Localization and Tracking of Implantable Biomedical Sensors
Abstract
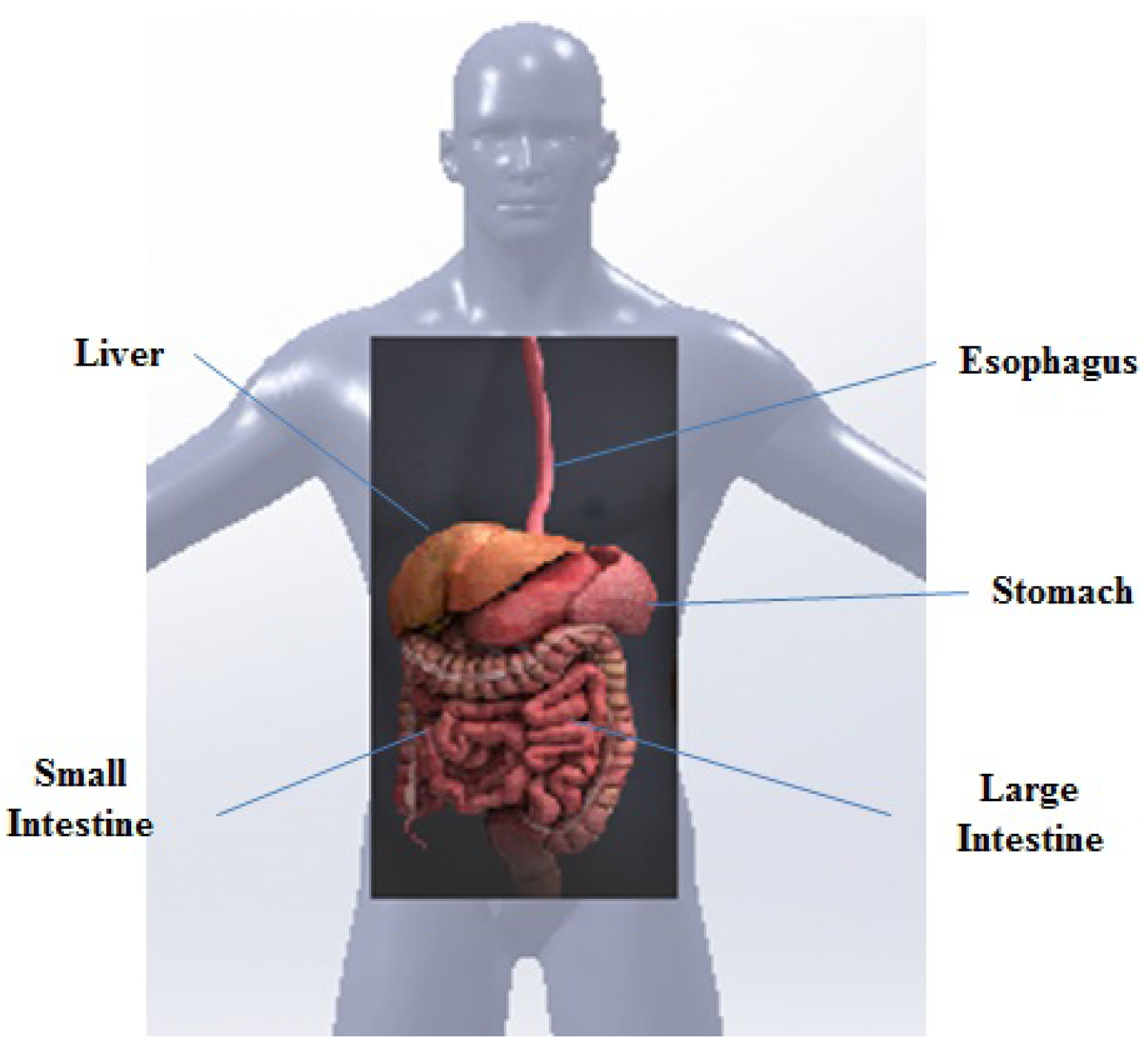
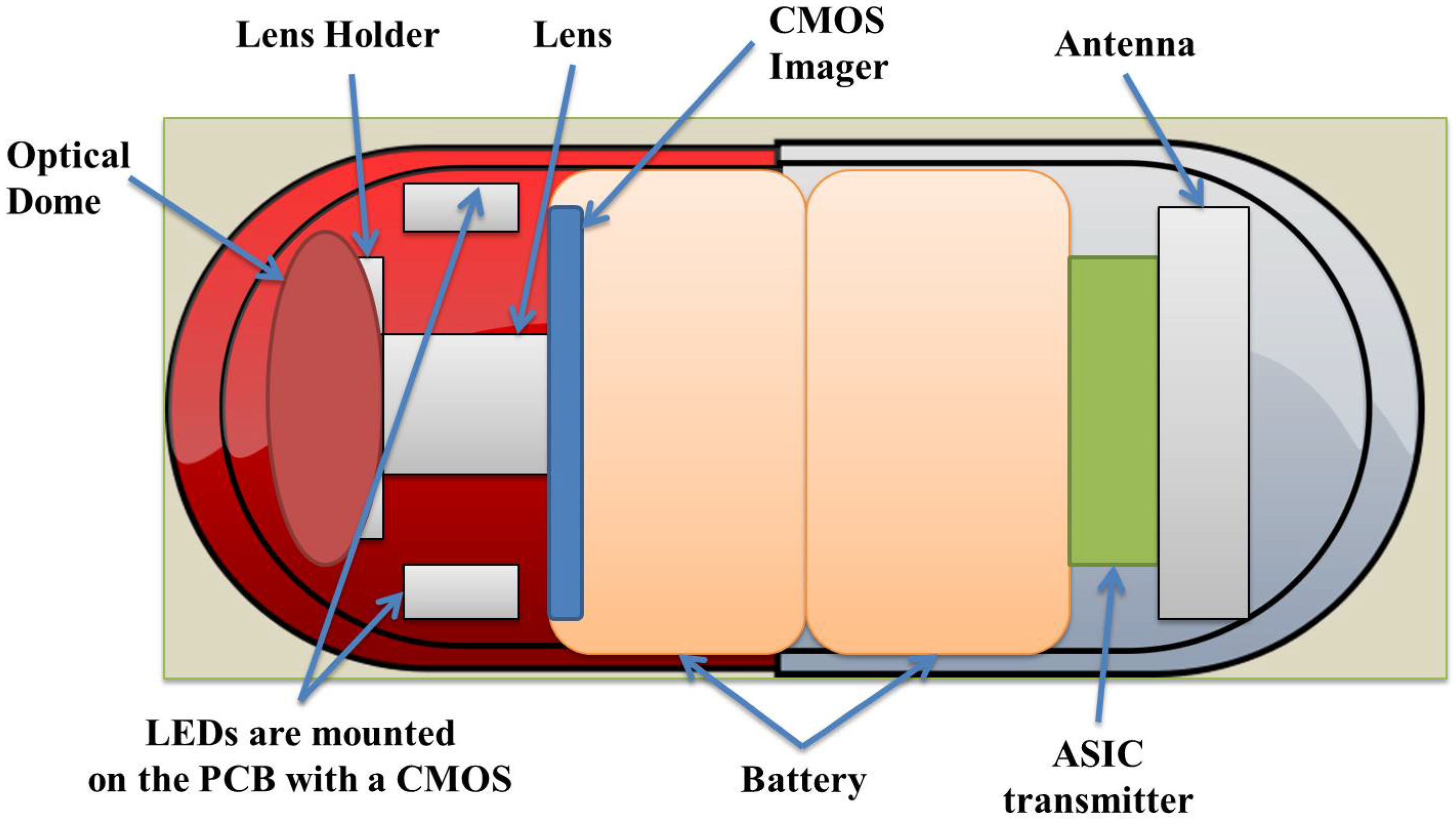
:1. Introduction
2. Radio-Frequency Electromagnetic Signal-Based Localization and Tracking
2.1. RSS Based Techniques
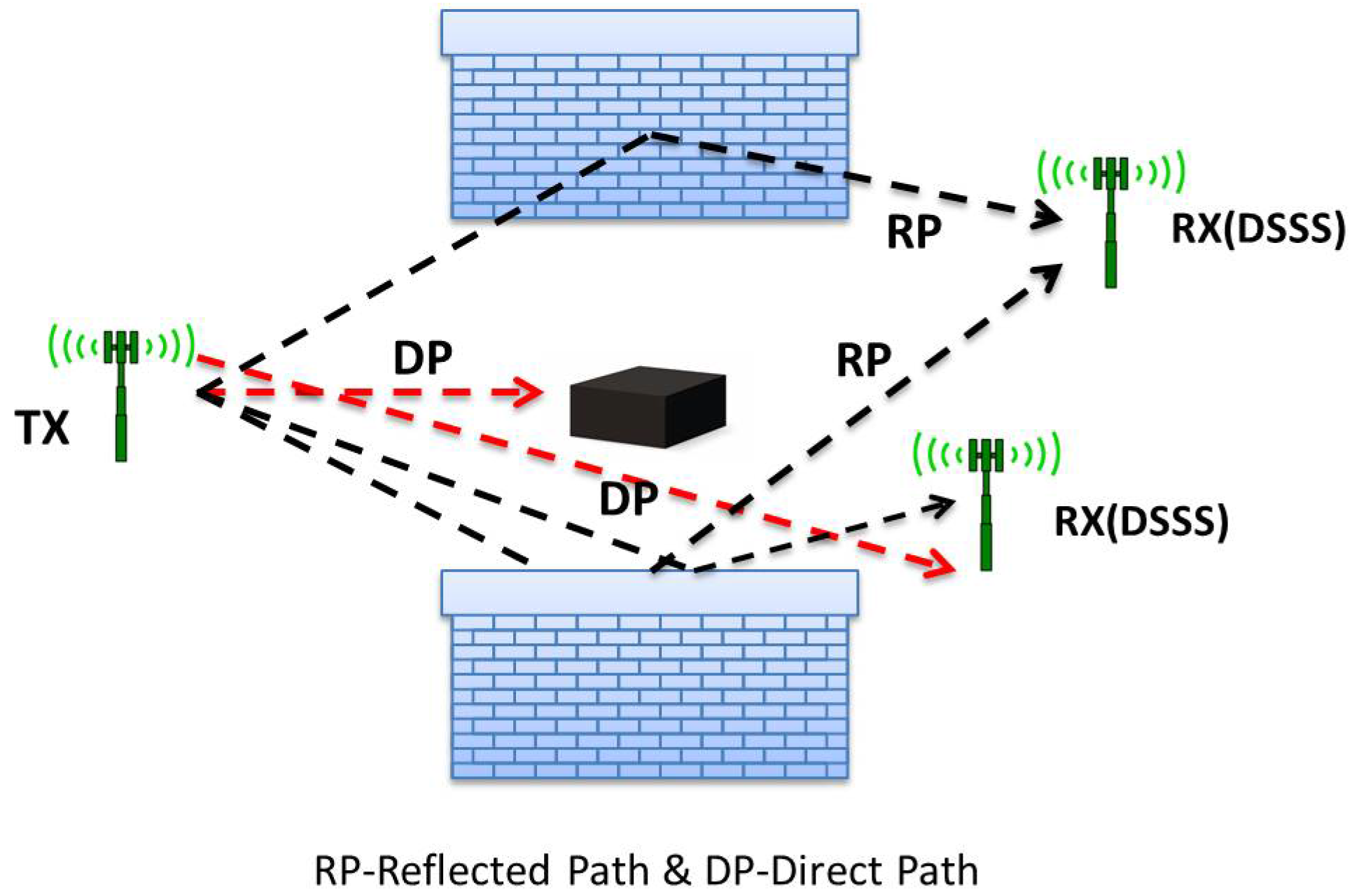
2.2. ToF-Based Techniques
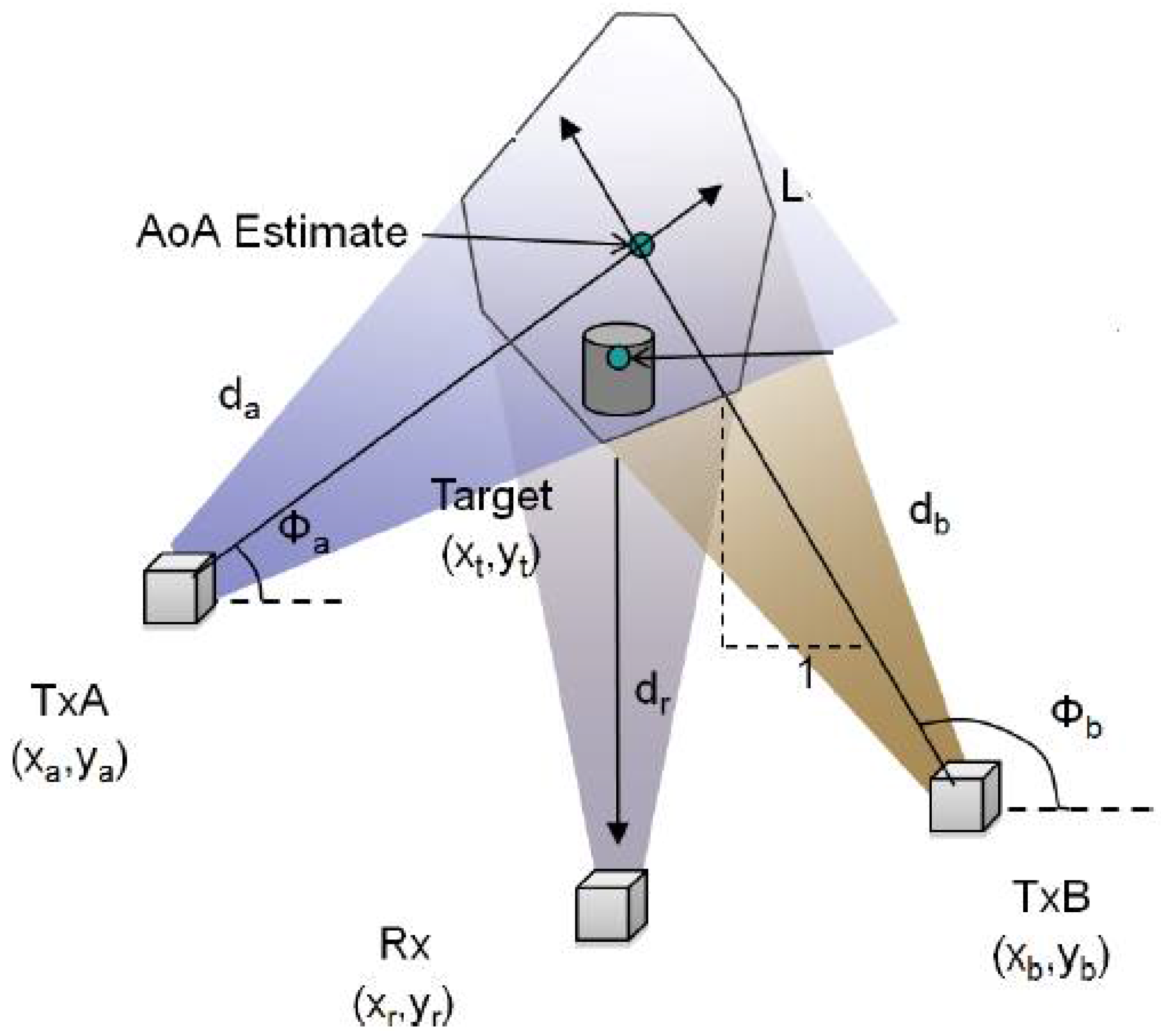
2.3. AoA-Based Techniques
2.4. RFID-Based Techniques
3. Magnetic-Signal-Based Localization and Tracking
3.1. Magnetic Localization and Tracking of Passive Sensors
3.2. Magnetic Localization and Tracking of Active Sensors
3.2.1. Alternating Magnetic Field-Based Techniques
3.2.2. Inertial Sensing-Based Techniques
3.2.3. Exterior Rotational Magnetic Field-Based Techniques
4. Localization and Tracking Algorithms
5. Hybrid Localization and Tracking Techniques
5.1. RF and Video Fusion-Based Techniques
5.2. RF and Magnetic Strength Fusion-Based Techniques
5.3. Magnetic Strength and Image Fusion-Based Techniques
6. Other Techniques
7. A Discussion on the Connection and Cooperation with Wearable Sensors
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ciuti, G. Frontiers of robotic endoscopic capsules: A review. J. Micro-Bio Robot. 2016, 11, 1–18. [Google Scholar] [CrossRef]
- Crohn’s and Colitis Foundation of Canada. Available online: http://www.isupportibd.ca/pdf/ccfc-ibd-impact-report-2012.pdf (accessed on 13 March 2017).
- Wang, A.; Banerjee, S.; Barth, B.A.; Bhat, Y.M.; Chauhan, S.; Gottlieb, K.T.; Konda, V.; Maple, J.T.; Murad, F.; Pfau, P.R.; et al. Wireless Capsule Endoscopy; Technical report; American Society for Capsule Endoscopy: Downers Grove, IL, USA, 2013. [Google Scholar]
- Ye, Y. Bounds on RF Cooperative Localization for Video Capsule Endoscopy. Ph.D. thesis, Worcester Polytechnic Institue, Worcester, MA, USA, 2013. [Google Scholar]
- Yu, M. M2A capsule endoscopy. Gastroenterol. Nurs. 2001, 25, 24–27. [Google Scholar] [CrossRef]
- Umay, I.; Fidan, B. Adaptive magnetic sensing based wireless capsule localization. In Proceedings of the 10th International Symposium on Medical Information and Communication Technology (ISMICT), Worcester, MA, USA, 20–23 March 2016; pp. 1–5.
- Than, T.D.; Alici, G.; Zhou, H.; Li, W. A review of localization systems for robotic endoscopic capsules. IEEE Trans. Biomed. Eng. 2012, 59, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, B.; Mackay, R.S. A pH-Endoradiosonde. Lancet 1957, 269, 1224. [Google Scholar] [CrossRef]
- Koulaouzidis, A.; Iakovidis, D.K.; Karargyris, A.; Rondonotti, E. Wireless endoscopy in 2020: Will it still be a capsule? World Gastroenterol. 2015, 21, 5119–5130. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.R.; Hasler, W.L. New vision in video capsule endoscopy: Current status and future directions. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Marya, N.; Karellas, A.; Foley, A.; Roychowdhury, A.; Cave, D. Computerized 3-dimensional localization of a video capsule in the abdominal cavity: Validation by digital radiography. Gastrointest. Endosc. 2014, 79, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Schreiber, R.; Levi, D.; Eliakim, R. Capsule endoscopy: The localization system. Gastrointestinal Endoscopy Clin. N. Am. 2004, 14, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Pahlavan, K. Design, implementation, and fundamental limits of image and RF based wireless capsule endoscopy hybrid localization. IEEE Trans. Mob. Comput. 2016, 15, 1951–1964. [Google Scholar] [CrossRef]
- Karargyris, A.; Koulaouzidis, A. OdoCapsule: Next-generation wireless capsule endoscopy with accurate lesion localization and video stabilization capabilities. IEEE Trans. Biomed. Eng. 2015, 62, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Dakurah, M.N.; Koo, C.; Choi, W.; Joung, Y.H. Implantable bladder sensors: A methodological review. Int. Neurourol. J. 2015, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Reilink, R.; Kappers, A.M.L.; Stramigioli, S.; Misra, S. Evaluation of robotically controlled advanced endoscopic instruments. Med. Robot. Comput. Assisted Surg. 2013, 9, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Vrooijink, G.J.; Ellenbroek, T.T.M.; Breedveld, P.; Grandjean, J.G.; Misra, S. A preliminary study on using a robotically-actuated delivery sheath (RADS) for transapical aortic valve implantation. In Proceedings of the IEEE International Conference on Robotics and Automation, Hong Kong, China, 31 May–7 June 2014; pp. 4380–4386.
- Arshak, K.; Adepoju, F. Adaptive linearized methods for tracking a moving telemetry capsule. In Proceedings of the IEEE International Symposium on Industrial Electronics, Vigo, Spain, 4–7 June 2007; pp. 2703–2708.
- Wang, L.; Li, L.; Hu, C.; Meng, M.Q.-H. A novel RF-based propagation model with tissue absorption for location of the GI tract. In Proceedings of the Annual Internatioanl Conference of the IEEE EMBS, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 654–657.
- Wang, L.; Li, L.; Hu, C.; Meng, M.Q.-H. A novel radio propagation radiation model for location of the capsule in GI tract. In Proceedings of the 2009 IEEE International Conference on Robotics and Biomimetics (ROBIO), Guilin, China, 19–23 December 2009; pp. 2332–2337.
- Shah, T.; Aziz, S.M.; Vaithianathan, T. Development of a tracking algorithm for an in-vivo RF capsule prototype. In Proceedings of the International Conference on Electrical and Computer Engineering, Dhaka, Bangladesh, 19–21 December 2006; pp. 173–176.
- Umay, I.; Fidan, B.; Yuce, M.R. Wireless capsule localization with unknown path loss coefficient and permittivity. In Proceedings of the IEEE International Conference on Advanced Robotics, Seattle, WA, USA, 26–30 May 2015; pp. 224–229.
- Wang, L.; Hu, C.; Li, M.; Tian, L.; Meng, M.Q.H. A novel radio propagation radiation model for location of the capsule in GI tract. In Proceedings of the IEEE International Conference on Robotics and Biomimetics, Guilin, China, 19–23 December 2009; pp. 2332–2337.
- Ye, Y.; Khan, U.; Yi, W.; Ruijun, F.; Pahlavan, K. Performance bounds for RF positioning of endoscopy camera capsules. In Proceedings of the Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS), Phoenix, AZ, USA, 16–19 January 2011; pp. 71–74.
- Ye, Y.; Khan, U.; Alsindi, N.; Fu, R.; Pahlavan, K. On the accuracy of RF positioning in multi-capsule endoscopy. In Proceedings of the IEEE 22nd International Symposium on Personal Indoor and Mobile Radio Communications (PIMRC), Toronto, ON, Canada, 11–14 September 2011; pp. 2173–2177.
- Camlica, A.; Fidan, B.; Yavuz, M. Implant localization in the human body using adaptive least square based algorithm. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, San Diego, CA, USA, 15–21 November 2013.
- Hekimian, C.W.; Grant, B.; Xiuwen, L.; Zhenghao, Z.; Kumar, P. Accurate localization of RFID tags using phase difference. In Proceedings of the 2010 IEEE International Conference on RFID, Orlando, FL, USA, 14–16 April 2010; pp. 89–96.
- Hou, J.; Zhu, Y.; Zhang, L.; Fu, Y.; Zhao, F.; Yang, L.; Rong, G. Design and implementation of a high resolution localization system for in-vivo capsule endoscopy. In Proceedings of the Eighth IEEE International Conference on Dependable, Autonomic and Secure Computing, Chengdu, China, 12–14 December 2009; pp. 209–214.
- Zhang, L.; Zhu, Y.; Mo, T.; Hou, J.; Hu, H. Design of 3D positioning algorithm based on RFID receiver array for in vivo micro-robot. In Proceedings of the Eighth IEEE International Conference on Dependable, Autonomic and Secure Computing, Chengdu, China, 12–14 December 2009; pp. 749–753.
- Wille, A.; Broll, M.; Winter, S. Phase difference based RFID navigation for medical applications. In Proceedings of the 2011 IEEE International Conference on RFID (RFID), Orlando, FL, USA, 12–14 April 2011; pp. 98–105.
- Zhang, L.; Zhu, Y.; Mo, T.; Hou, J.; Rong, G. Design and implementation of 3D positioning algorithms based on RF signal radiation patterns for in vivo micro-robot. In Proceedings of the 2010 International Conference on Body Sensor Networks (BSN), Singapore, 7–9 June 2010; pp. 255–260.
- Hashi, S.; Yabukami, S.; Kanetaka, H.; Ishiyama, K.; Arai, K.I. Numerical study on the improvement of detection accuracy for a wireless motion capture system. IEEE Trans. Magn. 2009, 45, 2736–2739. [Google Scholar] [CrossRef]
- Hosseini, S. Design, Fabrication and Control of a Magnetic Mapsule Robot for the Human Esophagus. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2009. [Google Scholar]
- Hosseini, S.; Khamesee, M.B. Design and control of a magnetically driven capsule robot for endoscopy and drug delivery. In Proceedings of the IEEE International Conference on Science and Technology for Humanity, Toronto, ON, Canada, 26–27 September 2009; pp. 697–702.
- Kim, M.G.; Hong, Y.S.; Lim, E.J. Position and orientation detection of capsule endoscopes in spiral motion. Precis. Eng. Manuf. 2010, 11, 31–37. [Google Scholar] [CrossRef]
- Popek, K.M.; Mahoney, A.W.; Abbott, J.J. Localization method for a magnetic capsule endoscope propelled by a rotating magnetic dipole field. In Proceedings of the IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 5348–5453.
- Hu, C.; Meng, Q.H.M.; Mandal, M. Efficient magnetic localization and orientation technique for capsule endoscopy. Int. J. Inf. Acquis. 2005, 2. [Google Scholar] [CrossRef]
- Hu, C.; Meng, M.Q.H.; Mandal, M. A linear algorithm for tracing magnet position and orientation by using three-axis magnetic sensors. IEEE Trans. Magn. 2007, 43, 4096–4101. [Google Scholar]
- Hu, C.; Li, M.; Song, S.; Yang, W.; Zhang, R.; Meng, M.Q.H. A cubic 3-axis magnetic sensor array for wirelessly tracking magnet position and orientation. IEEE Sens. J. 2010, 10, 903–913. [Google Scholar] [CrossRef]
- Hu, C. Locating intra-body capsule object by three-magnet sensing system. IEEE Sens. J. 2016, 16, 5167–5176. [Google Scholar] [CrossRef]
- Miller, V.; Mahoney, A.; Schmid, T.; Abbott, J.J. Proprioceptive magnetic-field sensing for closed-loop control of magnetic capsule endoscopes. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Algarve, Portugal, 7–12 October 2012; pp. 1994–1999.
- Natali, C.D.; Beccani, M.; Valdastri, P. Real-time pose detection for magnetic medical devices. IEEE Trans. Magn. 2013, 49, 3524–3527. [Google Scholar] [CrossRef]
- Natali, C.D.; Beccani, M.; Valdastri, P. Jacobian-based iterative method for magnetic localization in robotic capsule endoscopy. IEEE Trans. Robot. 2016, 32, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Ciuti, G.; Lucarini, G.; Rizzo, R.; Valdastri, P.; Menciassi, A.; Landi, A. A discrete-time localization method for capsule endoscopy based on on-board magnetic sensing. Meas. Sci. Technol. 2012, 23, 015701. [Google Scholar] [CrossRef]
- Yim, S.; Sitti, M. 3-D localization method for a magnetically actuated soft capsule endoscope and its applications. IEEE Trans. Robot. 2013, 29, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Pahlavan, K.; Mi, L. Hybrid localization of microrobotic endoscopic capsule inside small intestine by data fusion of vision and RF sensors. IEEE Sens. J. 2015, 15, 2669–2678. [Google Scholar] [CrossRef]
- Umay, I.; Fidan, B. Adaptive wireless biomedical capsule tracking based on magnetic sensing. Int. J. Wirel. Inf. Netw. 2016. submitted. [Google Scholar]
- Gumprecht, J.D.J.; Lueth, T.C.; Khamesee, M.B. Navigation of a robotic capsule endoscope with a novel ultrasound tracking system. Microsyst. Technol. 2013, 19, 1415–1423. [Google Scholar] [CrossRef]
- Chandra, M.; Johansson, A.J.; Tufvesson, F. Localization of an RF source inside the human body for wireless capsule endoscopy. In Proceedings of the 8th International Conference on Body Area Networks, Boston, MA, USA, 30 September–2 October 2013; pp. 48–54.
- Fluckiger, M.; Nelson, B.J. Ultrasound emitter localization in heterogeneous media. In Proceedings of the 29th Annual International Conference of the IEEE EMBS, Lyon, France, 22–26 August 2007; pp. 2867–2870.
- Krieger, A.; Susil, R.C.; Menard, C.; Coleman, J.A.; Fichtinger, G.; Atalar, E.; Whitcomb, L.L. Design of a novel MRI compatible manipulator for image guided prostate interventions. IEEE Trans. Biomed. Eng. 2005, 52, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Manchalapati, P.; Cave, D.R. Capsule retention: It’s not all bad! Vis. Hum. J. Endosc. 2010, 9. [Google Scholar]
- Martel, S.; Mohammadi, M.; Felfoul, O.; Lu, Z.; Pouponneau, P. Flagellated magnetotactic bacteria as controlled MRI-trackable propulsion and steering systems for medical nanorobots operating in the human microvasculature. Int. J. Robot. Res. 2009, 28, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Than, T.D.; Alici, G.; Harvey, S.; O’Keefe, G.; Zhou, H.; Li, W.; Cook, T.; Alam-Fotias, S. An effective localization method for robotic endoscopic capsules using multiple positron emission markers. IEEE Trans. Robot. 2014, 30, 1174–1186. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.; Yan, R. An electromagnetic localization method for medical micro-devices based on adaptive particle swarm optimization with neighborhood search. Measurement 2011, 44, 852–858. [Google Scholar] [CrossRef]
- Jacob, H. Localization of the given M2A ingestible capsule in the Given diagnostic imaging system. Am. J. Gastroenterol. 2001, 96, S106–S107. [Google Scholar] [CrossRef]
- Al-Busaidi, A.M.; Khriji, L. Wearable wireless medical sensors toward standards, safety and intelligence: A review. Int. J. Biomed. Eng. Technol. 2014, 14, 119–147. [Google Scholar] [CrossRef]
- Pourhomayoun, M.; Jin, Z.; Fowler, M.L. Accurate localization of in-body medical implants based on spatial sparsity. IEEE Trans. Biomed. Eng. 2014, 61, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q. Introduction to Body Area Communications. In Body Area Communications: Channel Modeling, Communication Systems, and EMC; John Wiley and Sons (Asia) Pte Ltd.: Singapore, 2012. [Google Scholar]
- Thotahewa, K.M.S.; Redoute, J.-M.; Yuce, M.R. Propagation, power absorption, and temperature analysis of UWB wireless capsule endoscopy devices operating in the human body. IEEE Trans. Microwave Theory Techn. 2015, 63, 3823–3833. [Google Scholar] [CrossRef]
- Fidan, B.; Umay, I. Adaptive environmental source localization and tracking with unknown permittivity and path loss coefficients. Sensors 2015, 15, 31125–31141. [Google Scholar] [CrossRef] [PubMed]
- Gezici, S. A survey on wireless position estimation. Wirel. Pers. Commun. 2008, 44, 263–282. [Google Scholar] [CrossRef]
- Mao, G.; Fidan, B. Localization Algorithms and Strategies for Wireless Sensor Networks; IGI Global Information Science Publishing: Hershey, PA, USA, 2009. [Google Scholar]
- So, H.C. Source localization: Algorithms and analysis. In Handbook of Position Location: Theory, Practice and Advances; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Chapter 2; pp. 813–836. [Google Scholar]
- Fidan, B.; Camlica, A.; Guler, S. Least-squares-based adaptive target localization by mobile distance measurement sensors. Int. J. Adapt. Control Signal Process. 2015, 29, 259–271. [Google Scholar] [CrossRef]
- Crohn’s and Colitis Foundation of America. IBD and Colorectal Cancer. 2014. Available online: http://www.ibdetermined.org/ibd-information/ibd-complications/colorectal-cancer.aspx (accessed on 10 October 2015).
- Hu, C.; Meng, M.Q.H.; Mandal, M. Efficient linear algorithm for magnetic localization and orientation in capsule endoscopy. In Proceedings of the 27th Annual International Conference of the IEEE EMBS, Cancun, Mexico, 17–18 January 2006; pp. 7143–7146.
- Son, D.; Yim, S.; Sitti, M. A 5-D Localization method for a magnetically manipulated untethered robot using a 2-D array of Hall-effect sensors. IEEE/ASME Trans. Mechatron. 2016, 21, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Valdastri, P.; Simi, M.; Webster R.J., III. Advanced technologies for gastrointestinal endoscopy. Annu. Rev. Biomed. Eng. 2012, 14, 397–429. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Meng, M.Q.H.; Mandal, M. The calibration of 3-axis magnetic sensor array system for tracking wireless capsule endoscope. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Beijing, China, 9–15 October 2006; pp. 162–167.
- Wang, X.; Meng, M.Q.H. Perspective of active capsule endoscope: Actuation and localisation. Mechatron. Autom. 2011, 1, 38–45. [Google Scholar] [CrossRef]
- Laulicht, B.; Gidmark, N.J.; Tripathi, A.; Mathiowitz, E. Localization of magnetic pills. Proc. Natl. Acad. Sci. USA 2011, 108, 2252–2257. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.Q.-H.; Hu, C.; Mandal, M. Efficient magnetic localization and orientation technique for capsule endoscopy. Inf. Acquis. 2005, 2, 23–36. [Google Scholar]
- Fidan, B.; Dasgupta, S.; Anderson, B.D.O. Adaptive range measurement-based target pursuit. Int. J. Adapt. Control Signal Process. 2013, 27, 66–81. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in C: The Art of Scientific Computing, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007; Chapter 10. [Google Scholar]
- Huyer, W.; Neumaier, A. Global optimization by multilevel coordinate search. Glob. Optim. 1999, 14, 331–355. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Fidan, B.; Dasgupta, S.; Anderson, B.D.O. Guaranteeing practical convergence in algorithms for sensor and source localization. IEEE Trans. Signal Process. 2008, 56, 4458–4469. [Google Scholar] [CrossRef]
- Mao, G.; Fidan, B.; Anderson, B.D.O. Wireless sensor network localization techniques. Comput. Netw. 2007, 51, 2529–2553. [Google Scholar] [CrossRef]
- Ioannou, P.A.; Fidan, B. Adaptive Control Tutorial; SIAM Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2006. [Google Scholar]
- Arshak, K.; Adepoju, F. Capsule tracking in the GI tract: A novel microcontroller based solution. In Proceedings of the IEEE Sensor Applications Symposium, Houston, TX, USA, 7–9 February 2006; pp. 186–191.
- Altun, K.; Barshan, B.; Tuncel, O. Comparative study on classifying human activities with miniature inertial and magnetic sensors. Pattern Recogn. 2010, 43, 3605–3620. [Google Scholar] [CrossRef] [Green Version]
- Crespo, C.; McNames, J.; Aboy, M. Review of recent patents on wearable movement sensors. Recent Patents Biomed. Eng. 2013, 6, 82–88. [Google Scholar]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. NeuroEng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuce, M.R.; Khan, J.Y. Wireless Body Area Networks Technology, Implementation and Applications; Pan Stanford Publishing: Singapore, 2012. [Google Scholar]
- Yurtman, A.; Barshan, B. Automated evaluation of physical therapy exercises using multi-template dynamic time warping on wearable sensor signals. Comput. Methods Programs Biomed. 2014, 117, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Bulling, A.; Blanke, U.; Schiele, B. A tutorial on human activity recognition using body-worn inertial sensors. ACM Comput. Surv. 2014, 46, 1–33. [Google Scholar] [CrossRef]
- Lara, O.D.; Labrador, M.A. A survey on human activity recognition using wearable sensors. IEEE Commun. Surv. & Tutor. 2013, 15, 1192–1209. [Google Scholar]
- Mukhopadhyay, S.C. Wearable sensors for human activity monitoring: A review. IEEE Sens. J. 2015, 15, 1321–1330. [Google Scholar] [CrossRef]
- Shoaib, M.; Bosch, S.; Durmaz Incel, O.; Scholten, H.; Havinga, P.J.M. A survey of online activity recognition using mobile phones. Sensors 2015, 15, 2059–2085. [Google Scholar] [CrossRef] [PubMed]
- Delahoz, Y.S.; Labrador, M.A. Survey on fall detection and fall prevention using wearable and external sensors. Sensors 2014, 14, 19806–19842. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, M.; Shao, L.; Seed, L. A survey on fall detection: Principles and approaches. Neurocomputing 2013, 100, 144–152. [Google Scholar] [CrossRef]
- Pannurat, N.; Thiemjarus, S.; Nantajeewarawat, E. Automatic fall monitoring: A review. Sensors 2014, 14, 12900–12936. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.; Mohktar, M.S.; Kamaruzzaman, S.B.; Lim, K.S.; Pin, T.M.; Ibrahim, F. Smartphone-based solutions for fall detection and prevention: Challenges and open issues. Sensors 2014, 14, 7181–7208. [Google Scholar]
- Altun, K.; Barshan, B. Pedestrian dead reckoning employing simultaneous activity recognition cues. Meas. Sci. Technol. 2012, 23, 025103. [Google Scholar] [CrossRef]
- Kangas, M. Comparison of real-life accidental falls in older people with experimental falls in middle-aged test subjects. Gait Posture 2012, 35, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis, P.; Pippa, E.; Ozdemir, A.T.; Barshan, B.; Megalooikonomou, V. Investigation of sensor placement for accurate fall detection. In Proceedings of the 6th EAI International Conference on Wireless Mobile Communication and Healthcare (MobiHealth), Milan, Italy, 14–16 November 2016.
- Ozdemir, A.T. An analysis on sensor locations of the human body for wearable fall detection devices: Principles and practice. Sensors 2016, 16, 1161. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, O.; Altun, K.; Barshan, B. Classifying human leg motions with uniaxial piezoelectric gyroscopes. Sensors 2009, 9, 8508–8546. [Google Scholar] [CrossRef] [PubMed]
- Barshan, B.; Yurtman, A. Investigating inter-subject and inter-activity variations in activity recognition using wearable motion sensors. Comput. J. 2016, 59, 1345–1362. [Google Scholar] [CrossRef]
- Darwish, A.; Hassanien, A.E. Wearable and implantable wireless sensor network solutions for healthcare monitoring. Sensors 2011, 11, 5561–5595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurtman, A.; Barshan, B. Activity recognition invariant to sensor orientation with wearable motion sensors. 2016; submitted. [Google Scholar]




| Implantable Biomedical Sensor Localization Techniques | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RF Electromagnetic Wave Based Techniques | Magnetic Field Strength Based Techniques | Hybrid Techniques | Other Techniques | |||||||
| RSS [4,12,18,19,20,21,22,23,24,25] | ToF and TDoA [4,18,26] | AoA [7] | RFID [27,28,29,30,31] | Active [6,32,33,34,35,36] | Passive [6,34,36,37,38,39,40,41,42,43,44,45] | RF and Video [13,46] | RF and Magnetic [6,13,47] | Magnetic and Video [48] | Ultrasound, MRI, CT [49,50,51,52,53,54] | X-Ray, γ-Ray, Visible Wave [51,53,54] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umay, I.; Fidan, B.; Barshan, B. Localization and Tracking of Implantable Biomedical Sensors. Sensors 2017, 17, 583. https://doi.org/10.3390/s17030583
Umay I, Fidan B, Barshan B. Localization and Tracking of Implantable Biomedical Sensors. Sensors. 2017; 17(3):583. https://doi.org/10.3390/s17030583
Chicago/Turabian StyleUmay, Ilknur, Barış Fidan, and Billur Barshan. 2017. "Localization and Tracking of Implantable Biomedical Sensors" Sensors 17, no. 3: 583. https://doi.org/10.3390/s17030583







