Rapid and Sensitive Lateral Flow Immunoassay Method for Procalcitonin (PCT) Based on Time-Resolved Immunochromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Buffer Solutions
2.3. Experiment Instruments
2.4. Preparation of Standard and Controls
2.5. Preparation of CM-EUs Coupled with Antibody
2.6. Preparation of the CM-EUs Test Strip
2.7. Serum Samples
2.8. Sample Detection and Analysis
2.9. Statistical Analysis
3. Results
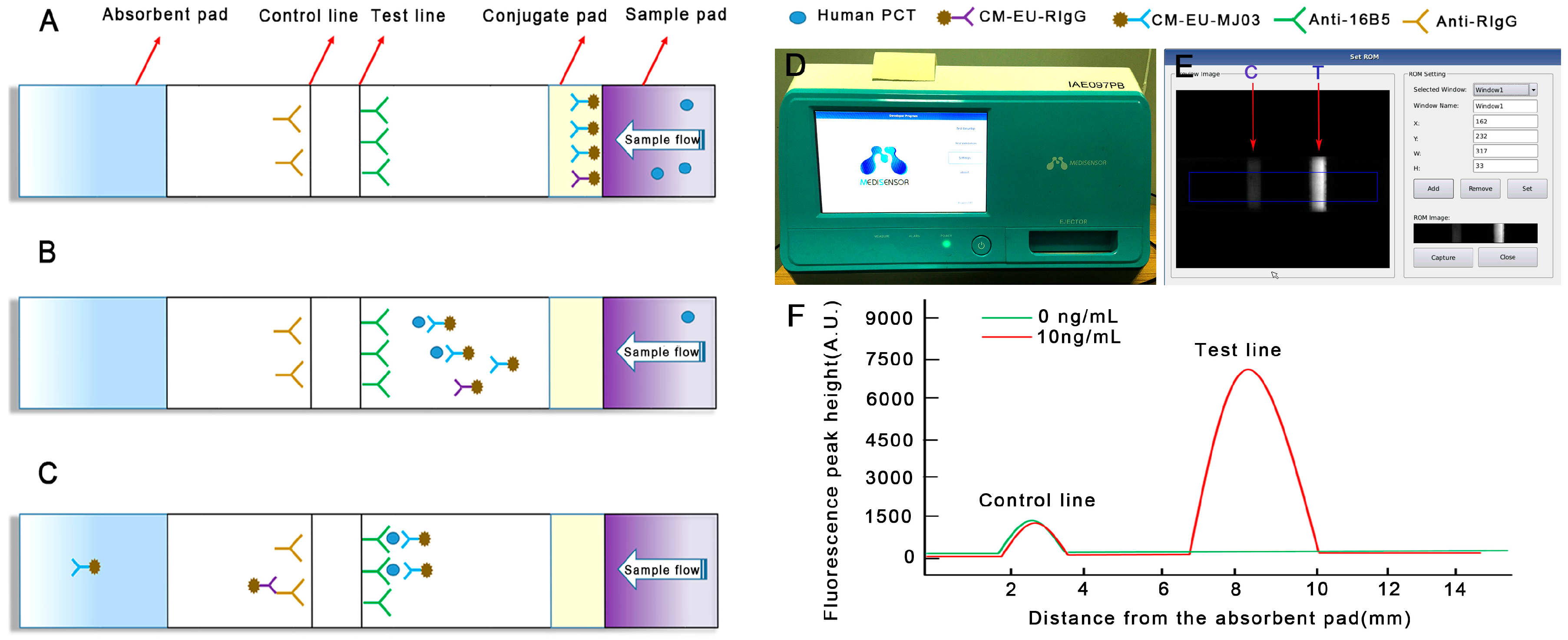
3.1. New Detecting System Establishment and Data Judgments
3.2. Optimization of Reaction Parameters
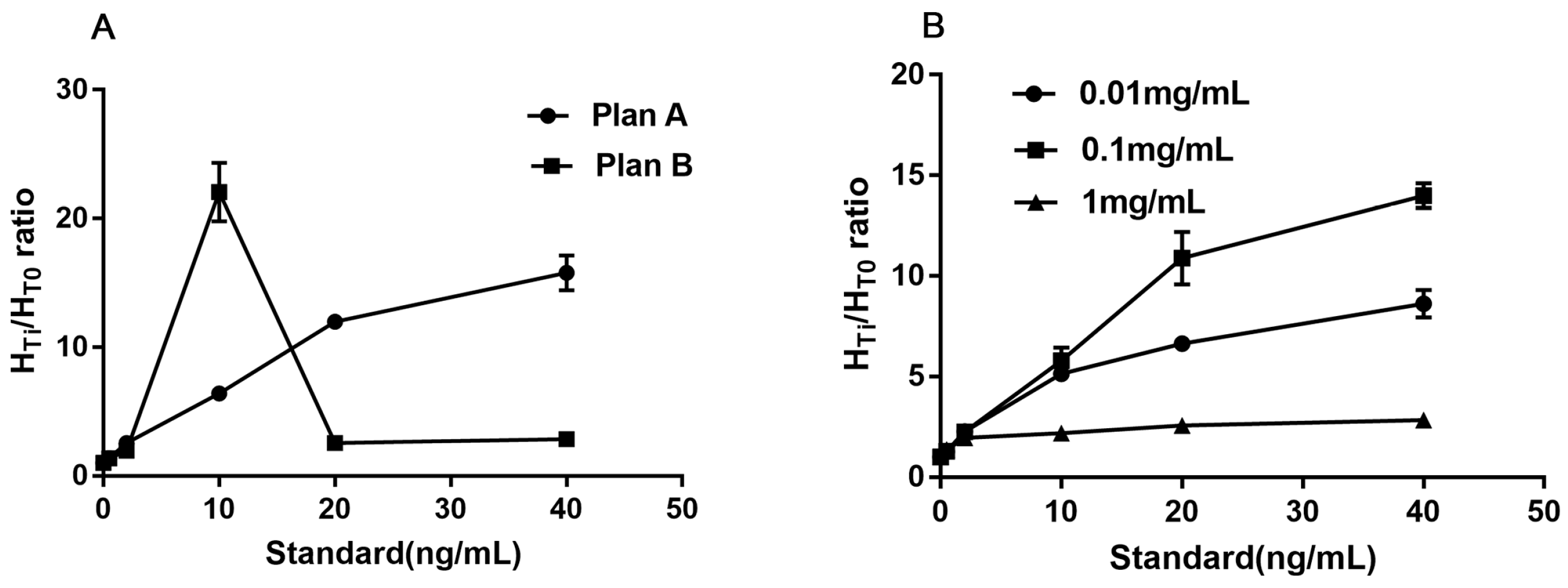
3.2.1. The Optimum Amount of Capture Antibody (16B5)
3.2.2. The Optimum Concentration of CM-EU-MJ03 Conjugates
3.3. Performance Evaluation
3.3.1. Analytical Sensitivity and Linearity
3.3.2. Accuracy
3.3.3. Precision
3.3.4. Specificity
3.4. Clinical Samples Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lipinska-Gediga, M.; Mierzchala-Pasierb, M.; Durek, G. Procalcitonin kinetics—Prognostic and diagnostic significance in septic patients. Arch. Med. Sci. 2016, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Pan, J.; Chen, D.; Li, Y. Serum procalcitonin and interleukin-6 levels may help to differentiate systemic inflammatory response of infectious and non-infectious origin. Chin. Med. J. 2003, 4, 538–542. [Google Scholar]
- Uzzan, B.; Cohen, R.; Nicolas, P.; Cucherat, M.; Perret, G.Y. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit. Care Med. 2006, 7, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin assay in systemic inflammation, infection, and sepsis: Clinical utility and limitations. Crit. Care Med. 2008, 3, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.; Melendez, J.H.; An, A.T.; Rosenbaum, J.E.; Zenilman, J.M. Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am. J. Clin. Pathol. 2011, 2, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Meisner, M.; Brunkhorst, F.M. Markers for sepsis diagnosis: What is useful? Crit. Care Clin. 2006, 3, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Schroeder, S.; Gehrt, A.; Bernhardt, K.; Bein, B.; Wegscheider, K.; Hochreiter, M. Procalcitonin-guided algorithm to reduce length of antibiotic therapy in patients with severe sepsis and septic shock. BMC Infect. Dis. 2013, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Bouadma, L.; Luyt, C.E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 9713, 463–474. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Muller, B. Procalcitonin in bacterial infections—Hype, hope, more or less? Swiss Med. Wkly. 2005, 31–32, 451–460. [Google Scholar]
- Charles, P.E.; Ladoire, S.; Aho, S.; Quenot, J.P.; Doise, J.M.; Prin, S.; Olsson, N.O.; Blettery, B. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect. Dis. 2008, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Novotny, A.; Emmanuel, K.; Matevossian, E.; Kriner, M.; Ulm, K.; Bartels, H.; Holzmann, B.; Weighardt, H.; Siewert, J.R. Use of procalcitonin for early prediction of lethal outcome of postoperative sepsis. Am. J. Surg. 2007, 1, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.E.; Fiechtl, J.F.; Brown, M.D.; Ballew, J.J.; Kline, J.A. Procalcitonin test in the diagnosis of bacteremia: A meta-analysis. Ann. Emerg. Med. 2007, 1, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Giannopoulou, P.; Grecka, P.; Voros, D.; Mandragos, K.; Giamarellou, H. Should procalcitonin be introduced in the diagnostic criteria for the systemic inflammatory response syndrome and sepsis? J. Crit. Care 2004, 3, 152–157. [Google Scholar] [CrossRef]
- Fu, Z.; Yan, F.; Liu, H.; Lin, J.; Ju, H. A channel-resolved approach coupled with magnet-captured technique for multianalyte chemiluminescent immunoassay. Biosens. Bioelectron. 2008, 10, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, D.; Wang, J.; Du, D.; Zou, Z.; Wang, H.; Smith, J.N.; Timchalk, C.; Liu, F.; Lin, Y. A novel immunochromatographic electrochemical biosensor for highly sensitive and selective detection of trichloropyridinol, a biomarker of exposure to chlorpyrifos. Biosens. Bioelectron. 2011, 6, 2835–2840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Pan, F.; Li, Y.; Lu, S.; Ren, H.; Shen, Q.; Li, Z.; Zhang, J.; Chen, Q.; et al. A competitive immunochromatographic assay based on a novel probe for the detection of mercury (II) ions in water samples. Biosens. Bioelectron. 2010, 11, 2534–2538. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Qian, G.; Yang, J.; Hu, B.; Fan, J.; Qin, N.; Li, G.; Wang, Y.; Liu, F. Development of an immunochromatographic assay for the rapid detection of chlorpyrifos-methyl in water samples. Biosens. Bioelectron. 2010, 1, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, M.; Chen, Y.; Li, J.; Wang, Y.; Qi, X.; Xue, Q. Lanthanide-labeled immunochromatographic strips for the rapid detection of Pantoea stewartii subsp. stewartii. Biosens. Bioelectron. 2014, 51, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, X.; Huang, X.; Yang, W.; Liu, C.; Lai, W.; Xu, H.; Xiong, Y. Ru(phen)3(2+) doped silica nanoparticle based immunochromatographic strip for rapid quantitative detection of beta-agonist residues in swine urine. Talanta 2013, 114, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xu, Y.; Ke, R.; Zhang, H.; Zou, M.; Yang, W.; Li, Q. A highly sensitive europium nanoparticle-based lateral flow immunoassay for detection of chloramphenicol residue. Anal. Bioanal. Chem. 2013, 23, 7541–7544. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xu, Y.; Zhao, X.; Li, Q. Lateral flow immunoassay using europium chelate-loaded silica nanoparticles as labels. Clin. Chem. 2009, 1, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.Y.; Jung, J.; Hwang, B.G.; Kim, W.J.; Kim, Y.S.; Kim, E.J.; Cho, M.Y.; Hwang, M.S.; Won, D.I.; Suh, J.S. Highly sensitive and novel point-of-care system, aQcare Chlamydia TRF kit for detecting Chlamydia trachomatis by using europium (Eu) (III) chelated nanoparticles. Ann. Lab. Med. 2015, 35, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M. Pathobiochemistry and clinical use of procalcitonin. Clin. Chim. Acta 2002, 1–2, 17–29. [Google Scholar] [CrossRef]
- Reinhart, K.; Karzai, W.; Meisner, M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 2000, 9, 1193–1200. [Google Scholar] [CrossRef]
- Liang, R.L.; Xu, X.P.; Liu, T.C.; Zhou, J.W.; Wang, X.G.; Ren, Z.Q.; Hao, F.; Wu, Y.S. Rapid and sensitive lateral flow immunoassay method for determining alpha fetoprotein in serum using europium (III) chelate microparticles-based lateral flow test strips. Anal. Chim. Acta 2015, 891, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.H.; Liang, R.L.; Liu, T.C.; Dong, Z.N.; Wu, Y.S.; Li, L.H. A Fluorescence Immunochromatographic Assay Using Europium (III) Chelate Microparticles for Rapid, Quantitative and Sensitive Detection of Creatine Kinase MB. J. Fluoresc. 2016, 26, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Hao, F.; Gao, X.; Wu, W.; Liang, M.; Liao, Z.; Luo, S.; Xu, W.; Li, D.; et al. Development of a colloidal gold kit for the diagnosis of severe fever with thrombocytopenia syndrome virus infection. Biomed. Res. Int. 2016, 530621, 1–6. [Google Scholar] [CrossRef] [PubMed]

| Sample | 1:2 | 1:4 | 1:8 |
|---|---|---|---|
| Expectation (ng/mL) | 31.40 | 15.70 | 7.85 |
| Mean value ± SD (ng/mL) | 29.96 ± 2.05 | 16.85 ± 1.05 | 7.94 ± 0.55 |
| Recovery | 1.05 | 0.93 | 0.99 |
| References (ng/mL) | Inter-Assay Precision (n = 5) | CV 2 % | Intra-Assay Precision (n = 3 × 2) | CV % | ||
|---|---|---|---|---|---|---|
| Mean (ng/mL) | SD 1 (ng/mL) | Mean (ng/mL) | SD (ng/mL) | |||
| 0.5 | 0.502 | 0.032 | 6.4 | 0.53 | 0.036 | 6.8 |
| 2.0 | 2.08 | 0.16 | 7.7 | 2.17 | 0.29 | 13.4 |
| 10. | 10.16 | 0.55 | 5.4 | 10.35 | 0.59 | 5.7 |
| Concentration of the Sample | The Specificity of the Sample | Test Results (ng/mL) |
|---|---|---|
| 50 ng/mL | CRP | <0.08 |
| 1 IU/mL | IL-6 | <0.08 |
| 60 ng/mL | Human calcitonin | <0.08 |
| 30 ng/mL | Human anti-calcium | <0.08 |
| CM-EU-Based Tests Trip Assay (ng/mL) | Roche Elecsys BRAHMS PCT Kit (ng/mL) | Total | |||
|---|---|---|---|---|---|
| <0.50 | 0.05 ≤ PCT ≤ 2.00 | 2.00 < PCT ≤ 10.00 | >10 | ||
| <0.50 | 83 | 0 | 0 | 0 | 83 |
| 0.05 ≤ PCT ≤ 2.00 | 0 | 5 | 8 | 0 | 13 |
| 2.00 < PCT ≤ 10.00 | 0 | 6 | 95 | 2 | 103 |
| >10.00 | 0 | 0 | 3 | 32 | 35 |
| Total | 0 | 11 | 106 | 34 | 234 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, X.-Y.; Wang, C.-R.; Xie, C.-M.; Wang, X.-G.; Liang, R.-L.; Xu, W.-W. Rapid and Sensitive Lateral Flow Immunoassay Method for Procalcitonin (PCT) Based on Time-Resolved Immunochromatography. Sensors 2017, 17, 480. https://doi.org/10.3390/s17030480
Shao X-Y, Wang C-R, Xie C-M, Wang X-G, Liang R-L, Xu W-W. Rapid and Sensitive Lateral Flow Immunoassay Method for Procalcitonin (PCT) Based on Time-Resolved Immunochromatography. Sensors. 2017; 17(3):480. https://doi.org/10.3390/s17030480
Chicago/Turabian StyleShao, Xiang-Yang, Cong-Rong Wang, Chun-Mei Xie, Xian-Guo Wang, Rong-Liang Liang, and Wei-Wen Xu. 2017. "Rapid and Sensitive Lateral Flow Immunoassay Method for Procalcitonin (PCT) Based on Time-Resolved Immunochromatography" Sensors 17, no. 3: 480. https://doi.org/10.3390/s17030480
APA StyleShao, X.-Y., Wang, C.-R., Xie, C.-M., Wang, X.-G., Liang, R.-L., & Xu, W.-W. (2017). Rapid and Sensitive Lateral Flow Immunoassay Method for Procalcitonin (PCT) Based on Time-Resolved Immunochromatography. Sensors, 17(3), 480. https://doi.org/10.3390/s17030480





