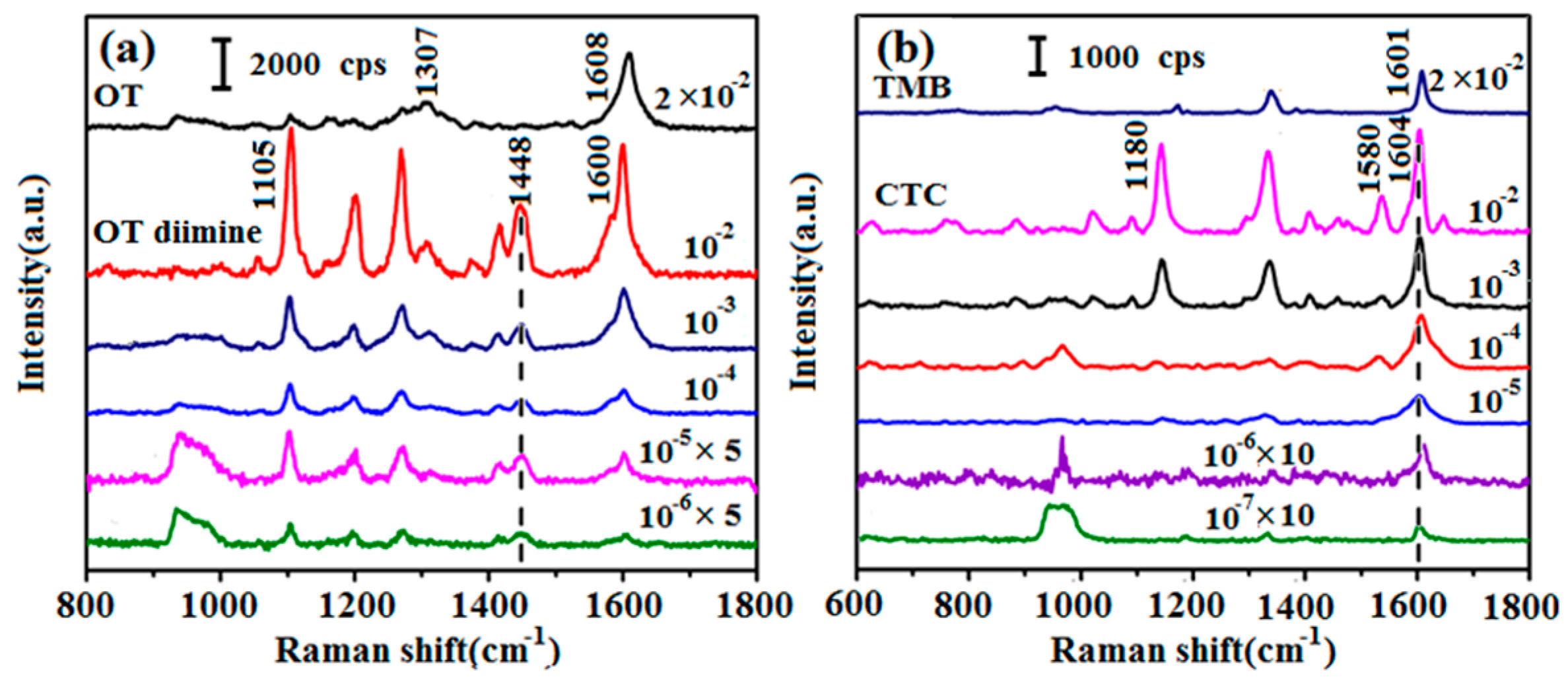
3.1. Chromogen Screening
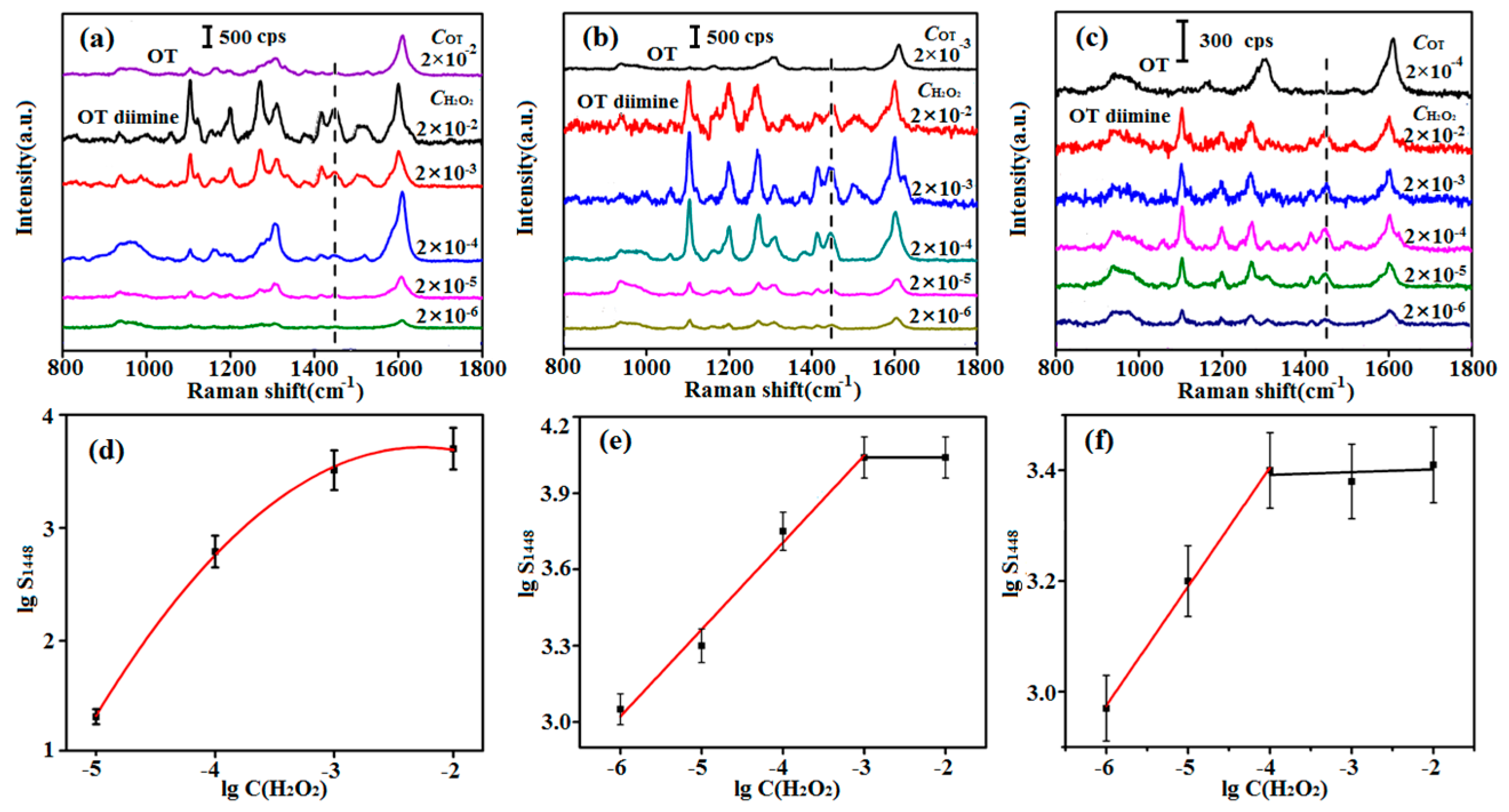
3.2. Optimization of the OT Concentration
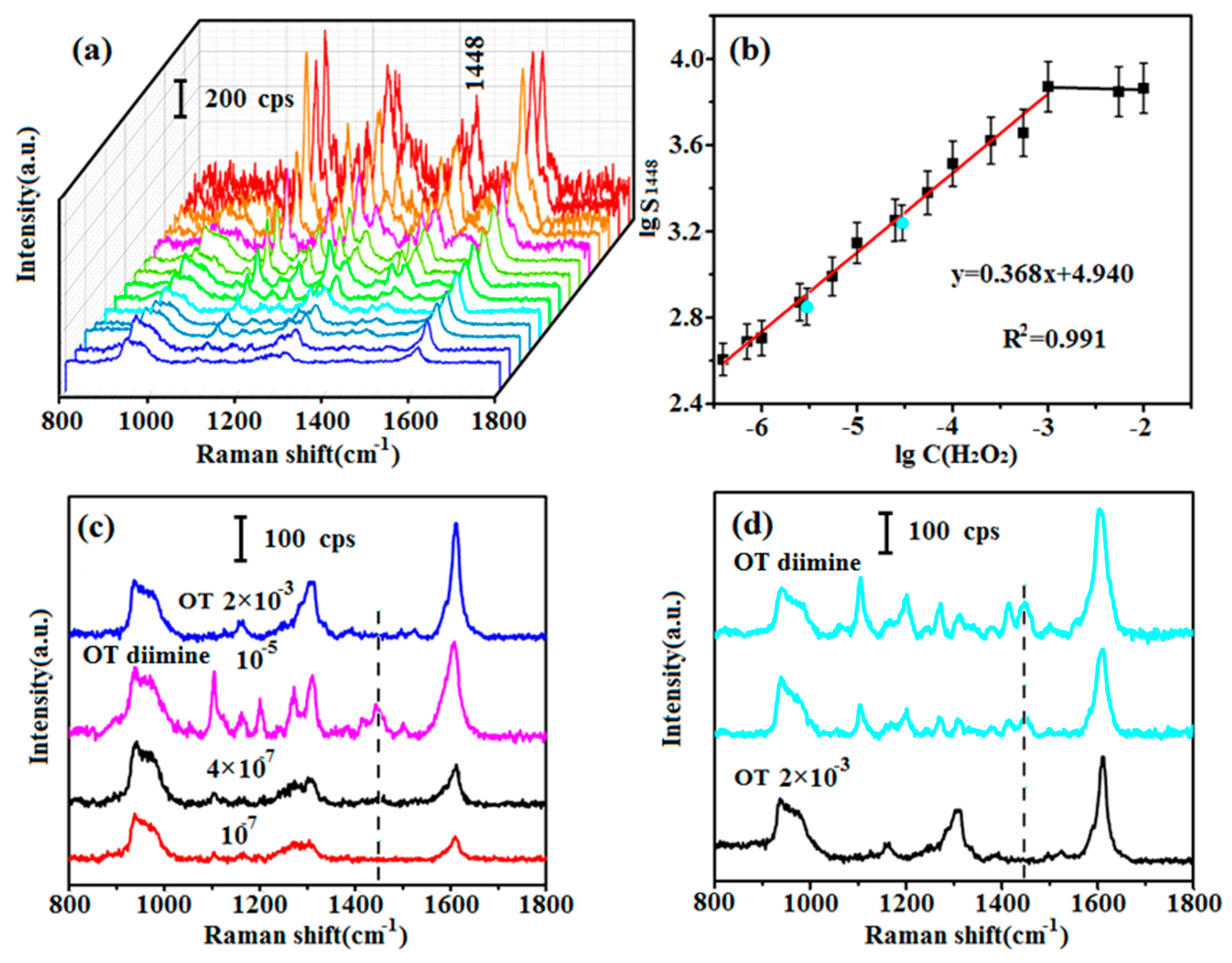
3.3. Establishment of the Relationship Curve
3.4. Validation of the Relationship Curve
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pollak, N.; Dolle, C.; Ziegler, M. The power to reduce: Pyridine nucleotides-small molecules with a multitude of functions. Biochem. J. 2007, 402, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, M.; Szabo, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Ma, W.; Li, D.W.; Sutherland, T.C.; Li, Y.; Long, Y.T.; Chen, H.Y. Reversible redox of NADH and NAD+ at a hybrid lipid bilayer membrane using ubiquinone. J. Am. Chem. Soc. 2011, 133, 12366–12369. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.T.; Martin, C.L.; Chen, D.H.; Nelson, P.; Sloan, D.L.; Callender, R. Raman spectroscopy of oxidized and reduced nicotinamide adenine dinucleotides. Biochemistry 1986, 25, 4941–4947. [Google Scholar] [CrossRef] [PubMed]
- Berrios-Rivera, S.J.; Bennett, G.N.; San, K.Y. Metabolic engineering of escherichia coli: Increase of NADH availability by over expressing an NAD+—Dependent formate dehydrogenase. Metab. Eng. 2002, 4, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Salis, A.; Curulli, A.; Palleschi, G. Chemical reversibility and stable low-potential NADH detection with nonconventional conducting polymer nanotubule modified glassy carbon electrodes. Anal. Chem. 2004, 76, 3244–3248. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, S.H.; Kim, J.H.; Park, C.B. Artificial photosynthesis on a chip: Microfluidic cofactor regeneration and photoenzymatic synthesis under visible light. Lab Chip 2011, 11, 2309–2311. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.O.; Ahn, J.Y.; Kim, S.; Yi, S.; Kim, M.H.; Jang, H.H.; Seo, S.H.; Eom, M.S.; Kim, S.K.; Ryu, D.H.; et al. Fluorescein derivative-based, selective and sensitive chemosensor for NADH. Tetrahedron Lett. 2010, 51, 3775–3778. [Google Scholar] [CrossRef]
- Ghukasyan, V.V.; Kao, F.J. Monitoring cellular metabolism with fluorescence lifetime of reduced nicotinamide adenine dinucleotide. J. Phys. Chem. C 2009, 113, 11532–11540. [Google Scholar] [CrossRef]
- Baron, R.; Lioubashevski, O.; Katz, E.; Niazov, T.; Willner, I. Elementary Arithmetic Operations by Enzymes: A Model for Metabolic Pathway Based Computing. Angew. Chem. Int. Ed. 2006, 45, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Radoi, A.; Compagnone, D.; Devic, E.; Pallechi, G. Low potential detection of NADH with Prussian Blue bulk modified screen-printed electrodes and recombinant NADH oxidase from thermus thermophilus. Sens. Actuators B 2007, 121, 501–506. [Google Scholar] [CrossRef]
- Harper, J.C.; Polsky, R.; Dirk, S.M.; Wheeler, D.R.; Brozik, S.M. Electroaddressable selective functionalization of electrode arrays: Catalytic NADH detection using aryl diazonium modified gold electrodes. Electroanalysis 2007, 19, 1268–1274. [Google Scholar] [CrossRef]
- Jochem, G.; Lehnert, R.J. On the potential of Raman microscopy for the forensic analysis of coloured textile fibres. Sci. Justice 2002, 42, 215–221. [Google Scholar] [CrossRef]
- Kasera, S.; Herrmann, L.O.; del Barrio, J.; Baumberg, J.J.; Scherman, O.A. Quantitative multiplexing with nano-self-assemblies in SERS. Sci. Rep. 2014, 4, 6785. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gu, C.; Qian, F.; Li, Y.; Zhang, J.Z. Highly sensitive detection of proteins and bacteria in aqueous solution using surface-enhanced Raman scatteringand optical fibers. Anal. Chem. 2011, 83, 5888–5894. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yin, H.J.; Lv, M.Y.; Xv, H.J.; Zhao, Y.M.; Zhang, X.; Wu, Z.L.; Liu, L.; Tan, T.W. Approach for determination of ATP: ADP molar ratio in mixed solution by surface-enhanced Raman scattering. Biosens. Bioelectron. 2015, 69, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Zhang, H.D.; Xu, S.P.; Gan, T.; Huang, K.J.; Liu, Y.M.; Zhang, X.; Liu, L.; Wang, M.L.; Xu, H.J. A sensitive and label-free electrochemical impedance biosensor for protein detection based on terminal protection of small molecule-linked DNA. Sens. Actuators B 2014, 194, 478–483. [Google Scholar] [CrossRef]
- Dasary, S.S.R.; Jones, Y.K.; Barnes, S.L.; Ray, P.C.; Singh, A.K. Alizarin dye based ultrasensitive plasmonic SERS probe for trace level cadmium detection in drinking water. Sens. Actuators B 2016, 224, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Z.B.; Peng, B.; Cao, C.; Zhang, C.; You, H.Y.; Xiong, Q.H.; Li, Z.Y.; Fang, J.X. Highly sensitive, uniform and reproducible surface-enhanced Raman spectroscopy from hollow Au-Ag Alloy nanourchins. Adv. Mater. 2014, 26, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.C.; Sivashanmugan, K.; Liao, J.D.; Yao, C.K.; Peng, H.C. Nanofabricated SERS-active substrates for single-molecule to virus detection in vitro: A review. Biosens. Bioelectron. 2014, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X. Theoretical study of coated spherical metallic nanoparticles for single molecule surface-enhanced spectroscopy. Appl. Phys. Lett. 2004, 85, 5980–5982. [Google Scholar] [CrossRef]
- Otto, A.; Mrozek, I.; Grabhorn, H.; Akemann, W. Surface-enhanced Raman scattering. J. Phys. Condens. Matter 1992, 4, 1143–1212. [Google Scholar] [CrossRef]
- Ward, D.R.; Grady, N.K.; Levin, C.S.; Halas, N.J.; Wu, Y.; Nordlander, P.; Natelson, D. Electromigrated nanoscale gaps for surface-enhanced Raman spectroscopy. Nano Lett. 2007, 7, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Volpe, G.; Draisci, R.; Palleschi, G.; Compagnone, D. 3,3′,5,5′-Tetramethylbenzidine as electrochemical substrate for horseradish peroxidase based enzyme immunoassays. A comparative study. Analyst 1998, 123, 1303–1307. [Google Scholar] [CrossRef]
- Zhang, C.X.; Liu, L.; Yin, H.J.; Fang, H.; Zhao, Y.M.; Chu, J.B.; Xu, H.J. Recyclable surface-enhanced Raman scattering template based on nanoporous gold film/Si nanowire arrays. Appl. Phys. Lett. 2014, 105, 011905. [Google Scholar]
- Bell, S.; Crayston, J.A.; Dines, T.J.; Ellahi, S.B. Resonance Raman, surface-enhanced resonance Raman, infrared, and ab initio vibrational spectroscopic study of tetraazaannulenes. J. Phys. Chem. 1996, 100, 5252–5260. [Google Scholar] [CrossRef]
- Laing, S.; Hernandez-Santana, A.; Sassmannshausen, J.; Asquith, D.L.; McInnes, I.B.; Faulds, K.; Graham, D. Quantitative detection of human tumor necrosis factor α by a resonance Raman enzyme-linked immunosorbent assay. Anal. Chem. 2011, 83, 297–302. [Google Scholar] [CrossRef] [PubMed]
| Sample | S1448 | lgC(NADH) | RSD (%) | Ζ (%) | |
|---|---|---|---|---|---|
| Actual | Calculated | ||||
| 1 | 1722 | −4.54 | −4.63 | 2.42 | 1.98 |
| 2 | 706 | −5.54 | −5.68 | 2.09 | 2.53 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, H.; Lv, M.; Liu, L.; Zhang, X.; Zhao, Y.; Wu, Z.; Xu, H. Quantitative Detection of NADH Using a Novel Enzyme-Assisted Method Based on Surface-Enhanced Raman Scattering. Sensors 2017, 17, 788. https://doi.org/10.3390/s17040788
Teng H, Lv M, Liu L, Zhang X, Zhao Y, Wu Z, Xu H. Quantitative Detection of NADH Using a Novel Enzyme-Assisted Method Based on Surface-Enhanced Raman Scattering. Sensors. 2017; 17(4):788. https://doi.org/10.3390/s17040788
Chicago/Turabian StyleTeng, Haiyan, Mingyang Lv, Luo Liu, Xin Zhang, Yongmei Zhao, Zhenglong Wu, and Haijun Xu. 2017. "Quantitative Detection of NADH Using a Novel Enzyme-Assisted Method Based on Surface-Enhanced Raman Scattering" Sensors 17, no. 4: 788. https://doi.org/10.3390/s17040788







