Rapid and Low-Cost CRP Measurement by Integrating a Paper-Based Microfluidic Immunoassay with Smartphone (CRP-Chip)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals for the CRP-Chip Test
2.2. Blood Samples
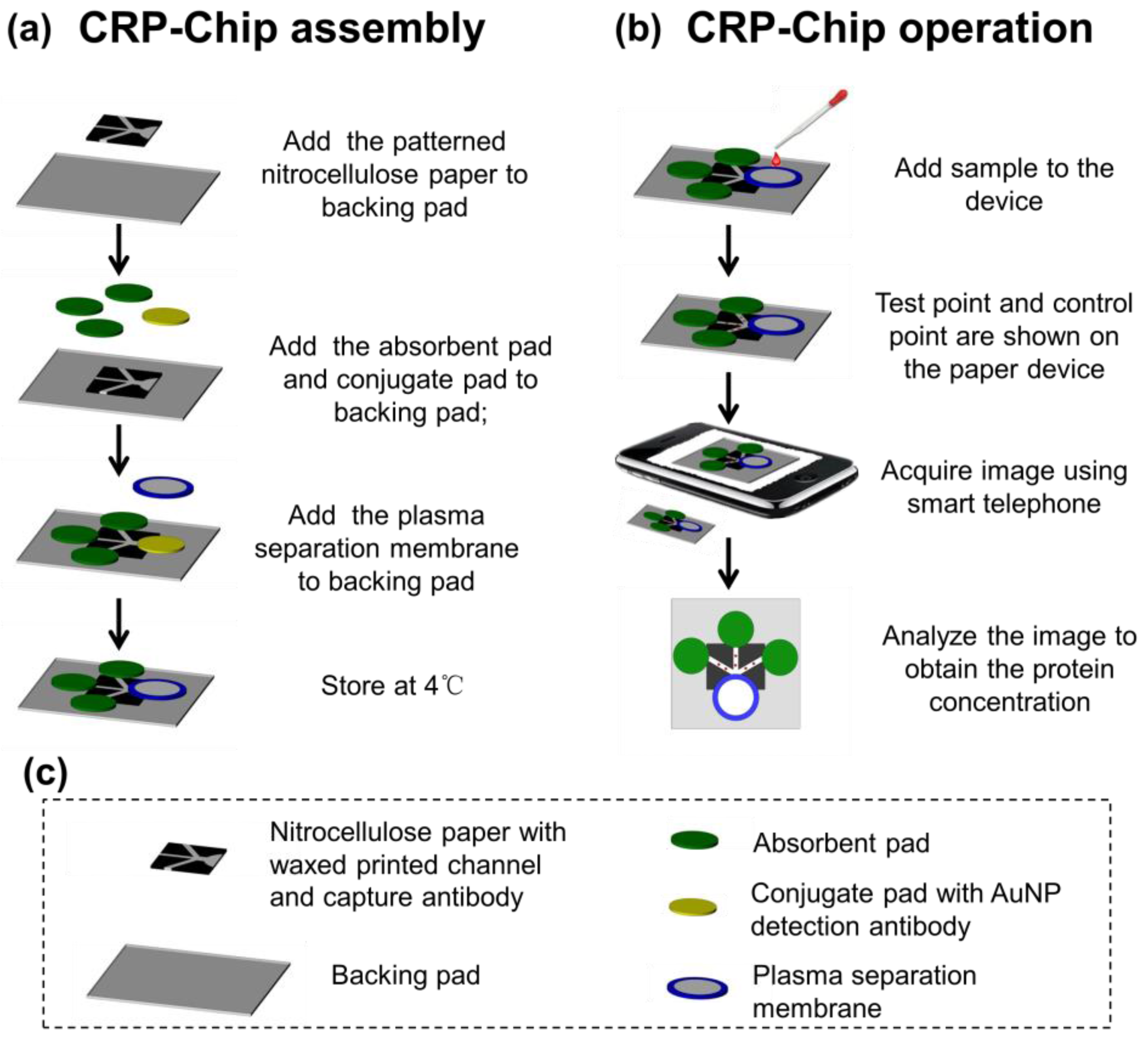
2.3. Paper Devices Fabrication
2.4. CRP-Chip Test Protocol
2.5. Polydimethylsiloxane (PDMS) Microlens Fabrication
2.6. Smartphone Imaging
2.7. Data Analysis
2.8. CRP Test Using Conventional ELISA Kit
3. Results
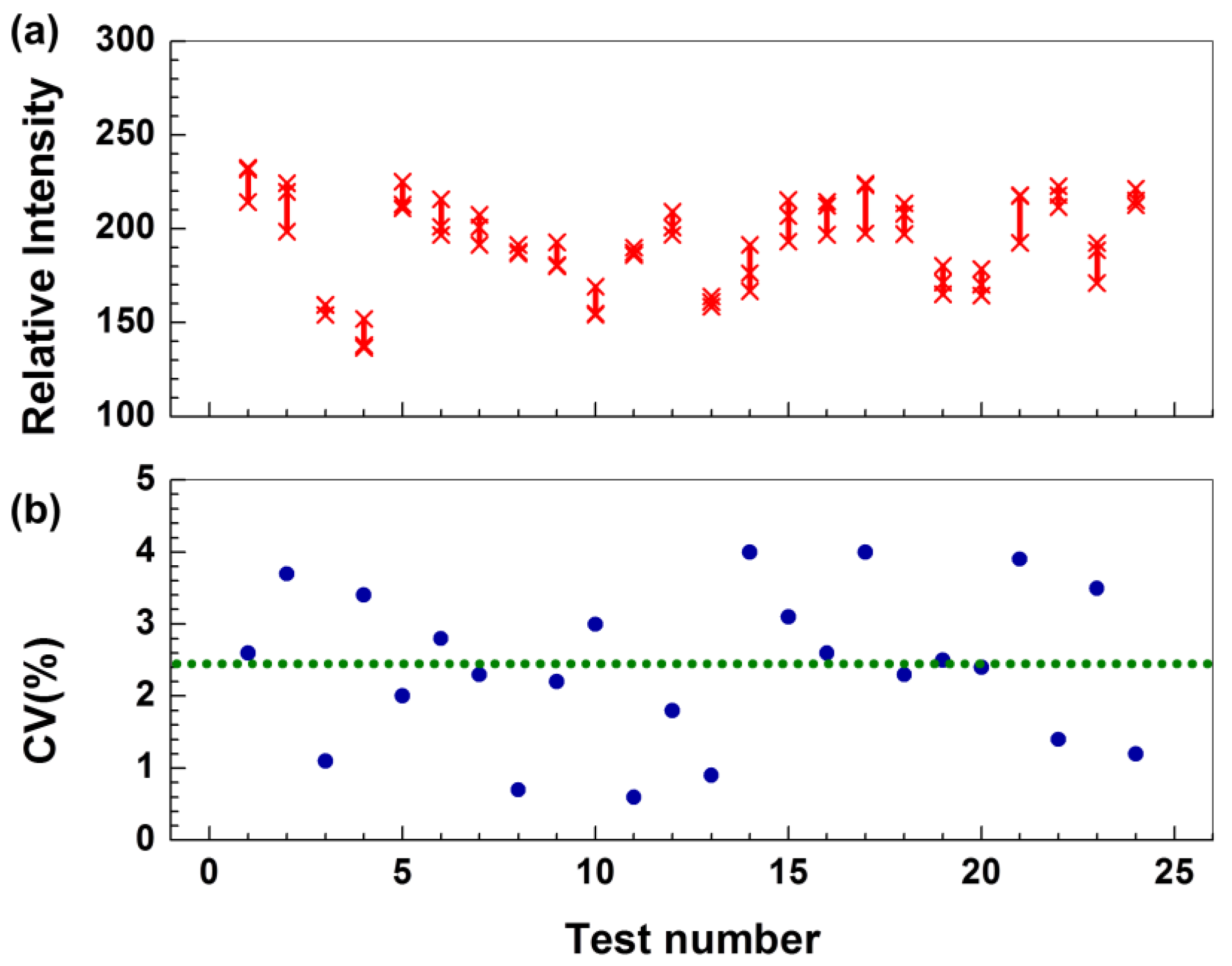
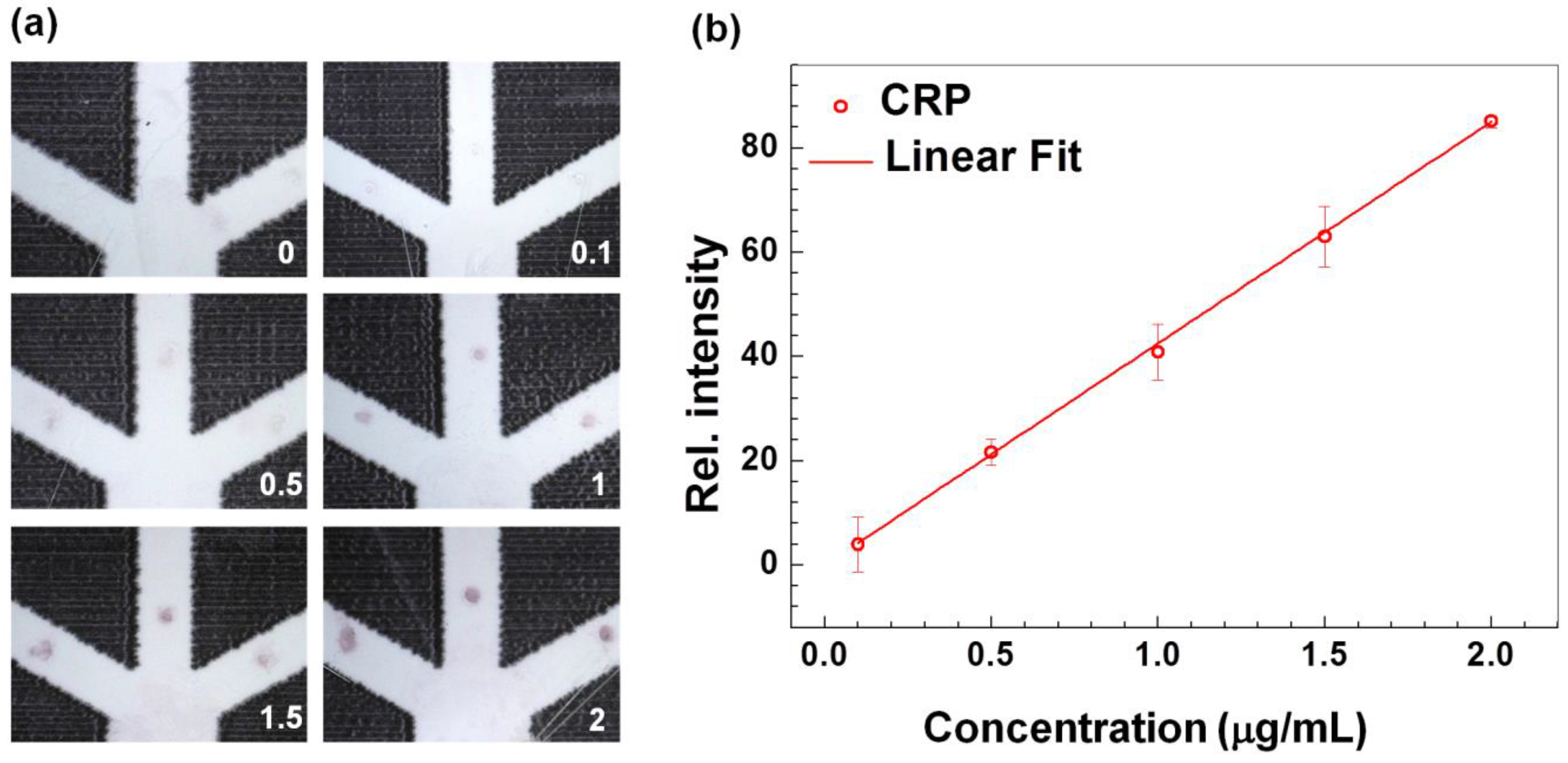
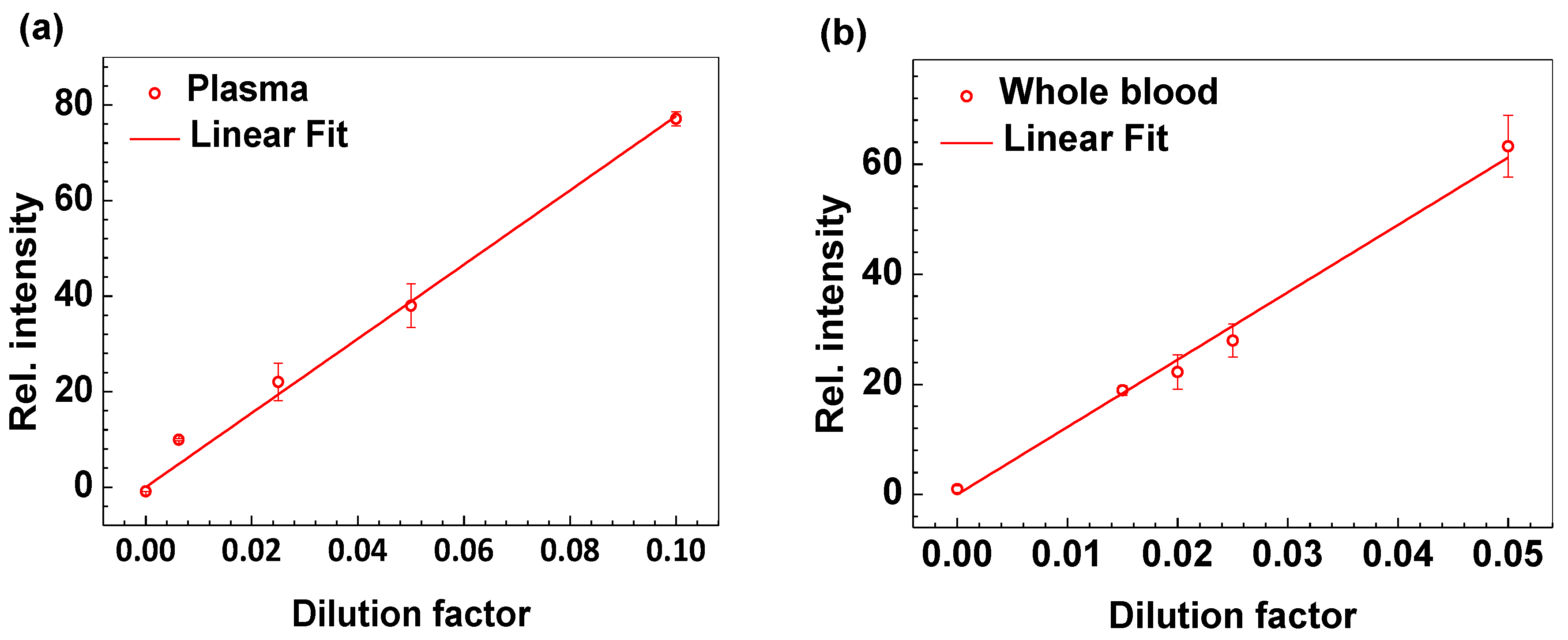
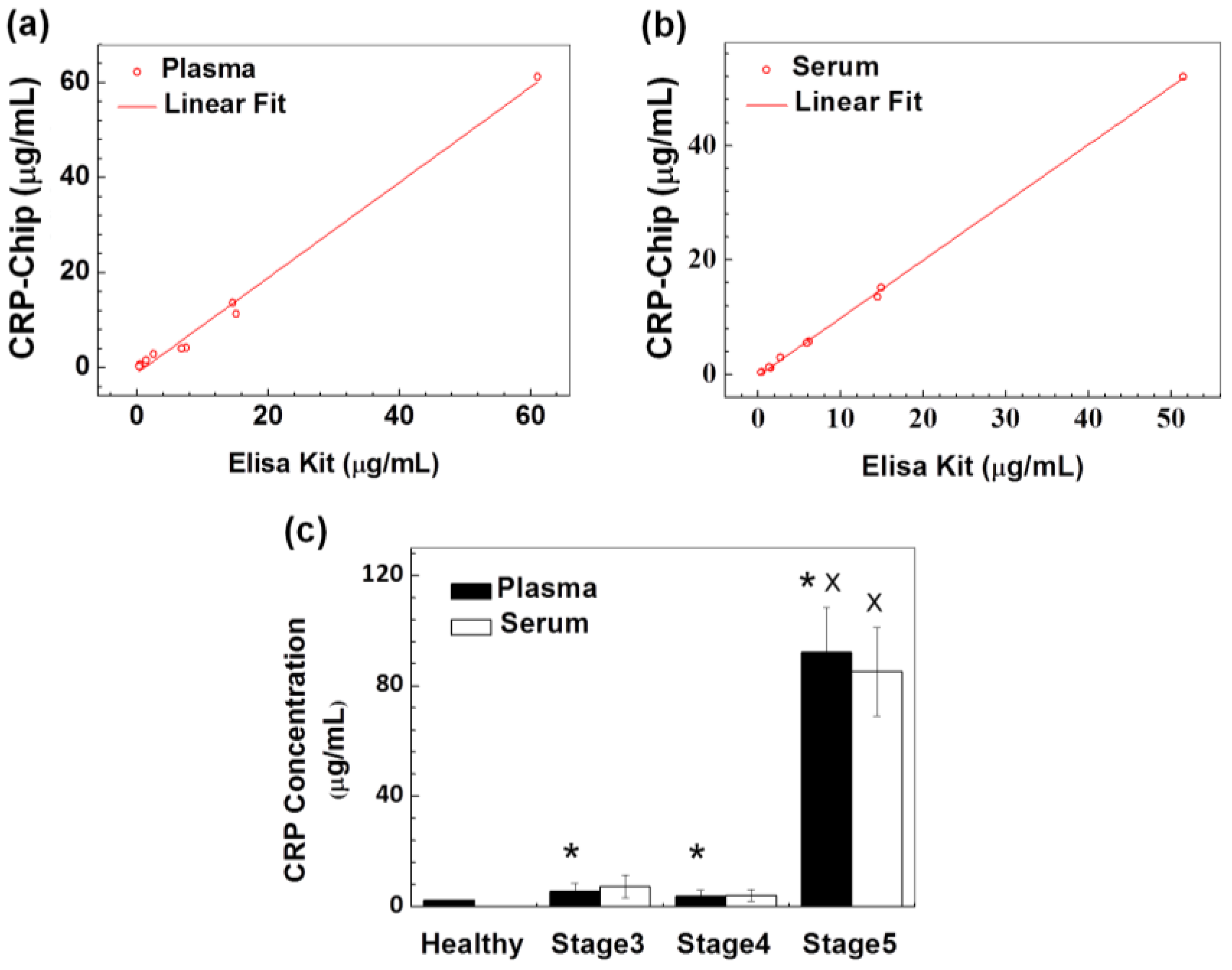
3.1. Technical Validation and Characterization of the CRP-Chip
3.2. Validation of the CRP-Chip for Testing Clinical CKD Patient Samples
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anderson, G.; Horvath, J. The growing burden of chronic disease in America. Public Health Rep. 2004, 119, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J.L. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef]
- Abegunde, D.O.; Mathers, C.D.; Adam, T.; Ortegon, M.; Strong, K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007, 370, 1929–1938. [Google Scholar] [CrossRef]
- An, W.S.; Kang, H.J.; Kim, K.H. Effect of low-dose niacin on dyslipidemia, serum phosphorus levels and adverse effects in patients with chronic kidney disease. Kidney Res. Clin. Pract. 2012, 31, A16. [Google Scholar]
- Golomb, B.A.; Evans, M.A. Statin adverse effects. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Wilson, P.W.F.; Larson, M.G.; Beiser, A.; Leip, E.P.; D’Agostino, R.B.; Levy, D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am. J. Cardiol. 2004, 94, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Li, L.; Greene, T.H.; Tin, A.; Sang, Y.; Kao, W.H.L.; Lipkowitz, M.S.; Wright, J.T.; Chang, A.R.; Astor, B.C.; et al. Estimating time to ESRD using kidney failure risk equations: Results from the African American Study of Kidney Disease and Hypertension (AASK). Am. J. Kidney Dis. 2015, 65, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Oehmichen, J.; Van Aken, H.; Reuter, S.; Pavenstädt, H.J.; Meersch, M.; Unruh, M.; Zarbock, A. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J. Clin. Investig. 2016, 126, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Nair, V.; Smith, S.; Zhu, L.; Shedden, K.; Song, P.X.K.; Mariani, L.H.; Eichinger, F.H.; Berthier, C.C.; Randolph, A.; et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci. Transl. Med. 2015, 7, 316ra193. [Google Scholar] [CrossRef] [PubMed]
- De Serres, S.A.; Varghese, J.C.; Levin, A. Biomarkers in native and transplant kidneys: Opportunities to improve prediction of outcomes in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Haubitz, M.; Brunkhorst, R.; Wrenger, E.; Froese, P.; Schulze, M.; Koch, K.-M. Chronic induction of C-reactive protein by hemodialysis, but not by peritoneal dialysis therapy. Perit. Dial. Int. 1996, 16, 158–162. [Google Scholar] [PubMed]
- Kuller, L.H.; Tracy, R.P.; Shaten, J.; Meilahn, E.N.; MRFIT Research Group. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Am. J. Epidemiol. 1996, 144, 537–547. [Google Scholar] [PubMed]
- Foudeh, A.M.; Fatanat Didar, T.; Veres, T.; Tabrizian, M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 2012, 12, 3249–3266. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Yu, J.; Ge, S.; Yan, M. Lab-on-paper-based devices using chemiluminescence and electrogenerated chemiluminescence detection. Anal. Bioanal. Chem. 2014, 406, 5613–5630. [Google Scholar] [CrossRef] [PubMed]
- Muluneh, M.; Issadore, D. Microchip-based detection of magnetically labeled cancer biomarkers. Adv. Drug Deliv. Rev. 2014, 66, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Henares, T.G.; Mizutani, F.; Hisamoto, H. Current development in microfluidic immunosensing chip. Anal. Chim. Acta 2008, 611, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Von Lode, P. Point-of-care immunotesting: Approaching the analytical performance of central laboratory methods. Clin. Biochem. 2005, 38, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2009, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Chen, Y.; Wu, H. New materials for microfluidics in biology. Curr. Opin. Biotechnol. 2014, 25, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Gan, X.; Fang, Y.; Zheng, S.; Dong, Q. A simple, rapid, and sensitive integrated protein microarray for simultaneous detection of multiple antigens and antibodies of five human hepatitis viruses (HBV, HCV, HDV, HEV, and HGV). Anal. Biochem. 2007, 362, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Apilux, A.; Ukita, Y.; Chikae, M.; Chailapakul, O.; Takamura, Y. Development of automated paper-based devices for sequential multistep sandwich enzyme-linked immunosorbent assays using inkjet printing. Lab Chip 2013, 13, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Songjaroen, T.; Dungchai, W.; Chailapakul, O.; Henry, C.S.; Laiwattanapaisal, W. Blood separation on microfluidic paper-based analytical devices. Lab Chip 2012, 12, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, B.; Dabiri, B.E.; Lockett, M.R.; Derda, R.; Campbell, P.; Parker, K.K.; Whitesides, G.M. Three-dimensional paper-based model for cardiac ischemia. Adv. Healthc. Mater. 2014, 3, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Peretz-Soroka, H.; Liu, Y.; Lin, F. Novel developments in mobile sensing based on the integration of microfluidic devices and smartphones. Lab Chip 2016, 16, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Huang, T.J. Microfluidic diagnostics for the developing world. Lab Chip 2012, 12, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.; Sindi, H.; Whitesides, G.M. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Latini, R.; Florea, V.G.; Kuskowski, M.A.; Rector, T.; Masson, S.; Signorini, S.; Mocarelli, P.; Hester, A.; Glazer, R. C-reactive protein in heart failure prognostic value and the effect of valsartan. Circulation 2005, 112, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Greene, T.; Wang, X.; Pereira, A.A.; Marcovina, S.M.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Sarnak, M.J. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005, 68, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.; Sundaram, V.; Sundaram, V.; Mathew, M.; Leslie, N.; Sathiah, V. C-reactive protein, a valuable predictive marker in chronic kidney disease. Saudi J. Kidney Dis. Transplant. 2009, 20, 811. [Google Scholar]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [PubMed]
- Tobe, S.W.; Clase, C.M.; Gao, P.; McQueen, M.; Grosshennig, A.; Wang, X.; Teo, K.K.; Yusuf, S.; Mann, J.F. ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: Results from the ONTARGET and TRANSCEND studies. Circulation 2011, 123, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Tobe, S.; Teo, K.K.; Yusuf, S. Is therapy of people with chronic kidney disease ONTARGET? Nephrol. Dial. Transplant. 2010, 25, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Schmieder, R.E.; McQueen, M.; Dyal, L.; Schumacher, H.; Pogue, J.; Wang, X.; Maggioni, A.; Budaj, A.; Chaithiraphan, S.; et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 2008, 372, 547–553. [Google Scholar] [CrossRef]
- Bowling, C.B.; O’Hare, A.M. Managing Older Adults With CKD: Individualized Versus Disease-Based Approaches. Am. J. Kidney Dis. 2012, 59, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Kinjo, K.; Iseki, C.; Takishita, S. Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am. J. Kidney Dis. 2004, 44, 806–814. [Google Scholar] [CrossRef]
- Ishani, A.; Grandits, G.A.; Grimm, R.H.; Svendsen, K.H.; Collins, A.J.; Prineas, R.J.; Neaton, J.D.; MRFIT Research Group. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J. Am. Soc. Nephrol. 2006, 17, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Gervais, L.; de Rooij, N.; Delamarche, E. Microfluidic chips for point-of-care immunodiagnostics. Adv. Mater. 2011, 23, H151–H176. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Yang, S.T.; Lai, S.; Bai, Y.; Huang, W.C.; Juang, Y.J. Microfluidic enzyme-linked immunosorbent assay technology. Adv. Clin. Chem. 2006, 42, 255–295. [Google Scholar] [PubMed]
- Ng, A.H.; Uddayasankar, U.; Wheeler, A.R. Immunoassays in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Apilux, A.; Ukita, Y.; Chikae, M.; Chailapakul, O.; Takamura, Y. Development of automated paper-based devices for sequential multistep sandwich enzyme-linked immunosorbent assays using inkjet printing. Lab Chip 2012, 13, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Joung, H.-A.; Kim, S.; Kim, M.-G. Vertical flow immunoassay (VFA) biosensor for a rapid one-step immunoassay. Lab Chip 2013, 13, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.-L.; Wen, C.-Y.; Tang, M.; Wu, L.-L.; Liu, C.; Zhu, L.; Pang, D.-W. Sensitive and Quantitative Detection of C-Reaction Protein Based on Immunofluorescent Nanospheres Coupled with Lateral Flow Test Strip. Anal. Chem. 2016, 88, 6577–6584. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.-A.; Oh, Y.K.; Kim, M.-G. An automatic enzyme immunoassay based on a chemiluminescent lateral flow immunosensor. Biosens. Bioelectron. 2014, 53, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Choi, S.; Jang, S.H.; Chang, H.J.; Kim, J.H.; Nahm, K.B.; Oh, S.W.; Choi, E.Y. Development of a point-of-care assay system for high-sensitivity C-reactive protein in whole blood. Clin. Chim. Acta 2003, 332, 51–59. [Google Scholar] [CrossRef]


| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma (ELISA kit) | 15.1 | 1.24 | 6.8 | 0.45 | 14.6 | 2.52 | 7.51 | 1.39 | 0.32 | 61.1 |
| Plasma (CKD-Chip) | 11.3 | 0.92 | 4.10 | 0.65 | 13.6 | 2.90 | 4.13 | 1.60 | 0.28 | 61.2 |
| Serum (ELISA kit) | 14.9 | 1.56 | 5.9 | 0.41 | 14.5 | 2.73 | 6.15 | 1.35 | 0.33 | 51.5 |
| Serum (CKD-Chip) | 15.18 | 1.14 | 5.51 | 0.40 | 13.53 | 2.96 | 5.75 | 1.25 | 0.35 | 52 |
| CKD Stage | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 5 |
| Gender | Fasting | Dialysis | CKD stage | eGFR* | uACR# | |
|---|---|---|---|---|---|---|
| 1 | F | N | Y | STAGE 3 | 33 | 60.7 |
| 2 | F | Y | N | STAGE 3 | 40 | 2.3 |
| 3 | M | N | N | STAGE 3 | 36 | 4.1 |
| 4 | F | N | N | STAGE 4 | 22 | 452.3 |
| 5 | F | N | Y | STAGE 4 | 23 | 0.8 |
| 6 | M | N | N | STAGE 4 | 27 | 27 |
| 7 | F | Y | N | STAGE 4 | 26 | 0.6 |
| 8 | M | N | N | STAGE 4 | 24 | 11.2 |
| 9 | F | N | Y | STAGE 4 | 22 | 334.5 |
| 10 | M | N | Y | STAGE 5 | 13 | 47.9 |
| 11 | F | Y | Y | STAGE 5 | 8 | 502.7 |
| 12 | M | N | Y | STAGE 5 | 11 | 302.2 |
| 13 | F | N | Y | STAGE 5 | 11 | 698.4 |
| 14 | M | Y | N | STAGE 5 | 9 | 144.8 |
| 15 | M | N | Y | STAGE 5 | 12 | 433 |
| 16 | M | Y | Y | STAGE 5 | 11 | >656.7 |
| 17 | F | Y | N | STAGE 5 | 10 | 156.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, M.; Wu, J.; Ma, Z.; Peretz-Soroka, H.; Zhang, M.; Komenda, P.; Tangri, N.; Liu, Y.; Rigatto, C.; Lin, F. Rapid and Low-Cost CRP Measurement by Integrating a Paper-Based Microfluidic Immunoassay with Smartphone (CRP-Chip). Sensors 2017, 17, 684. https://doi.org/10.3390/s17040684
Dong M, Wu J, Ma Z, Peretz-Soroka H, Zhang M, Komenda P, Tangri N, Liu Y, Rigatto C, Lin F. Rapid and Low-Cost CRP Measurement by Integrating a Paper-Based Microfluidic Immunoassay with Smartphone (CRP-Chip). Sensors. 2017; 17(4):684. https://doi.org/10.3390/s17040684
Chicago/Turabian StyleDong, Meili, Jiandong Wu, Zimin Ma, Hagit Peretz-Soroka, Michael Zhang, Paul Komenda, Navdeep Tangri, Yong Liu, Claudio Rigatto, and Francis Lin. 2017. "Rapid and Low-Cost CRP Measurement by Integrating a Paper-Based Microfluidic Immunoassay with Smartphone (CRP-Chip)" Sensors 17, no. 4: 684. https://doi.org/10.3390/s17040684






